Acute Phase Proteins and Vitamin D Seasonal Variation in End-Stage Renal Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Assessment and Questionnaire
2.3. Laboratory Tests
2.4. Nutritional Status Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Vitamin D Seasonal Variation
4.2. Acute Phase Proteins’ Seasonal Variation
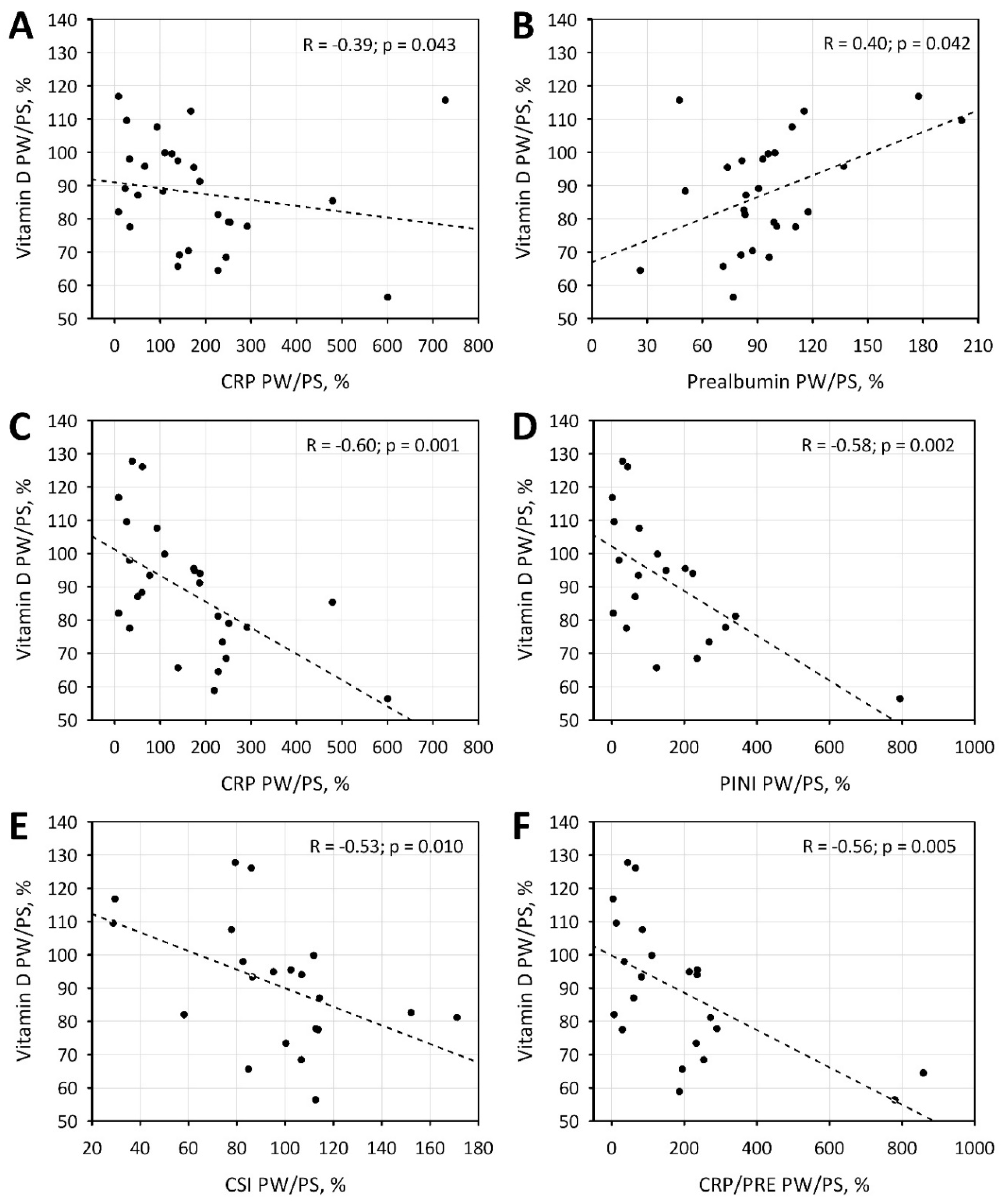
4.3. Relationship between Vitamin D and Acute Phase Proteins
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bednarek-Skublewska, A.; Smoleń, A.; Jaroszyński, A.; Załuska, W.; Ksia̧żek, A. Effects of vitamin D3 on selected biochemical parameters of nutritional status, inflammation, and cardiovascular disease in patients undergoing long-term hemodialysis. Pol. Arch. Med. Wewn. 2010, 120, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Oh, I.H.; Baek, H.J.; Lee, C.H.; Lee, S.S. Effects of sun exposure and dietary vitamin D intake on serum 25-hydroxyvitamin D status in hemodialysis patients. Nutr. Res. Pract. 2015, 9, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Maseeh, A. Vitamin D: The sunshine vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Gois, P.H.F.; Wolley, M.; Ranganathan, D.; Seguro, A.C. Vitamin D deficiency in chronic kidney disease: Recent evidence and controversies. Int. J. Environ. Res. Public Health 2018, 15, 1–16. [Google Scholar]
- Guinsburg, A.M.; Usvyat, L.A.; Etter, M.; Xu, X.; Thijssen, S.; Marcelli, D.; Canaud, B.; Marelli, C.; Barth, C.; Wang, Y.; et al. Seasonal variations in mortality and clinical indicators in international hemodialysis populations from the MONDO registry. BMC Nephrol. 2015, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Elder, G.J. Vitamin D levels, bone turnover and bone mineral density show seasonal variation in patients with chronic kidney disease stage 5. Nephrology 2007, 12, 90–94. [Google Scholar]
- Rosa Diez, G.J.; Fassi, J.; Crucelequi, S.; Rivera Nuñez, H.; Trillini, M.; Furci, A.; Algranati, S.; Plantalech, L. 25-Hydroxy vitamin D levels in hemodialysis-requiring end-stage renal disease patients. Dial. Transplant. 2008, 37, 388–390. [Google Scholar] [CrossRef]
- Argilés, À.; Lorho, R.; Servel, M.F.; Couret, I.; Chong, G.; Mourad, G. Blood pressure is correlated with vitamin D3 serum levels in dialysis patients. Blood Purif. 2002, 20, 370–375. [Google Scholar] [CrossRef]
- Tolouian, R.; Rao, D.S.; Goggins, M.; Bhat, S.; Gupta, A. Seasonal variation of vitamin D in patients on hemodialysis. Clin. Nephrol. 2010, 74, 19–24. [Google Scholar] [CrossRef]
- Chang, J.H.; Ro, H.; Kim, S.; Lee, H.H.; Chung, W.; Jung, J.Y. Study on the relationship between serum 25-hydroxyvitamin D levels and vascular calcification in hemodialysis patients with consideration of seasonal variation in vitamin D levels. Atherosclerosis 2012, 220, 563–568. [Google Scholar] [CrossRef]
