Evaluating the Efficacy of L-N-acetylcysteine and Dexamethasone in Combination to Provide Otoprotection for Electrode Insertion Trauma
Abstract
1. Introduction
2. Materials and Methods
2.1. Organ of Corti (OC) Dissections
2.2. FITC-Phalloidin Staining
2.3. Oxidative Stress Determination
2.4. Determination of Cytokine Production
2.5. Measurement of Nitric Oxide (NO) Release
2.6. Statistical Analysis
3. Results
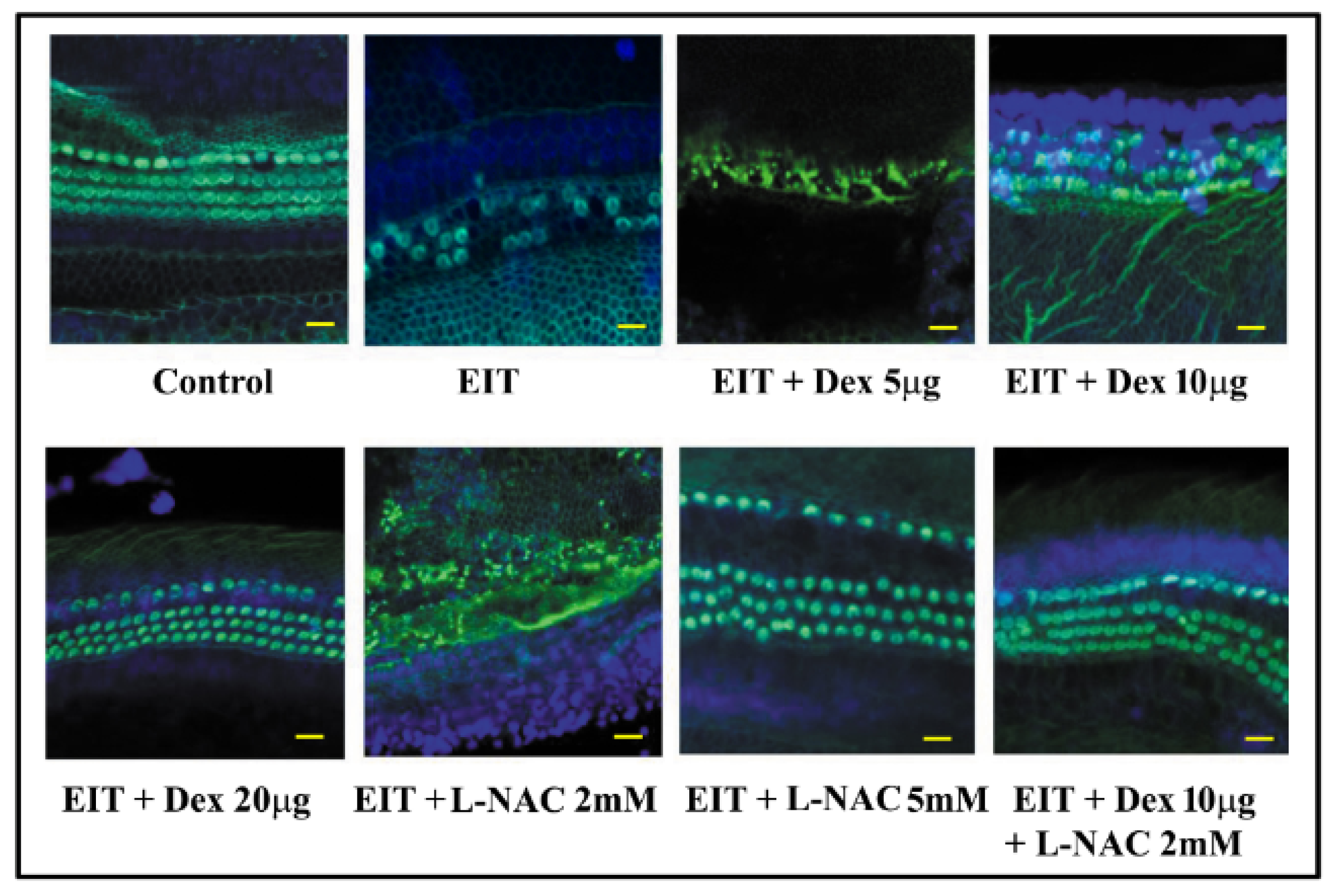
3.1. Effect of L-NAC and Dex on Hair Cell Viability
3.2. L-NAC and Dex Significantly Downregulate Oxidative Stress
3.3. L-NAC and Dex Significantly Decrease the Production of Proinflammatory Cytokines
3.4. L-NAC and Dex Attenuate NO Production
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eshraghi, A.A.; Nazarian, R.; Telischi, F.F.; Rajguru, S.M.; Truy, E.; Gupta, C. The cochlear implant: Historical aspects and future prospects. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2012, 295, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.C.; Walker, E.A.; Gogel, S.; Van Voorst, T.; Hansen, M.; Gantz, B.J. Bilateral Cochlear Implants Using Two Electrode Lengths in Infants With Profound Deafness. Otol. Neurotol. 2019, 40, e267–e276. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Deafness and Other Communication Disorders. Cochlear Implants. Available online: https://www.nidcd.nih.gov/health/cochlear-implants#c (accessed on 20 August 2019).
