Interstitial Lung Disease Associated with Lung Cancer: A Case–Control Study
Abstract
1. Introduction
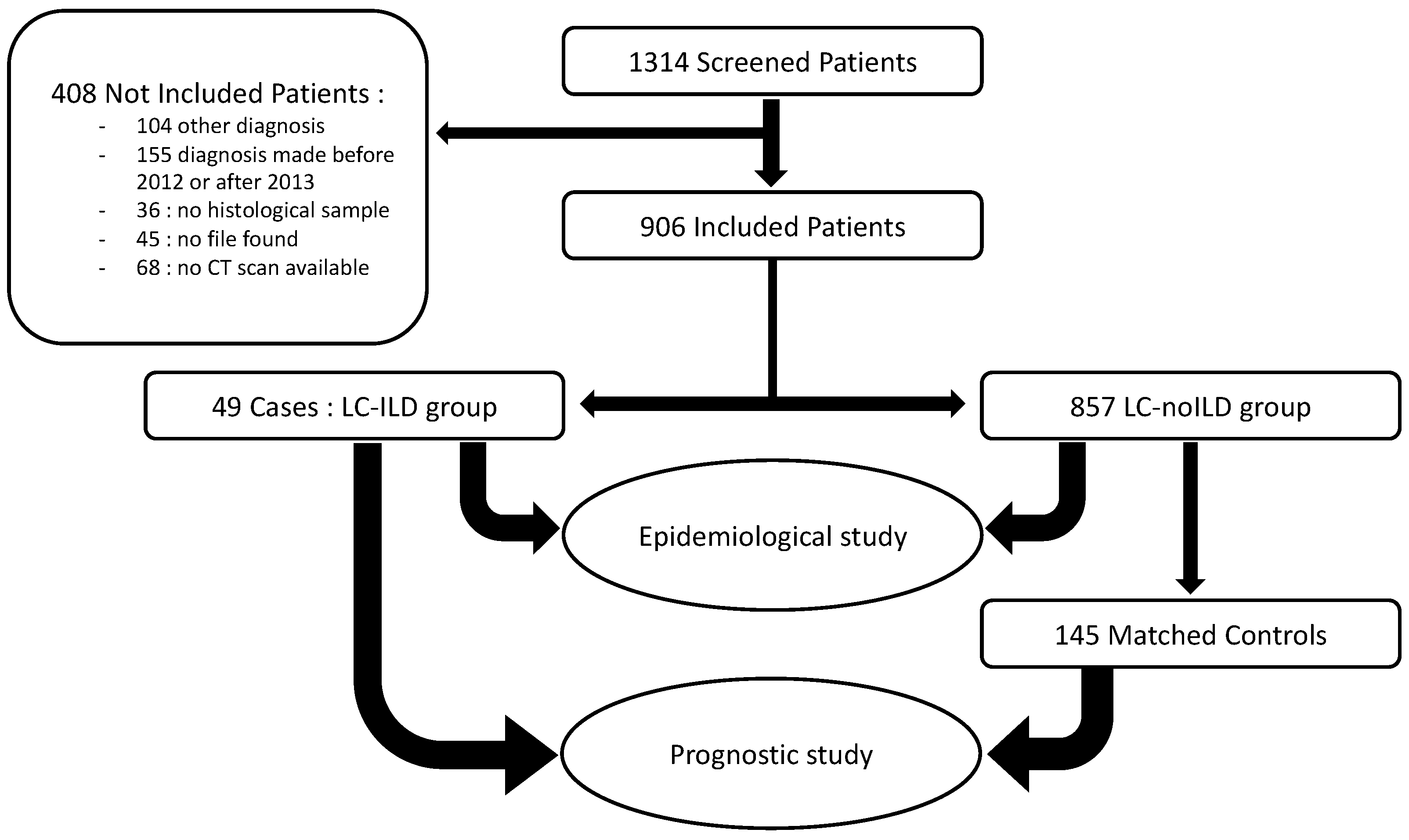
2. Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Study Design
2.4. Statistical Analyses
3. Results
3.1. ILD Frequency and Characteristics
3.2. LC–ILD and LC–noILD Patients’ Comparisons
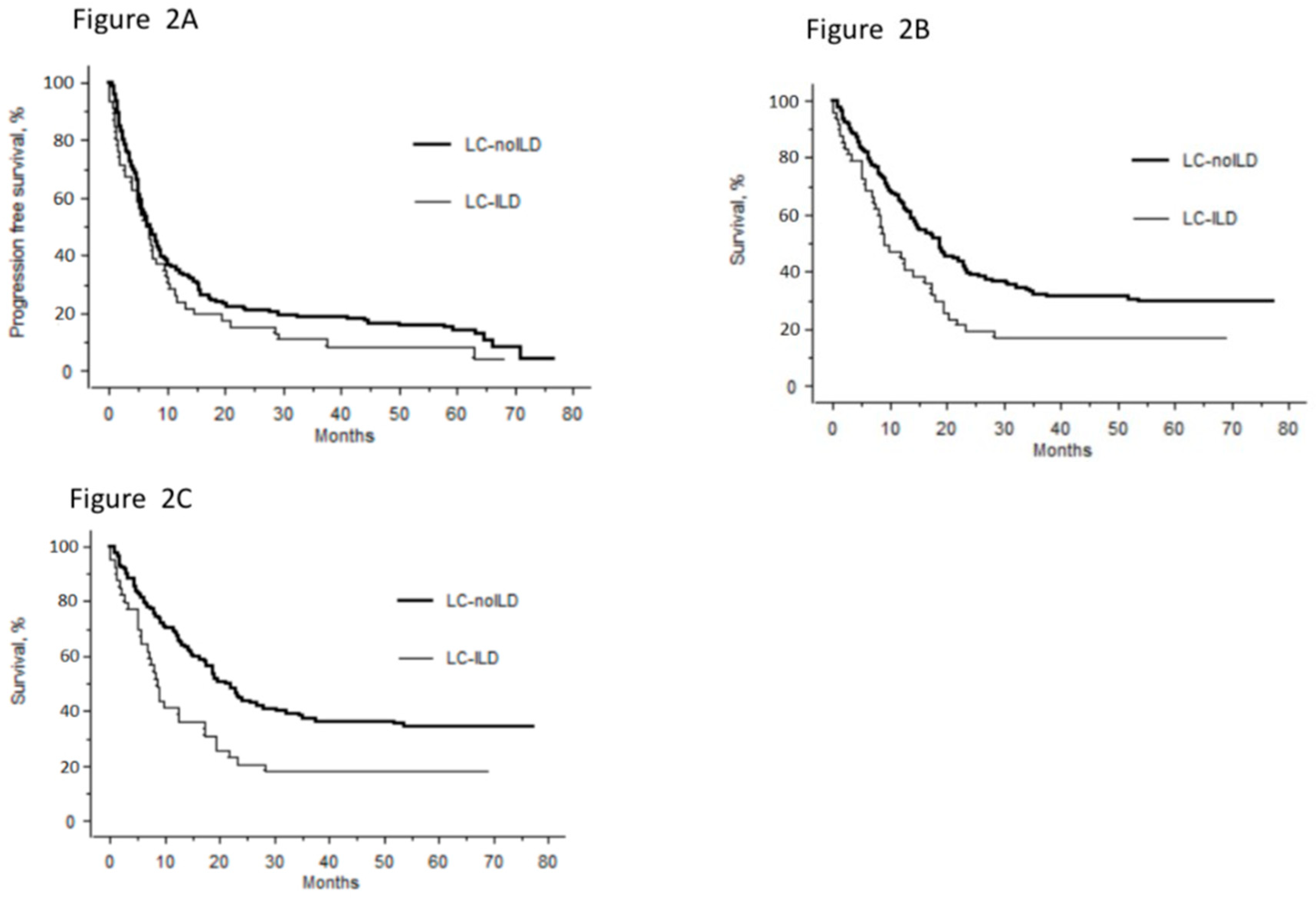
3.3. Case-Control Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mizushima, Y.; Kobayashi, M. Clinical characteristics of synchronous multiple lung cancer associated with idiopathic pulmonary fibrosis. A review of Japanese cases. Chest 1995, 108, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Turner-Warwick, M.; Lebowitz, M. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980, 35, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Bouros, D.; Hatzakis, K. Association of malignancy with diseases causing interstitial pulmonary changes. Chest 2002, 121, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Failla, M. Idiopathic pulmonary fibrosis: A disease with similarities and links to cancer biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef]
- Scrimini, S.; Pons, H. Expansion of myeloid-derived suppressor cells in chronic obstructive pulmonary disease and lung cancer: Potential link between inflammation and cancer. Cancer Immunol. Immunother. 2015, 64, 1261–1270. [Google Scholar] [CrossRef]
- Naccache, J.M.; Gibiot, Q. Lung cancer and interstitial lung disease: A literature review. J. Thorac. Dis. 2018, 10, 3829–3844. [Google Scholar] [CrossRef]
- Hubbard, R.; Venn, A. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am. J. Respir. Crit. Care Med. 2000, 161, 5–8. [Google Scholar] [CrossRef]
- Le Jeune, I.; Gribbin, J. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir. Med. 2007, 101, 2534–2540. [Google Scholar] [CrossRef]
- Harris, J.M.; Johnston, I.D.A. Cryptogenic fibrosing alveolitis and lung cancer: The BTS study. Thorax 2010, 65, 70–76. [Google Scholar] [CrossRef]
- Hironaka, M.; Fukayama, M. Pulmonary fibrosis and lung carcinoma: A comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol. Int. 1999, 49, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Gurioli, C. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015, 147, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, D.S. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2001, 17, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Ushijima, T. The frequency of epidermal growth factor receptor mutation of nonsmall cell lung cancer according to the underlying pulmonary diseases. Pulm. Med. 2011, 2011, 290132. [Google Scholar] [CrossRef]
