Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Study Protocol
2.3. Salt Sensitive/Resistant Status Classification
2.4. 24-h Urine Samples Analysis and Blood Pressure Measurement
2.5. Assessment of Skin Microcirculatory Blood Flow
2.6. Venous Blood Samples Analysis
2.7. Measurement of Thiobarbituric Acid Reactive Substances (TBARS) and Ferric-Reducing Ability of Plasma (FRAP)
2.8. Measurement of Intracellular Reactive Oxygen Species (ROS) Production
2.9. Measurement of Serum 8-iso Prostaglandin F2α (8-iso-PGF2a) Protein Concentration
2.10. Measurement of Serum Cu/Zn Superoxide Dismutase (Cu/Zn SOD), Glutathione Peroxidase 1 (GPx1) and Catalase Protein Concentrations
2.11. Measurement of Matrix Metalloproteinase 9 (MMP-9) Protein Concentration
2.12. Statistical Analysis
3. Results
3.1. Anthropometric, Hemodynamic, and Biochemical Parameters
3.2. Post-Occlusive Reactive Hyperemia, Acetylcholine-Induced Dilation, and Sodium Nitroprusside-Induced Dilation of Forearm Skin Microcirculation
3.3. Markers of Oxidative Stress and Antioxidative Defense
3.4. Serum Protein Concentration of Matrix Metalloproteinase 9 (MMP-9)
3.5. Correlation Between Microvascular Reactivity and Salt Intake/RAS Suppression/Oxidative Stress and Antioxidative Defense Following the High-Salt Diet
4. Discussion
4.1. High-Salt Diet Impairs Microvascular Function
4.2. High-Salt Diet and Vascular Structural Remodeling
4.3. High-Salt Diet Increases Oxidative Stress due to a Decrease of Antioxidative Capacity
4.4. Increase in Antioxidative Capacity by Vitamin C and E Peroral Administration Restores Microvascular Reactivity in Healthy Individuals
4.5. The Relationship between the Renin–Angiotensin System, Oxidative Stress and Microvascular Function
Author Contributions
Funding
Conflicts of Interest
References
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt Sensitivity, Pulse Pressure, and Death in Normal and Hypertensive Humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.M.Y.; Arcand, J.; Leung, A.A.; Thout, S.R.; Campbell, N.R.C.; Webster, J. The science of salt: A regularly updated systematic review of salt and health outcomes (December 2015-March 2016). J. Clin. Hypertens. 2017, 19, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.J.; MacGregor, G.A. Effect of modest salt reduction on blood pressure: A meta-analysis of randomized trials. Implications for public health. J. Hum. Hypertens. 2002, 16, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intersalt Cooperative Research Group. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, P.; Stamler, J.; Nichols, R.; Dyer, A.R.; Stamler, R.; Kesteloot, H.; Marmot, M. Intersalt revisited: Further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 1996, 312, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Eguchi, K.; Murakami, T.; Arakawa, K.; Tsuchihashi, T.; Kario, K. High Salt Intake Is Independently Associated With Hypertensive Target Organ Damage. J. Clin. Hypertens. 2016, 18, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holowatz, L.A.; Thompson-Torgerson, C.S.; Kenney, W.L. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol. 2008, 105, 370–372. [Google Scholar] [CrossRef] [Green Version]
- Cavka, A.; Jukic, I.; Ali, M.; Goslawski, M.; Bian, J.T.; Wang, E.; Drenjancevic, I.; Phillips, S.A. Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J. Hypertens. 2016, 34, 676–684. [Google Scholar] [CrossRef]
- DuPont, J.J.; Greaney, J.L.; Wenner, M.M.; Lennon-Edwards, S.L.; Sanders, P.W.; Farquhar, W.B.; Edwards, D.G. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J. Hypertens. 2013, 31, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Lennon-Edwards, S.; Ramick, M.G.; Matthews, E.L.; Brian, M.S.; Farquhar, W.B.; Edwards, D.G. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. Endothelial function is impaired after a high-salt meal in healthy subjects. Am. J. Clin. Nutr. 2011, 93, 500–505. [Google Scholar] [CrossRef]
- Boegehold, M.A.; Drenjancevic, I.; Lombard, J.H. Salt, Angiotensin II, Superoxide, and Endothelial Function. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 215–254. [Google Scholar]
