Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and methods
2.1. Patients
2.2. Treatment, Efficacy Evaluation, and Assessment of Baseline Tumor Burden
2.3. PET Imaging and Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Assessment of SUVmax, MTV, and TLG on 18F-FDG Uptake
3.2. Patient Demographics
3.3. Survival and 18F-FDG-PET:
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Kaira, K.; Oriuchi, N.; Otani, Y.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Ishizuka, T.; Endo, K.; Mori, M. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest 2007, 131, 1019–1027. [Google Scholar] [CrossRef]
- Kaira, K.; Endo, M.; Abe, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Takahashi, T.; Murakami, H.; Tsuya, A.; Nakamura, Y.; et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J. Clin. Oncol. 2010, 28, 3746–3753. [Google Scholar] [CrossRef]
- Takada, K.; Toyokawa, G.; Okamoto, T.; Baba, S.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017, 6, 2552–2561. [Google Scholar] [CrossRef]
- Kasahara, N.; Kaira, K.; Yamaguchi, K.; Masubuchi, H.; Tsurumaki, H.; Hara, K.; Koga, Y.; Sakurai, R.; Higuchi, T.; Handa, T.; et al. Fluorodeoxyglucose uptake is associated with low tumor-infiltrating lymphocyte levels in patients with small cell lung cancer. Lung Cancer 2019, 134, 180–186. [Google Scholar] [CrossRef]
- Kaira, K.; Shimizu, K.; Kitahara, S.; Yajima, T.; Atsumi, J.; Kosaka, T.; Ohtaki, Y.; Higuchi, T.; Oyama, T.; Asao, T.; et al. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur. J. Cancer 2018, 101, 181–190. [Google Scholar] [CrossRef]
- Kaira, K.; Higuchi, T.; Naruse, I.; Arisaka, Y.; Tokue, A.; Altan, B.; Suda, S.; Mogi, A.; Shimizu, K.; Sunaga, N.; et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 56–66. [Google Scholar] [CrossRef]
- Im, H.J.; Pak, K.; Cheon, G.J.; Kang, K.W.; Kim, S.J.; Kim, I.J.; Chung, J.K.; Kim, E.E.; Lee, D.S. Prognostic value of volumetric parameters of 18F-FDG PET in non-small-cell lung cancer: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, M.; Nagano, T.; Tachihara, M.; Kiriu, T.; Furukawa, K.; Koyama, K.; Otoshi, T.; Sekiya, R.; Hazama, D.; Tamura, D.; et