- González-Parra, E.; Avila, P.J.; Mahillo-Fernández, I.; Lentisco, C.; Gracia, C.; Egido, J.; Ortiz, A. High prevalence of winter 25-hydroxyvitamin D deficiency despite supplementation according to guidelines for hemodialysis patients. Clin. Exp. Nephrol. 2012, 16, 945–951. [Google Scholar] [CrossRef]
- Ostrowska, M.M.; Szulc-Mysińska, E.; Bobilewicz, D.; Faryna, M.; Laboratoryjnej, Z.D.; Nauki, W.; Warszawskiego, Z. Stężenie witaminy D (25-OH) u pacjentów hospitalizowanych, ambulatoryjnych i dializowanych w zależności od pory roku. J. Lab. Diagn. Diagn Lab. 2015, 51, 209–212. [Google Scholar]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–775. [Google Scholar] [CrossRef]
- Manson, J.A.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. New Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Aleksova, J.; Kurniawan, S.; Elder, G.J. The trabecular bone score is associated with bone mineral density, markers of bone turnover and prevalent fracture in patients with end stage kidney disease. Osteoporos. Int. 2018, 29, 1447–1455. [Google Scholar] [CrossRef]
- Cesareo, R.; Iozzino, M.; D’onofrio, L.; Terrinoni, I.; Maddaloni, E.; Casini, A.; Campagna, G.; Santonati, A.; Palermo, A. Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis. Minerva Endocrinol. 2015, 40, 231–237. [Google Scholar]
- Morrone, L.F.; Bolasco, P.; Camerini, C.; Cianciolo, G.; Cupisti, A.; Galassi, A.; Mazzaferro, S.; Russo, D.; Russo, L.; Cozzolino, M. Vitamin D in patients with chronic kidney disease: A position statement of the Working Group “Trace Elements and Mineral Metabolism” of the Italian Society of Nephrology. J. Nephrol. 2016, 29, 305–328. [Google Scholar] [CrossRef]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Galassi, A.; Ciceri, P.; Cozzolino, M. Clinica Chimica Acta Nutritional vitamin D in CKD: Should we measure? Should we treat? Clin. Chim. Acta 2020, 501, 186–197. [Google Scholar] [CrossRef]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Burif. 2001, 19, 53–61. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, Y.K.; Jin Cho, A.; Park, H.C.; Han, C.H.; Choi, M.J.; Koo, J.R.; Yoon, J.W.; Noh, J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Interrelationships in hemodialysis patients. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Sueta, D.; Hokimoto, S.; Sakamoto, K.; Akasaka, T.; Tabata, N.; Kaikita, K.; Honda, O.; Naruse, M.; Ogawa, H. Validation of the high mortality rate of Malnutrition-Inflammation-Atherosclerosis syndrome: –Community-based observational study–. Int. J. Cardiol. 2017, 230, 97–102. [Google Scholar] [CrossRef]