- Cavka, A.; Cosic, A.; Jukic, I.; Jelakovic, B.; Lombard, J.H.; Phillips, S.A.; Seric, V.; Mihaljevic, I.; Drenjancevic, I. The role of cyclo-oxygenase-1 in high-salt diet-induced microvascular dysfunction in humans. J. Physiol. 2015, 593, 5313–5324. [Google Scholar] [CrossRef] [PubMed]
- Barić, L.; Drenjančević, I.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Lenasi, H.; Šerić, V.; Stupin, A. Seven-Day Salt Loading Impairs Microvascular Endothelium-Dependent Vasodilation without Changes in Blood Pressure, Body Composition and Fluid Status in Healthy Young Humans. Kidney Blood Press. Res. 2019, 44, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Greaney, J.L.; DuPont, J.J.; Lennon-Edwards, S.L.; Sanders, P.W.; Edwards, D.G.; Farquhar, W.B. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: Role of oxidative stress. J. Physiol. 2012, 590, 5519–5528. [Google Scholar] [CrossRef]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljevic, Z.; Novak, S.; Vukovic, R.; Drenjancevic, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Drenjancevic-Peric, I.; McEwen, S.; Friesema, J.; Schulta, D.; Yu, M.; Roman, R.J.; Lombard, J.H. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca 2+ signaling and NO production in rat aorta. Am. J. Physiol. Circ. Physiol. 2006, 291, H929–H938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Mori, T.; Huang, T.; Lombard, J.H. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am. J. Physiol. Circ. Physiol. 2004, 286, H575–H583. [Google Scholar] [CrossRef] [PubMed]
- Matic, A.; Jukic, I.; Stupin, A.; Baric, L.; Mihaljevic, Z.; Unfirer, S.; Tartaro Bujak, I.; Mihaljevic, B.; Lombard, J.H.; Drenjancevic, I. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am. J. Physiol. Circ. Physiol. 2018, 315, H718–H730. [Google Scholar] [CrossRef] [PubMed]
- Nurkiewicz, T.R.; Boegehold, M.A. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R1550–R1556. [Google Scholar] [CrossRef]
- Rosenbaugh, E.G.; Savalia, K.K.; Manickam, D.S.; Zimmerman, M.C. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R917–R928. [Google Scholar] [CrossRef]
- Radimer, K. Dietary Supplement Use by US Adults: Data from the National Health and Nutrition Examination Survey, 1999-2000. Am. J. Epidemiol. 2004, 160, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesso, H.D. Vitamins E and C in the Prevention of Cardiovascular Disease in Men. JAMA 2008, 300, 2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitz, R.; Mensink, G.; Fischer, B.; Thamm, M. Vitamins—Dietary intake and intake from dietary supplements in Germany. Eur. J. Clin. Nutr. 2002, 56, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamant, M.; Placier, S.; Dubroca, C.; Esposito, B.; Lopes, I.; Chatziantoniou, C.; Tedgui, A.; Dussaule, J.-C.; Lehoux, S. Role of Matrix Metalloproteinases in Early Hypertensive Vascular Remodeling. Hypertension 2007, 50, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Hua, Q.; Xing, X.; Wen, J.; Liu, R.; Yang, Z. Impact of the Metalloproteinase-9/Tissue Inhibitor of Metalloproteinase-1 System on Large Arterial Stiffness in Patients with Essential Hypertension. Hypertens. Res. 2007, 30, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Lehoux, S.; Lemarié, C.A.; Esposito, B.; Lijnen, H.R.; Tedgui, A. Pressure-Induced Matrix Metalloproteinase-9 Contributes to Early Hypertensive Remodeling. Circulation 2004, 109, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Cavka, A.; Cosic, A.; Grizelj, I.; Koller, A.; Jelakovic, B.; Lombard, J.H.; Phillips, S.A.; Drenjancevic, I. Effects of AT1 Receptor Blockade on Plasma Thromboxane A 2 (TXA 2) Level and Skin Microcirculation in Young Healthy Women on Low Salt Diet. Kidney Blood Press. Res. 2013, 37, 432–442. [Google Scholar] [CrossRef]