- Kinoshita, T.; Azuma, K. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol. Lett. 2012, 4, 477–482. [Google Scholar] [CrossRef][Green Version]
- Sato, T.; Watanabe, A.; Kondo, H.; Kanzaki, M.; Okubo, K.; Yokoi, K.; Matsumoto, K.; Marutsuka, T.; Shinohara, H.; Teramukai, S.; et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J. Thorac. Cardiovasc. Surg. 2015, 149, 64–69. [Google Scholar] [CrossRef]
- Kumar, P.; Goldstraw, P. Pulmonary fibrosis and lung cancer: Risk and benefit analysis of pulmonary resection. J. Thorac. Cardiovasc. Surg. 2003, 125, 1321–1327. [Google Scholar] [CrossRef]
- Khan, K.A.; Kennedy, M.P. Radiological characteristics, histological features and clinical outcomes of lung cancer patients with coexistent idiopathic pulmonary fibrosis. Lung 2015, 193, 71–77. [Google Scholar] [CrossRef]
- Vallières, E.; Shepherd, F.A. The IASLC Lung Cancer Staging Project: Proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 1049–1059. [Google Scholar] [CrossRef]
- Brambilla, E.; Travis, W.D. The new World Health Organization classification of lung tumours. Eur. Respir. J. 2001, 18, 1059–1068. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.I.S.; Müller, N.L. Nonspecific interstitial pneumonia and idiopathic pulmonary fibrosis: Changes in pattern and distribution of disease over time. Radiology 2008, 247, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Adjei, A.A. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Crinò, L.; Weder, W. ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.; Pijls-Johannesma, M. ESMO Guidelines Working Group. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 120–125. [Google Scholar]
- Voltolini, L.; Bongiolatti, S. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: Analysis of risk factors. Eur. J. Cardio-Thorac. Surg. 2013, 43, e17–e23. [Google Scholar] [CrossRef]
- Salvatore, M.; Henschke, C.I. Evidence of Interstitial Lung Disease on Low-Dose Chest CT Images: Prevalence, Patterns, and Progression. AJR Am. J. Roentgenol. 2016, 206, 487–494. [Google Scholar] [CrossRef]