- Miyamoto, T.; Carrero, J.J.; Stenvinkel, P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 662–668. [Google Scholar] [CrossRef]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib. Nephrol. 2017, 191, 32–43. [Google Scholar]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of Vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Hoe, E.; Nathanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-inflammatory effects of vitamin D on human immune cells in the context of bacterial infection. Nutrients 2016, 8, 806. [Google Scholar] [CrossRef] [Green Version]
- Gemelli, C.; Martello, A.; Montanari, M.; Zanocco Marani, T.; Salsi, V.; Zappavigna, V.; Parenti, S.; Vignudelli, T.; Selmi, T.; Ferrari, S.; et al. The Orosomucoid 1 protein is involved in the vitamin D - mediated macrophage de-activation process. Exp. Cell Res. 2013, 319, 3201–3213. [Google Scholar] [CrossRef]
- Rangé, H.; Poitou, C.; Boillot, A.; Ciangura, C.; Katsahian, S.; Lacorte, J.M.; Czernichow, S.; Meilhac, O.; Bouchard, P.; Chaussain, C. Orosomucoid, a New Biomarker in the Association between Obesity and Periodontitis. PLoS ONE 2013, 8, e57645. [Google Scholar] [CrossRef]
- Hochepied, T.; Van Molle, W.; Berger, F.G.; Baumann, H.; Libert, C. Involvement of the acute phase protein α1-acid glycoprotein in nonspecific resistance to a lethal Gram-negative infection. J. Biol. Chem. 2000, 275, 14903–14909. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.J. Control of malaria virulence by α1-acid glycoprotein (orosomucoid), an acute-phase (inflammatory) reactant. Proc. Natl. Acad. Sci. USA 1983, 80, 5421–5424. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.F.; Rosenfeld, M.R.; Gribbon, P.M.; Winlove, C.P.; Tsai, C.M. Alpha-1-acid (AAG, orosomucoid) glycoprotein: Interaction with bacterial lipopolysaccharide and protection from sepsis. Inflammation 1997, 21, 69–82. [Google Scholar] [CrossRef]
- Taguchi, K.; Nishi, K.; Giam Chuang, V.T.; Maruyama, T.; Otagiri, M. Molecular Aspects of Human Alpha-1 Acid Glycoprotein—structure and Function. In Acute Phase Proteins; IntechOpen: London, UK, 2013; pp. 139–162. [Google Scholar] [CrossRef] [Green Version]
- Shemyakin, I.G.; Pukhalsky, A.L.; Stepanshina, V.N.; Shmarina, G.V.; Aleshkin, V.A.; Afanas’ev, S.S. Preventive and therapeutic effects of α1-acid glycoprotein in mice infected with B. Anthracis. Bull. Exp. Biol. Med. 2005, 140, 439–444. [Google Scholar] [CrossRef]
- Zeki Tonbul, H.; Demir, M.; Altintepe, L.; Güney, I.; Yeter, E.; Türk, S.; Yeksan, M.; Yildiz, A. Malnutrition-inflammation-atherosclerosis (MIA) syndrome components in hemodialysis and peritoneal dialysis patients. Ren. Fail. 2006, 28, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Kundin, W.D.; Mechali, P.; Hollinshead, A.C.; Miller, H.; Bensimon, H. Cancer serum index: A useful nonspecific test as a parameter in multimodality screening and assessment of patients with cancer of the prostate. Prostate 1981, 2, 207–217. [Google Scholar] [CrossRef]