al. Baseline tumor size as predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res. 2019, 39, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Kawamura, K.; Ichikado, K.; Shingu, N.; Yasuda, Y.; Eguchi, Y.; Hisanaga, J.; Nitawaki, T.; Iio, M.; Sekido, Y.; et al. Comparisons between tumor burden and other prognostic factors that influence survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac. Cancer 2019, 10, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Ammari, S.; Champiat, S.; Massard, C.; Ferté, C.; Taihi, L.; Seban, R.D.; Aspeslagh, S.; Mahjoubi, L.; Kamsu-Kom, N.; et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long-term clinical benefit on anti-PD-1/L1 therapy. Eur. J. Cancer 2016, 65, 33–42. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics; opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 29, 325–340. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 1, 115. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwart, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumour: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Jreige, M.; Letovanec, I.; Chaba, K.; Renaud, S.; Rusakiewicz, S.; Cristina, V.; Peters, S.; Krueger, T.; de Leval, L.; Kandalaft, L.E.; et al. 18F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1859–1868. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. Cell death and immunity in cancer from danger signals to mimicry of pathogen defense responses. Immmunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef]
- Gkogkou, C.; Frangia, K.; Saif, M.W.; Trigidou, R.; Syrigos, K. Necrosis and apoptotic index as prognostic factors in non-small cell lung cancer: A review. Springerplus 2014, 3, 120. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhao, N.; Wu, Z.; Pan, N.; Shen, X.; Liu, T.; Wei, F.; You, J.; Xu, W.; Ren, X. New insight on the correlation of metabolic status on 18F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, in press. [Google Scholar] [CrossRef] [PubMed]
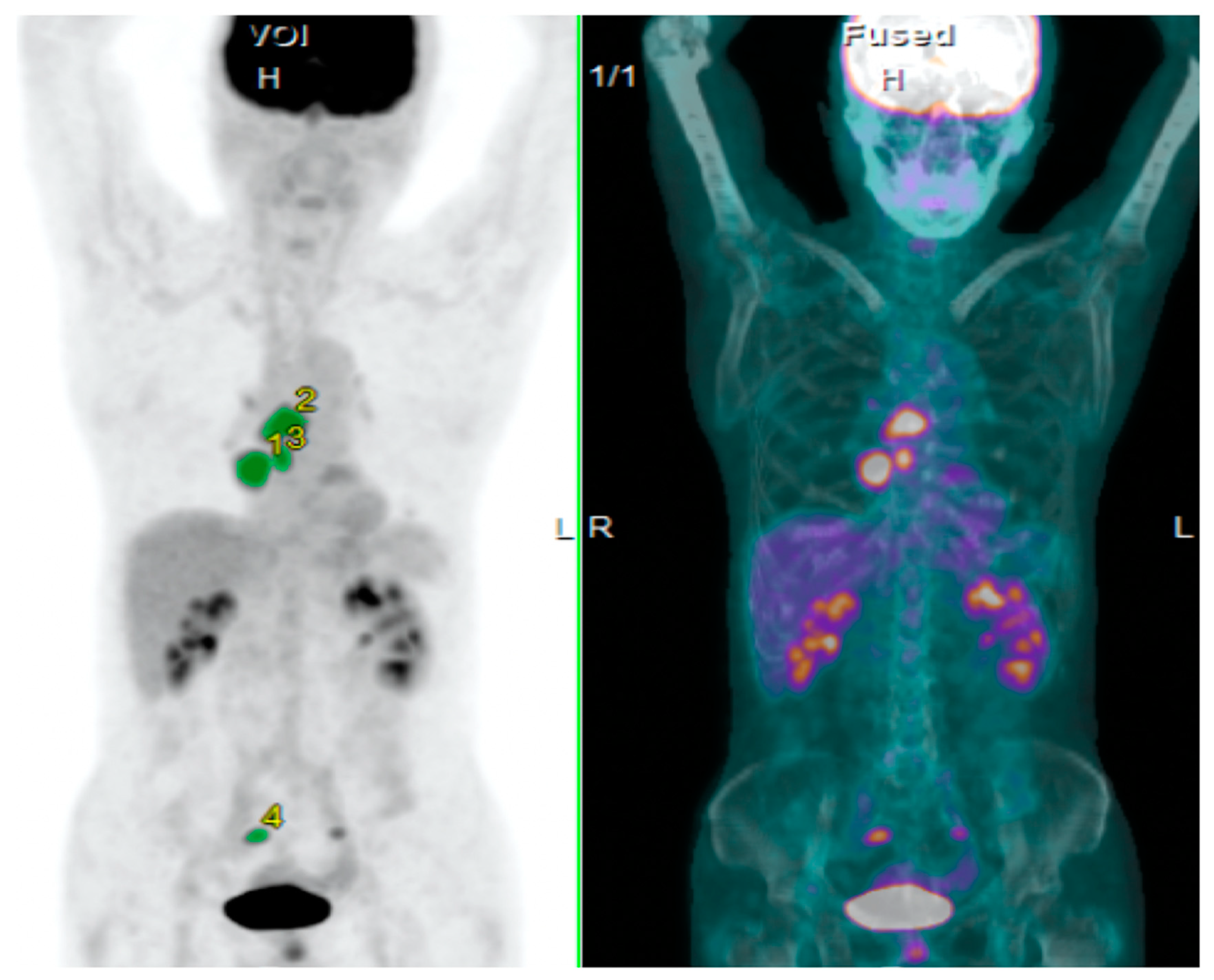

| Variables | Total (n = 85) | TLG | MTV | SUVmax | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High (n = 59) | Low (n = 26) | p-Value | High (n = 58) | Low (n = 27) | p-Value | High (n =5 2) | Low (n = 33) | p-Value | ||