- Voltz, J.W.; Card, J.W. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 45–52. [Google Scholar] [CrossRef]
- Girard, N.; Marchand-Adam, S. Lung cancer in combined pulmonary fibrosis and emphysema: A series of 47 Western patients. J. Thorac. Oncol. 2014, 9, 1162–1170. [Google Scholar] [CrossRef]
- Watanabe, N.; Taniguchi, H. Efficacy of chemotherapy for advanced non-small cell lung cancer with idiopathic pulmonary fibrosis. Respir. Int. Rev. Thorac. Dis. 2013, 85, 326–331. [Google Scholar] [CrossRef]
- Okuda, K.; Hirose, T. Evaluation of the safety and efficacy of combination chemotherapy with vinorelbine and platinum agents for patients with non-small cell lung cancer with interstitial lung disease. Anticancer Res. 2012, 32, 5475–5480. [Google Scholar] [PubMed]
- Goto, T.; Maeshima, A. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int. J. Clin. Oncol. 2014, 19, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kawai, Y. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann. Thorac. Surg. 2011, 92, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.; Park, C.-M. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir. Med. 2014, 108, 524–530. [Google Scholar] [CrossRef]
- Sekihara, K.; Aokage, K. Long-term survival after complete resection of non-small-cell lung cancer in patients with interstitial lung disease. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 638–643. [Google Scholar] [CrossRef]
- Kanaji, N.; Tadokoro, A. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J. Cancer Res. Clin. Oncol. 2016, 142, 1855–1865. [Google Scholar] [CrossRef]
- Nishino, M.; Cardarella, S. Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival. Eur. J. Radiol. 2015, 84, 998–1004. [Google Scholar] [CrossRef]
- Yano, M.; Yoshida, J. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2015, 47, 135–142. [Google Scholar] [CrossRef]
- Fujimoto, T.; Okazaki, T. Operation for lung cancer in patients with idiopathic pulmonary fibrosis: Surgical contraindication? Ann. Thorac. Surg. 2003, 76, 1674–1678. [Google Scholar] [CrossRef]
- Onishi, H.; Yamashita, H. Stereotactic Body Radiation Therapy for Patients with Pulmonary Interstitial Change: High Incidence of Fatal Radiation Pneumonitis in a Retrospective Multi-Institutional Study. Cancers 2018, 10, 257. [Google Scholar] [CrossRef]