- Dessì, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, Y.; Xu, Z.; Yang, Y.; Kuang, D.; You, H.; Ma, S.; Hao, C.; Gu, Y.; Lin, S.; et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol. 2011, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguchi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Kitazono, T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol. Ren. Physiol. 2014, 306, 1418–1428. [Google Scholar] [CrossRef]
- Mukai, H.; Villafuerte, H.; Qureshi, A.R.; Lindholm, B.; Stenvinkel, P. Serum albumin, inflammation, and nutrition in end-stage renal disease: C-reactive protein is needed for optimal assessment. Semin. Dial. 2018, 31, 435–439. [Google Scholar] [CrossRef]
- Yanai, M.; Satomura, A.; Uehara, Y.; Murakawa, M.; Takeuchi, M.; Kumasaka, K. Circannual rhythm of laboratory test parameters among chronic haemodialysis patients. Blood Purif. 2008, 26, 196–203. [Google Scholar] [CrossRef]
- Gama-Axelsson, T.; Heimbürger, O.; Stenvinkel, P.; Bárány, P.; Lindholm, B.; Qureshi, A.R. Serum albumin as predictor of nutritional status in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1446–1453. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.H.; Kwon, O.; Kim, S.U.; Kim, R.H.; Cho, Y.W.; Jung, H.Y.; Choi, J.Y.; Kim, C.D.; Kim, Y.L.; et al. Low prealbumin levels are independently associated with higher mortality in patients on peritoneal dialysis. Kidney Res. Clin. Pract. 2016, 35, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Chertow, G.M.; Ackert, K.; Lew, N.L.; Lazarus, J.M.; Lowrie, E.G. Prealbumin is as important as albumin in the nutritional assessment of hemodialysis patients. Kidney Int. 2000, 58, 2512–2517. [Google Scholar] [CrossRef] [Green Version]
- Vasson, M.P.; Baguet, J.C.; Arveiller, M.R.; Bargnoux, P.J.; Giroud, J.P.; Raichvarg, D. Serum and urinary alpha-1 acid glycoprotein in chronic renal failure. Nephron 1993, 65, 299–303. [Google Scholar] [CrossRef]
- Luo, Z.; Lei, H.; Sun, Y.; Liu, X.; Su, D.F. Orosomucoid, an acute response protein with multiple modulating activities. J. Physiol. Biochem. 2015, 71, 329–340. [Google Scholar] [CrossRef]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. α1-acid glycoprotein: An acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Zhang, Y.; Ishimi, Y.; Tabata, I.; Higuchi, M. Association between serum 25-hydroxyvitamin d and inflammatory cytokines in healthy adults. Nutrients 2014, 6, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, S.A.; Marie, M.; Ashraf, M.; Hussein, M.; Almalki, N. Is there an association between Vitamin D level and inflammatory markers in hemodialysis patients? A cross-sectional study. Saudi J. Kidney Dis. Transpl. 2016, 27, 460–466. [Google Scholar]