| Age ≤ 70/>70 (range: 38–86 years) | 42/43 | 29/30 | 13/13 | >0.99 | 25/33 | 17/10 | 0.11 | 26/26 | 16/17 | >099 |
| Gender Male/Female | 65/20 | 47/12 | 18/8 | 0.41 | 46/12 | 19/8 | 0.42 | 42/10 | 23/10 | 0.29 |
| Smoking history Yes/No | 71/14 | 51/8 | 10/16 | <0.01 | 50/8 | 21/6 | 0.35 | 47/5 | 24/9 | 0.04 |
| Performance status 0 or 1/2 or 3 | 79/6 | 53/6 | 26/0 | 0.17 | 52/6 | 27/0 | 0.17 | 46/6 | 33/0 | 0.07 |
| Histological type AC/Non-AC | 51/34 | 33/26 | 18/8 | 0.34 | 32/26 | 19/8 | 0.24 | 27/25 | 24/9 | 0.07 |
| EGFR mutation Yes/No | 17/68 | 12/47 | 5/21 | >0.99 | 12/47 | 5/22 | >0.99 | 10/42 | 7/26 | >0.99 |
| Response to ICI # CR or PR/SD or PD CR, PR or SD/PD | 29/51 53/27 | 20/35 34/21 | 9/16 19/6 | >0.99 0.31 | 20/34 36/18 | 9/17 17/9 | >0.99 >0.99 | 21/27 34/15 | 8/24 19/12 | 0.10 0.47 |
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n = 85) | Patients without EGFR Mutation (n = 70) | All Patients (n = 85) | Patients without EGFR Mutation (n = 70) | |||||
| MST (days) | p-Value | MST (days) | p-Value | MST (days) | p-Value | MST (days) | p-Value | |
| Age (≤70/>70) | 737/693 | 0.73 | 865/693 | 0.85 | 164/161 | 0.88 | 181/180 | 0.92 |
| Gender (Male/Female) | 716/737 | 0.85 | 693/837 | 0.59 | 181/75 | 0.67 | 172/420 | 0.23 |
| Smoking (Yes/No) | 716/737 | 0.53 | 716/637 | 0.74 | 181/72 | 0.09 | 181/272 | 0.85 |
| PS (0 or 1/2 or 3) | 724/115 | <0.01 | 716/74 | <0.01 | 172/40 | <0.01 | 200/25 | 0.10 |
| Histological type (AC/Non-AC) | 724/716 | 0.84 | 693/716 | 0.66 | 146/161 | 0.76 | 220/161 | 0.39 |
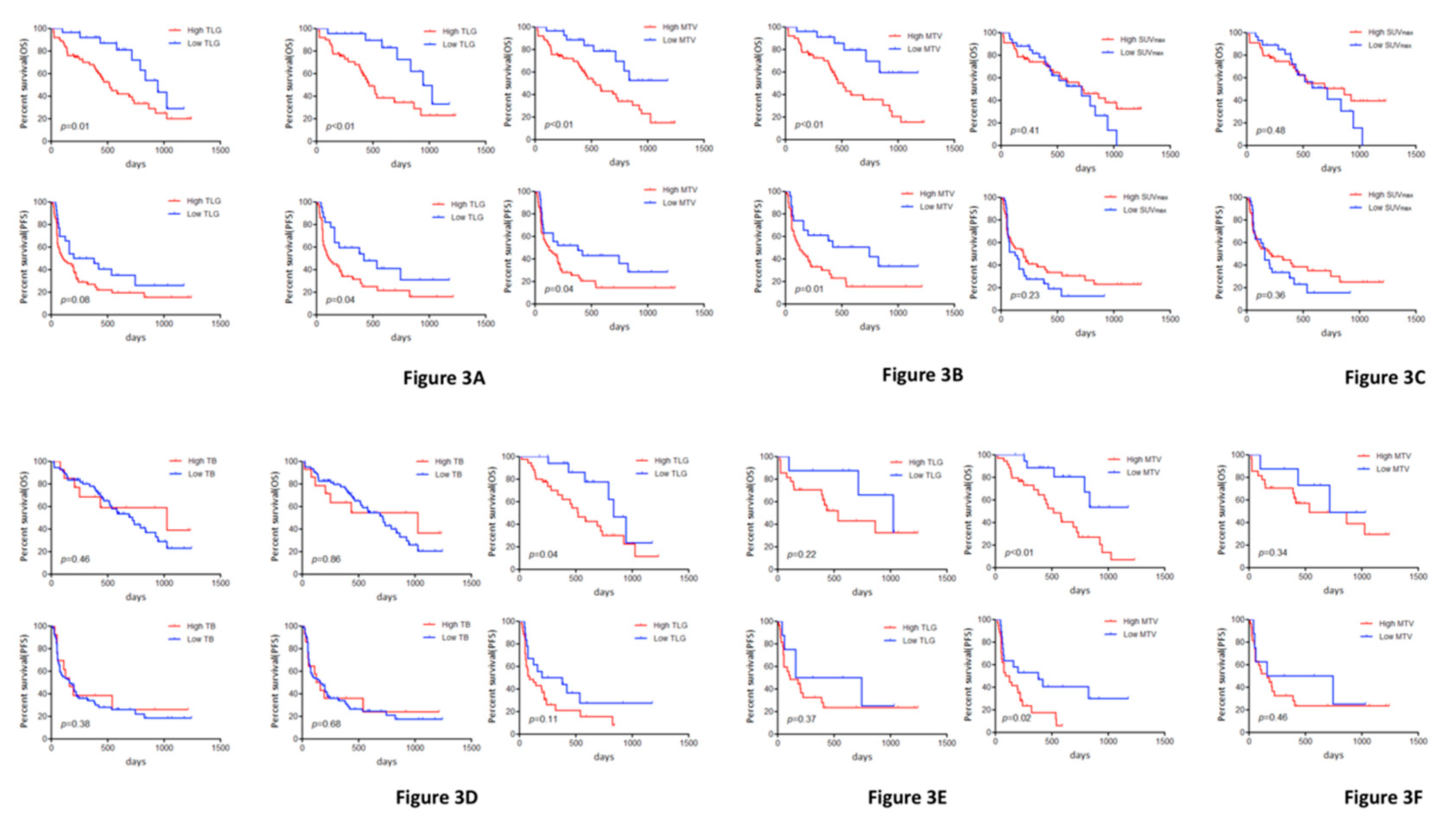
| TLG (High/Low) | 516/945 | 0.01 | 465/945 | <0.01 | 114/291 | 0.08 | 114/420 | 0.04 |
| MTV (High/Low) | 536/NR | <0.01 | 465/NR | <0.01 | 125/382 | 0.04 | 127/382 | 0.02 |
| SUVmax (High/Low) | 724/716 | 0.41 | 865/716 | 0.48 | 201/125 | 0.23 | 204/161 | 0.36 |
| TB (High/Low) | 793/693 | 0.46 | 837/693 | 0.86 | 204/137 | 0.38 | 182/181 | 0.68 |
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n = 85) | Patients without EGFR Mutation (n = 70) | All Patients (n = 85) | Patients without EGFR Mutation (n = 70) | |||||