- Quesnel, A.M.; Nakajima, H.H.; Rosowski, J.J.; Hansen, M.R.; Gantz, B.J.; Nadol, J.B., Jr. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear. Res. 2016, 333, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.A.; Ahmed, J.; Krysiak, E.; Ila, K.; Ashman, P.; Telischi, F.F.; Angeli, S.; Prentiss, S.; Martinez, D.; Valendia, S. Clinical, surgical, and electrical factors impacting residual hearing in cochlear implant surgery. Acta Otolaryngol. 2017, 137, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.A.; Lang, D.M.; Roell, J.; Van De Water, T.R.; Garnham, C.; Rodrigues, H.; Guardiola, M.; Gupta, C.; Mittal, J. Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma. Acta Otolaryngol. 2015, 135, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.; Chambers, S.; Hampson, A.; Eastwood, H.; Campbell, L.; O’Leary, S. Delayed low frequency hearing loss caused by cochlear implantation interventions via the round window but not cochleostomy. Hear. Res. 2016, 333, 49–57. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Francois, F.; Uziel, A.; Puel, J.L.; Venail, F. Molecular and cellular mechanisms of loss of residual hearing after cochlear implantation. Ann. Otol. Rhinol. Laryngol. 2013, 122, 33–39. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Roell, J.; Shaikh, N.; Telischi, F.F.; Bauer, B.; Guardiola, M.; Bas, E.; Van De Water, T.; Rivera, I.; Mittal, J. A novel combination of drug therapy to protect residual hearing post cochlear implant surgery. Acta Otolaryngol. 2016, 136, 420–424. [Google Scholar] [CrossRef]
- Zhou, L.; Friedmann, D.R.; Treaba, C.; Peng, R.; Roland, J.T., Jr. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope 2015, 125, 966–971. [Google Scholar] [CrossRef]
- Bas, E.; Gupta, C.; Van De Water, T.R. A novel organ of corti explant model for the study of cochlear implantation trauma. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2012, 295, 1944–1956. [Google Scholar] [CrossRef]
- Li, C.; Kuhlmey, M.; Kim, A.H. Electroacoustic Stimulation. Otolaryngol. Clin. N. Am. 2019, 52, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Gautschi-Mills, K.; Khoza-Shangase, K.; Pillay, D. Preservation of residual hearing after cochlear implant surgery: An exploration of residual hearing function in a group of recipients at cochlear implant units. Braz. J. Otorhinolaryngol. 2019, 85, 310–318. [Google Scholar] [CrossRef]
- Balkany, T.J.; Connell, S.S.; Hodges, A.V.; Payne, S.L.; Telischi, F.F.; Eshraghi, A.A.; Angeli, S.I.; Germani, R.; Messiah, S.; Arheart, K.L. Conservation of residual acoustic hearing after cochlear implantation. Otol. Neurotol. 2006, 27, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Gantz, B.J.; Dunn, C.C.; Oleson, J.; Hansen, M.R. Acoustic plus electric speech processing: Long-term results. Laryngoscope 2018, 128, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Haake, S.M.; Dinh, C.T.; Chen, S.; Eshraghi, A.A.; Van De Water, T.R. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear. Res. 2009, 255, 22–32. [Google Scholar] [CrossRef]
- Rybak, L.P.; Dhukhwa, A.; Mukherjea, D.; Ramkumar, V. Local Drug Delivery for Prevention of Hearing Loss. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Kopke, R.D.; Coleman, J.K.; Liu, J.; Campbell, K.C.; Riffenburgh, R.H. Candidate’s thesis: Enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope 2002, 112, 1515–1532. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: Oxidative stress and ROS signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Eshraghi, A.A. Prevention of cochlear implant electrode damage. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 323–328. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, C.H. Noise-Induced Neural Degeneration and Therapeutic Effect of Antioxidant Drugs. J. Audiol. Otol. 2015, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.I.; Abi-Hachem, R.N.; Vivero, R.J.; Telischi, F.T.; Machado, J.J. L-N-Acetylcysteine treatment is associated with improved hearing outcome in sudden idiopathic sensorineural hearing loss. Acta Otolaryngol. 