- Yonemura, K.; Fujimoto, T.; Fujigaki, Y.; Hishida, A. Vitamin D deficiency is implicated in reduced serum albumin concentrations in patients with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 337–344. [Google Scholar] [CrossRef]
- Rao, M.; Guo, D.; Perianayagam, M.C.; Tighiouart, H.; Jaber, B.L.; Pereira, B.J.G.; Balakrishnan, V.S. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2005, 45, 324–333. [Google Scholar] [CrossRef]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef]
- Lin, R. Crosstalk between Vitamin D metabolism, VDR signalling, and innate immunity. Biomed Res. Int. 2016, 2016, 1375858. [Google Scholar] [CrossRef] [Green Version]
- Meireles, M.S.; Kamimura, M.A.; Dalboni, M.A.; Giffoni de Carvalho, J.T.; Aoike, D.T.; Cuppari, L. Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: A randomized controlled trial. Clin. Nutr. 2016, 35, 1251–1258. [Google Scholar] [CrossRef]
- Ammar, Y.A.; Nienaa, Y.A.; El-Banawy, S.S.; Moghazy, T.F.; Kandil, N.S.; El-Sayed, A.A. Association of vitamin D deficiency with renal anemia and erythropoietin hyporesponsiveness in hemodialysis patients. J. Egypt. Soc. Nephrol. Transplant. 2017, 17, 125. [Google Scholar]


| Characteristics | The Study Group; n = 59 |
|---|---|
| Age, years | 57.9 ± 14.1 |
| Female, n (%) | 24 (40.7) |
| BMI, kg/m2 | 25.1 ± 5.3 |
| Dialysis duration time, months | 137 (39; 392) |
| Treatment with phosphate binders, n (%) | 46 (77.9) |
| Calcium carbonate, n (%) | 37 (62.7) |
| Aluminium hydroxide, n (%) | 7 (11.9) |
| Sevelamer, n (%) | 2 (3.4) |
| Vitamin D supplementation; n (%) | 25 (42.4) |
| Treatment with erythropoietin analogues, n (%) | 28 (47.5) |
| Epoietin, n (%) | 14 (23.7) |
| Darbepoietin α, n (%) | 6 (10.2) |
| Methoxy polyethylene glycol-epoetin β, n (%) | 8 (13.6) |
| Low dose *, n (%) | 21 (35.6) |
| High dose, n (%) | 7 (11.9) |
| Iron supplementation, n (%) | 25 (42.4) |
| Status post kidney transplantation; n (%) | 12 (20.3) |
| Hyperparathyroidism; n (%) | 13 (22) |
| Diabetes, n (%) | 21 (35.6) |
| Physical activity level | |
| Low, n (%) | 47 (79.7) |
| Moderate, n (%) | 12 (20.3) |
| Vitamin D (ng/mL) | 22.44 (15.59; 34.39) |
| Albumin (g/L) | 40 (37; 42) |
| Prealbumin (g/L) | 0.39 ± 0.11 |
| CRP (mg/L) | 3.16 (1.25; 9.27) |
| AGP (g/L) | 1.44 (1.25; 1.67) |
| Total calcium (mmol/L) | 2.19 (2.06; 2.41) |
| Phosphates (mmol/L) | 1.70 ± 0.55 |
| Intact PTH (pg/mL) | 316.7 (119.5; 636.8) |