| HR 95% CI | p-Value | HR 95% CI | p-Value | HR 95% CI | p-Value | HR 95% CI | p-Value | |
| Survival Analysis Including TLG | ||||||||
| Age (≤ 70/> 70) | 1.02 0.74–1.39 | 0.91 | 1.02 0.72–1.44 | 0.90 | 0.95 0.74–1.23 | 0.74 | 0.98 0.73–1.31 | 0.89 |
| Gender (Male/Female) | 1.11 0.76–1.55 | 0.55 | 0.95 0.55–1.48 | 0.85 | 1.07 0.79–1.41 | 0.61 | 0.81 0.49–1.19 | 0.31 |
| PS (0 or 1/2 or 3) | 1.68 1.05–2.51 | 0.03 | 2.22 1.06–3.93 | 0.03 | 1.63 1.02–2.36 | 0.04 | 1.41 0.68–2.43 | 0.31 |
| TLG (High/Low) | 1.47 1.03–2.21 | 0.03 | 1.63 1.10–2.60 | 0.01 | 1.21 0.92–1.63 | 0.16 | 1.32 0.97–1.86 | 0.07 |
| Survival Analysis Including MTV | ||||||||
| Age (≤ 70/> 70) | 0.92 0.68–1.26 | 0.64 | 0.87 0.61–1.25 | 0.46 | 0.91 0.71–1.18 | 0.51 | 0.91 0.67–1.21 | 0.51 |
| Gender (Male/Female) | 1.09 0.75–1.52 | 0.61 | 0.97 0.56–1.51 | 0.91 | 1.06 0.78–1.40 | 0.66 | 0.80 0.49–1.18 | 0.28 |
| PS (0 or 1/2 or 3) | 1.69 1.06–2.51 | 0.02 | 2.23 1.06–3.94 | 0.03 | 1.59 1.02–2.33 | 0.04 | 1.41 0.68–2.41 | 0.31 |
| MTV (High/Low) | 1.59 1.09–2.45 | 0.01 | 1.83 1.19–3.04 | <0.01 | 1.28 0.97–1.73 | 0.07 | 1.45 1.05–2.05 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Murayama, Y.; Kobayashi, K.; Kagamu, H.; Kuji, I. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 725. https://doi.org/10.3390/jcm9030725
Hashimoto K, Kaira K, Yamaguchi O, Mouri A, Shiono A, Miura Y, Murayama Y, Kobayashi K, Kagamu H, Kuji I. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2020; 9(3):725. https://doi.org/10.3390/jcm9030725
Chicago/Turabian StyleHashimoto, Kosuke, Kyoichi Kaira, Ou Yamaguchi, Atsuto Mouri, Ayako Shiono, Yu Miura, Yoshitake Murayama, Kunihiko Kobayashi, Hiroshi Kagamu, and Ichiei Kuji. 2020. "Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer" Journal of Clinical Medicine 9, no. 3: 725. https://doi.org/10.3390/jcm9030725
APA StyleHashimoto, K., Kaira, K., Yamaguchi, O., Mouri, A., Shiono, A., Miura, Y., Murayama, Y., Kobayashi, K., Kagamu, H., & Kuji, I. (2020). Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 9(3), 725. https://doi.org/10.3390/jcm9030725