- Kanai, O.; Kim, Y.H. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac. Cancer 2018, 9, 847–855. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | LC–ILD (n = 49) | LC–noILD (n = 857) | p Value |
|---|---|---|---|
| Males, n (%) | 41 (83.7) | 567 (66.2) | 0.017 |
| Mean age at diagnosis, years, ± SD | 66.4 ± 8.8 | 64.7 ± 11.3 | 1 |
| Smoking history | |||
| Non-smoker/ever-smoker, % | 8.2/91.8 | 12.7/87.3 | 0.47 |
| Current smoker/ex-smoker, % | 60/40 | 60.3/39.7 | 1 |
| Mean pack-years, ± SD | 44.4 ± 22.0 | 45.4 ± 25.9 | 1 |
| Performans status: 0–1/2–4/U, % | 47/25/29 | 59/20/22 | 0.19 |
| Asbestos: NE/U/ARW/E, % | 59.2/18.4/6.1/16.3 | 59.4/17.0/10.7/12.8 | 0.70 |
| Lung-cancer histology, n a | 51 | 866 | 0.08 |
| Adenocarcinoma, % | 47.1 | 58.7 | |
| Squamous carcinoma, % | 19.6 | 19.6 | |
| Undifferentiated carcinoma, % | 13.7 | 4.5 | |
| Small-cell carcinoma, % | 15.7 | 12.0 | |
| Others b, % | 4.0 | 5.2 | |
| Lung cancer stage, % | |||
| I/II/III/IV NSCLCs | 20.9/11.6/20.9/46.5 | 14.4/8.1/22.0/55.4 | 0.35 |
| LS/ES SCLCs | 50/50 | 30.8/69.2 | 0.50 |
| Synchronous LC, n (%) | 2 (4.1) | 9 (1.1) | 0.1 |
| Mutation analysis, n subjects c | 23 | 438 | 0.56 |
| Unknown status, n (%) | 6 (26.1) | 63 (14.4) | |
| Wild-type, n (%) | 10 (43.5) | 172 (39.3) | |
| Mutation+, n (%) | 7 (30.4) | 199 (45.4) | |
| EGFR/KRAS/ALK, n (%) | 1 (4.3)/4(17.4)/1 (4.3) | 59 (13.5)/101(23.1)/20 (4.6) | |
| Rare mutations *, n (%) | 1 (4.3) | 23 (5.3) |
| Characteristic | LC–ILD Cases (n = 49) | LC–noILD Controls (n = 145) |
|---|---|---|
| Males, n (%) | 41 (83.7) | 121 (83.4) |
| Mean age at diagnosis, years, ± SD | 66.4 ± 8.8 | 66.4 ± 11.5 |
| Smoking | ||
| Ever-smoker, % | 92 | 89 |
| Mean pack-years, ± SD | 44 ± 22 | 49 ± 27 |
| Mean body mass index, ± SD | 23.8 ± 4.3 | 24.5 ± 4.9 |
| Mean performance status, ± SD | 1 ± 0.9 | 1 ± 1 |
| Comorbidities | ||
| Chronic obstructive lung disease | 10 (20.4%) | 21 (14.5%) |
| Diabetes | 8 (16.3%) | 17 (11.7%) |
| Cardiovascular | 26 (53.1%) | 63 (43.4%) |
| Lung-cancer histology, n | 49 | 145 |
| Adenocarcinoma, (%) | 23 (46.9%) | 69 (47.6%) |
| Squamous carcinoma, (%) | 10 (20.4%) | 30 (20.7%) |
| Undifferentiated carcinoma, (%) | 6 (12.2%) | 17 (11.7%) |