2012, 132, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Elbini Dhouib, I.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, H.; Chen, G.D.; Longo-Guess, C.; Muthaiah, V.P.; Tian, C.; Sheppard, A.; Salvi, R.; Johnson, K.R. N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in gamma-glutamyl transferase 1 deficient mice. Aging 2016, 8, 730–750. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Ding, X.; Zhao, Q.; Chen, J.; Tian, K.; Qiu, Y.; Lu, L. N-Acetylcysteine protects inner ear hair cells and spiral ganglion neurons from manganese exposure by regulating ROS levels. Toxicol. Lett. 2017, 279, 77–86. [Google Scholar] [CrossRef]
- Ada, S.; Hanci, D.; Ulusoy, S.; Vejselova, D.; Burukoglu, D.; Muluk, N.B.; Cingi, C. Potential protective effect of N-acetyl cysteine in acoustic trauma: An experimental study using scanning electron microscopy. Adv. Clin. Exp. Med. 2017, 26, 893–897. [Google Scholar] [CrossRef]
- Tillinger, J.A.; Gupta, C.; Ila, K.; Ahmed, J.; Mittal, J.; Van De Water, T.R.; Eshraghi, A.A. l-N-acetylcysteine protects outer hair cells against TNFalpha initiated ototoxicity in vitro. Acta Otolaryngol. 2018, 138, 676–684. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Matsumoto, K.; Takeshita, I.; Horie, T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br. J. Pharmacol. 2001, 132, 270–276. [Google Scholar] [CrossRef]
- Habybabady, R.H.; Mortazavi, S.B.; Khavanin, A.; Mirzaei, R.; Arab, M.R.; Mesbahzadeh, B.; Hoseini, M.; Mohammadi, M. Protective Effects of N-Acetyl-L-Cysteine on the Density of Spiral Ganglion Cells and Histological Changes Induced by Continuous Noise Exposure in Rats. Malays. J. Med. Sci. 2018, 25, 48–58. [Google Scholar] [CrossRef]
- Terakado, M.; Kumagami, H.; Takahashi, H. Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. Hear. Res. 2011, 280, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Curtis, L.M.; Yao, X.; ten Cate, W.J.; Bagger-Sjoback, D.; Hultcrantz, M.; Rarey, K.E. Glucocorticoid receptor expression in the postnatal rat cochlea. Hear. Res. 1995, 87, 220–227. [Google Scholar] [CrossRef]
- Lee, S.H.; Lyu, A.R.; Shin, S.A.; Jeong, S.H.; Lee, S.A.; Park, M.J.; Park, Y.H. Cochlear Glucocorticoid Receptor and Serum Corticosterone Expression in a Rodent Model of Noise-induced Hearing Loss: Comparison of Timing of Dexamethasone Administration. Sci. Rep. 2019, 9, 12646. [Google Scholar] [CrossRef] [PubMed]
- Tahera, Y.; Meltser, I.; Johansson, P.; Bian, Z.; Stierna, P.; Hansson, A.C.; Canlon, B. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. J. Neurosci. Res. 2006, 83, 1066–1076. [Google Scholar] [CrossRef]
- Morawski, K.; Telischi, F.F.; Bohorquez, J.; Niemczyk, K. Preventing hearing damage using topical dexamethasone during reversible cochlear ischemia: An animal model. Otol. Neurotol. 2009, 30, 851–857. [Google Scholar] [CrossRef]
- Heinrich, U.R.; Strieth, S.; Schmidtmann, I.; Stauber, R.; Helling, K. Dexamethasone prevents hearing loss by restoring glucocorticoid receptor expression in the guinea pig cochlea. Laryngoscope 2016, 126, E29–E34. [Google Scholar] [CrossRef]
- El Sabbagh, N.G.; Sewitch, M.J.; Bezdjian, A.; Daniel, S.J. Intratympanic dexamethasone in sudden sensorineural hearing loss: A systematic review and meta-analysis. Laryngoscope 2017, 127, 1897–1908. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Yamashita, D.; Minami, S.B.; Yamasoba, T.; Miller, J.M. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 2007, 226, 22–43. [Google Scholar] [CrossRef]
- Dinh, C.T.; Bas, E.; Chan, S.S.; Dinh, J.N.; Vu, L.; Van De Water, T.R. Dexamethasone treatment of tumor necrosis factor-alpha challenged organ of Corti explants activates nuclear factor kappa B signaling that induces changes in gene expression that favor hair cell survival. Neuroscience 2011, 188, 157–167. [Google Scholar] [CrossRef]
- Gumrukcu, S.S.; Topaloglu, I.; Salturk, Z.; Tutar, B.; Atar, Y.; Berkiten, G.; Goker, A.E. Effects of intratympanic dexamethasone on noise-induced hearing loss: An experimental study. Am. J. Otolaryngol. 2018, 39, 71–73. [Google Scholar] [CrossRef]