| Ca × P (mmol2/L2) | 3.74 ± 1.26 |
| PINI | 0.29 (0.1; 1.23) |
| CSI | 3.49 (2.70; 4.84) |
| CRP/PRE (×10−3) | 7.57 (2.77; 12.79) |
| Hemoglobin (g/dL) | 11.11 ± 1.15 |
| Characteristics | Points | The Study Group; n = 59 |
|---|---|---|
| Frequency of sun exposure: | ||
| Every day, n (%) | 3 | 37 (62.7) |
| 3–4 times a week, n (%) | 2 | 13 (22.0) |
| 1–2 times a week or lower, n (%) | 1 | 9 (15.2) |
| Duration of sun exposure session: | ||
| ≥30 min, n (%) | 3 | 40 (67.8) |
| 15–30 min, n (%) | 2 | 9 (15.3) |
| <15 min, n (%) | 1 | 10 (16.9) |
| Body surface exposed to sun: | ||
| More than face, forearms and lower legs, n (%) | 3 | 4 (6.8) |
| Face, forearms and lower legs, n (%) | 2 | 40 (67.8) |
| Smaller, n (%) | 1 | 15 (25.4) |
| Sun exposure index, points | sum of above | 7 (6; 8) |
| Use of sun protection filter creams or lotions: | ||
| Yes, n (%) | - | 9 (15.2) |
| No, n (%) | 50 (84.7) |
| Post-Summer | Post-Winter | p | |
|---|---|---|---|
| Energy (kcal/day) | 1335 (1016; 1556) | 1387 (1199; 1663) | 0.043 |
| Proteins (g/day) | 51.0 ± 17.0 | 54.8 ± 16.4 | 0.1 |
| Fat (g/day) | 46.34 (36.41; 60.03) | 48.09 (37.33; 60.99) | 0.6 |
| Carbohydrates (g/day) | 184.2 (139.9; 225.9) | 204.3 (152.7; 238.6) | 0.09 |
| Calcium (mg/day) | 22.3 (170.8; 323.8) | 249.1 (175.4; 355.7) | 0.2 |
| Phosphorus (mg/day) | 711.2 (563.0; 853.6) | 731.9 (655.4; 888.9) | 0.5 |
| Magnesium (mg/day) | 169.61 ± 50.67 | 178.48 ± 65.61 | 0.4 |
| Zinc (mg/day) | 6.55 ± 2.27 | 6.88 ± 2.34 | 0.4 |
| Potassium (mg/day) | 1897.1 ± 626.9 | 1993.8 ± 675.7 | 0.9 |
| Iron (mg/day) | 7.07 ± 2.24 | 7.42 ± 2.31 | 0.3 |
| Vitamin D (µg/day) | 1.32 (0.80; 2.71) | 1.44 (0.83; 2.58) | 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraj, M.; Hetwer, P.; Dumnicka, P.; Ceranowicz, P.; Mazur-Laskowska, M.; Ząbek-Adamska, A.; Warzecha, Z.; Kuśnierz-Cabala, B.; Kuźniewski, M. Acute Phase Proteins and Vitamin D Seasonal Variation in End-Stage Renal Disease Patients. J. Clin. Med. 2020, 9, 807. https://doi.org/10.3390/jcm9030807
Maraj M, Hetwer P, Dumnicka P, Ceranowicz P, Mazur-Laskowska M, Ząbek-Adamska A, Warzecha Z, Kuśnierz-Cabala B, Kuźniewski M. Acute Phase Proteins and Vitamin D Seasonal Variation in End-Stage Renal Disease Patients. Journal of Clinical Medicine. 2020; 9(3):807. https://doi.org/10.3390/jcm9030807
Chicago/Turabian StyleMaraj, Małgorzata, Paulina Hetwer, Paulina Dumnicka, Piotr Ceranowicz, Małgorzata Mazur-Laskowska, Anna Ząbek-Adamska, Zygmunt Warzecha, Beata Kuśnierz-Cabala, and Marek Kuźniewski. 2020. "Acute Phase Proteins and Vitamin D Seasonal Variation in End-Stage Renal Disease Patients" Journal of Clinical Medicine 9, no. 3: 807. https://doi.org/10.3390/jcm9030807