| Small-cell carcinoma, (%) | 8 (16.3%) | 24 (16.6%) |
| Others, (%) | 2 (4.1%) | 5 (3.4%) |
| Lung cancer stage, % | ||
| I/II/III/IV NSCLCs | 17/10/22/51 | 16/12/21/1 |
| LS/ES SCLCs | 50/50 | 50/50 |
| Univariate | Multivariate (n = 177) | Multivariate Descending Stepwise (n = 193) | ||||
|---|---|---|---|---|---|---|
| Factor | n | p | HR 95% CI | p | HR 95% CI | p |
| Age | 194 | 0.01 | 1.01 (0.99–1.03) | 0.23 | ||
| Sex | ||||||
| Female | 32 | 0.31 | ||||
| Male | 162 | |||||
| Body mass index | 178 | 0.097 | 0.96 (0.92–1.00) | 0.08 | ||
| Smoker | ||||||
| No | 20 | 0.05 | Reference | 0.01 | ||
| Yes | 174 | 2.67 (1.22–5.81) | ||||
| Chronic obstructive lung disease | ||||||
| No | 163 | 0.4 | ||||
| Yes | 31 | |||||
| Diabetes mellitus | ||||||
| No | 169 | 0.09 | Reference | |||
| Yes | 25 | 0.57 (0.32–1.02) | 0.06 | |||
| Cardiovascular comorbidities | ||||||
| No | 105 | 0.56 | ||||
| Yes | 89 | |||||
| Interstitial lung disease | ||||||
| No | 145 | 0.059 | Reference | |||
| Yes | 49 | 1.80 (1.21–2.67) | 0.004 | 1.81 (1.24–2.64) | 0.002 | |
| Standard-of-care management | ||||||
| No | 44 | 0.002 | Reference | |||
| Yes | 149 | 0.61 (0.39–0.97) | 0.04 | 0.5 (0.34–0.73) | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibiot, Q.; Monnet, I.; Levy, P.; Brun, A.-L.; Antoine, M.; Chouaïd, C.; Cadranel, J.; Naccache, J.-M. Interstitial Lung Disease Associated with Lung Cancer: A Case–Control Study. J. Clin. Med. 2020, 9, 700. https://doi.org/10.3390/jcm9030700
Gibiot Q, Monnet I, Levy P, Brun A-L, Antoine M, Chouaïd C, Cadranel J, Naccache J-M. Interstitial Lung Disease Associated with Lung Cancer: A Case–Control Study. Journal of Clinical Medicine. 2020; 9(3):700. https://doi.org/10.3390/jcm9030700
Chicago/Turabian StyleGibiot, Quentin, Isabelle Monnet, Pierre Levy, Anne-Laure Brun, Martine Antoine, Christos Chouaïd, Jacques Cadranel, and Jean-Marc Naccache. 2020. "Interstitial Lung Disease Associated with Lung Cancer: A Case–Control Study" Journal of Clinical Medicine 9, no. 3: 700. https://doi.org/10.3390/jcm9030700
APA StyleGibiot, Q., Monnet, I., Levy, P., Brun, A.-L., Antoine, M., Chouaïd, C., Cadranel, J., & Naccache, J.-M. (2020). Interstitial Lung Disease Associated with Lung Cancer: A Case–Control Study. Journal of Clinical Medicine, 9(3), 700. https://doi.org/10.3390/jcm9030700