- Han, M.A.; Back, S.A.; Kim, H.L.; Park, S.Y.; Yeo, S.W.; Park, S.N. Therapeutic Effect of Dexamethasone for Noise-induced Hearing Loss: Systemic Versus Intratympanic Injection in Mice. Otol. Neurotol. 2015, 36, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Harrop-Jones, A.; Wang, X.; Fernandez, R.; Dellamary, L.; Ryan, A.F.; LeBel, C.; Piu, F. The Sustained-Exposure Dexamethasone Formulation OTO-104 Offers Effective Protection against Noise-Induced Hearing Loss. Audiol. Neurotol. 2016, 21, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Harrop-Jones, A.; Wang, X.; Dellamary, L.; LeBel, C.; Piu, F. The Sustained-Exposure Dexamethasone Formulation OTO-104 Offers Effective Protection against Cisplatin-Induced Hearing Loss. Audiol. Neurotol. 2016, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, X.; Chen, D.; Lin, X.; Yu, D.; Wu, H. Dexamethasone loaded nanoparticles exert protective effects against Cisplatin-induced hearing loss by systemic administration. Neurosci. Lett. 2016, 619, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, E.C.; Kobel, M.J. Advances and Challenges in Pharmaceutical Therapies to Prevent and Repair Cochlear Injuries From Noise. Front. Cell. Neurosci. 2019, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, R.; Ogita, K. Enhanced biosynthesis of glutathione in the spiral ganglion of the cochlea after in vivo treatment with dexamethasone in mice. Brain Res. 2006, 1117, 101–108. [Google Scholar] [CrossRef]
- Kuthubutheen, J.; Joglekar, S.; Smith, L.; Friesen, L.; Smilsky, K.; Millman, T.; Ng, A.; Shipp, D.; Coates, H.; Arnoldner, C.; et al. The Role of Preoperative Steroids for Hearing Preservation Cochlear Implantation: Results of a Randomized Controlled Trial. Audiol. Neurotol. 2017, 22, 292–302. [Google Scholar] [CrossRef]
- Hamid, M.; Trune, D. Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 434–440. [Google Scholar] [CrossRef]
- Spear, S.A.; Schwartz, S.R. Intratympanic steroids for sudden sensorineural hearing loss: A systematic review. Otolaryngol. Head Neck Surg. 2011, 145, 534–543. [Google Scholar] [CrossRef]
- Kopke, R.D.; Jackson, R.L.; Coleman, J.K.; Liu, J.; Bielefeld, E.C.; Balough, B.J. NAC for noise: From the bench top to the clinic. Hear. Res. 2007, 226, 114–125. [Google Scholar] [CrossRef]
- Eastwood, H.; Pinder, D.; James, D.; Chang, A.; Galloway, S.; Richardson, R.; O’Leary, S. Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation. Hear. Res. 2010, 259, 24–30. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshraghi, A.A.; Shahal, D.; Davies, C.; Mittal, J.; Shah, V.; Bulut, E.; Garnham, C.; Sinha, P.; Mishra, D.; Marwede, H.; et al. Evaluating the Efficacy of L-N-acetylcysteine and Dexamethasone in Combination to Provide Otoprotection for Electrode Insertion Trauma. J. Clin. Med. 2020, 9, 716. https://doi.org/10.3390/jcm9030716
Eshraghi AA, Shahal D, Davies C, Mittal J, Shah V, Bulut E, Garnham C, Sinha P, Mishra D, Marwede H, et al. Evaluating the Efficacy of L-N-acetylcysteine and Dexamethasone in Combination to Provide Otoprotection for Electrode Insertion Trauma. Journal of Clinical Medicine. 2020; 9(3):716. https://doi.org/10.3390/jcm9030716
Chicago/Turabian StyleEshraghi, Adrien A., David Shahal, Camron Davies, Jeenu Mittal, Viraj Shah, Erdogan Bulut, Carolyn Garnham, Priyanka Sinha, Dibyanshi Mishra, Hannah Marwede, and et al. 2020. "Evaluating the Efficacy of L-N-acetylcysteine and Dexamethasone in Combination to Provide Otoprotection for Electrode Insertion Trauma" Journal of Clinical Medicine 9, no. 3: 716. https://doi.org/10.3390/jcm9030716
APA StyleEshraghi, A. A., Shahal, D., Davies, C., Mittal, J., Shah, V., Bulut, E., Garnham, C., Sinha, P., Mishra, D., Marwede, H., & Mittal, R. (2020). Evaluating the Efficacy of L-N-acetylcysteine and Dexamethasone in Combination to Provide Otoprotection for Electrode Insertion Trauma. Journal of Clinical Medicine, 9(3), 716. https://doi.org/10.3390/jcm9030716