- Stupin, M.; Stupin, A.; Rasic, L.; Cosic, A.; Kolar, L.; Seric, V.; Lenasi, H.; Izakovic, K.; Drenjancevic, I. Acute exhaustive rowing exercise reduces skin microvascular dilator function in young adult rowing athletes. Eur. J. Appl. Physiol. 2018, 118, 461–474. [Google Scholar] [CrossRef]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Slovinski, A.P.; Hajjar, L.A.; Ince, C. Microcirculation in Cardiovascular Diseases. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Miličić, D.; Jakuš, N.; Fabijanović, D. Microcirculation and Heart Failure. Curr. Pharm. Des. 2018, 24, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Selthofer-Relatic, K.; Mihalj, M.; Kibel, A.; Stupin, A.; Stupin, M.; Jukic, I.; Koller, A.; Drenjancevic, I. Coronary Microcirculatory Dysfunction in Human Cardiomyopathies. Cardiol. Rev. 2017, 25, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.M.; Nelson, R.; Brown, M.; Wood, M.; Wood, A.J.J. Dietary Sodium Intake Modulates Vasodilation Mediated by Nitroprusside but Not by Methacholine in the Human Forearm. Hypertension 1995, 25, 1220–1223. [Google Scholar] [CrossRef]
- Tzemos, N.; Lim, P.O.; Wong, S.; Struthers, A.D.; MacDonald, T.M. Adverse Cardiovascular Effects of Acute Salt Loading in Young Normotensive Individuals. Hypertension 2008, 51, 1525–1530. [Google Scholar] [CrossRef] [Green Version]
- Kalani, A.; Pushpakumar, S.B.; Vacek, J.C.; Tyagi, S.C.; Tyagi, N. Inhibition of MMP-9 attenuates hypertensive cerebrovascular dysfunction in Dahl salt-sensitive rats. Mol. Cell. Biochem. 2016, 413, 25–35. [Google Scholar] [CrossRef]
- Lenda, D.M.; Sauls, B.A.; Boegehold, M.A. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am. J. Physiol. Circ. Physiol. 2000, 279, H7–H14. [Google Scholar] [CrossRef]
- Lenda, D.M.; Boegehold, M.A. Effect of a High Salt Diet on Microvascular Antioxidant Enzymes. J. Vasc. Res. 2002, 39, 41–50. [Google Scholar] [CrossRef]
- Lenda, D.M.; Boegehold, M.A. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am. J. Physiol. Circ. Physiol. 2002, 282, H395–H402. [Google Scholar] [CrossRef] [Green Version]
- Drenjancevic-Peric, I.; Lombard, J.H. Reduced Angiotensin II and Oxidative Stress Contribute to Impaired Vasodilation in Dahl Salt-Sensitive Rats on Low-Salt Diet. Hypertension 2005, 45, 687–691. [Google Scholar] [CrossRef] [Green Version]
- Drenjancevic-Peric, I.; Phillips, S.A.; Falck, J.R.; Lombard, J.H. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13 BN rats. Am. J. Physiol. Circ. Physiol. 2005, 289, H188–H195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, T.; Lombard, J.H. Effect of High-Salt Diet on Vascular Relaxation and Oxidative Stress in Mesenteric Resistance Arteries. J. Vasc. Res. 2007, 44, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.J.; Lombard, J.H. Low-Dose Angiotensin II Infusion Restores Vascular Function in Cerebral Arteries of High Salt-Fed Rats by Increasing Copper/Zinc Superoxide Dimutase Expression. Am. J. Hypertens. 2013, 26, 739–747. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.T.; Schmidt, J.R.; Somberg, L.; de la Cruz, L.; Lombard, J.H. Time-Course and Mechanisms of Restored Vascular Relaxation by Reduced Salt Intake and Angiotensin II Infusion in Rats Fed a High-Salt Diet. Microcirculation 2009, 16, 220–234. [Google Scholar] [CrossRef]
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A 2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131. [Google Scholar] [CrossRef] [Green Version]
- Benndorf, R.A.; Schwedhelm, E.; Gnann, A.; Taheri, R.; Kom, G.; Didié, M.; Steenpass, A.; Ergün, S.; Böger, R.H. Isoprostanes Inhibit Vascular Endothelial Growth Factor–Induced Endothelial Cell Migration, Tube Formation, and Cardiac Vessel Sprouting In Vitro, As Well As Angiogenesis In Vivo via Activation of the Thromboxane A 2 Receptor. Circ. Res. 2008, 103, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Rietjens, I.M.C.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- DuPont, J.J.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. Ascorbic acid or l -arginine improves cutaneous microvascular function in chronic kidney disease. J. Appl. Physiol. 2011, 111, 1561–1567. [Google Scholar] [CrossRef] [Green Version]
- Ghiadoni, L.; Cupisti, A.; Huang, Y.; Mattei, P.; Cardinal, H.; Favilla, S.; Rindi, P.; Barsotti, G.; Taddei, S.; Salvetti, A. Endothelial dysfunction and oxidative stress in chronic renal failure. J. Nephrol. 2004, 17, 512–519. [Google Scholar]
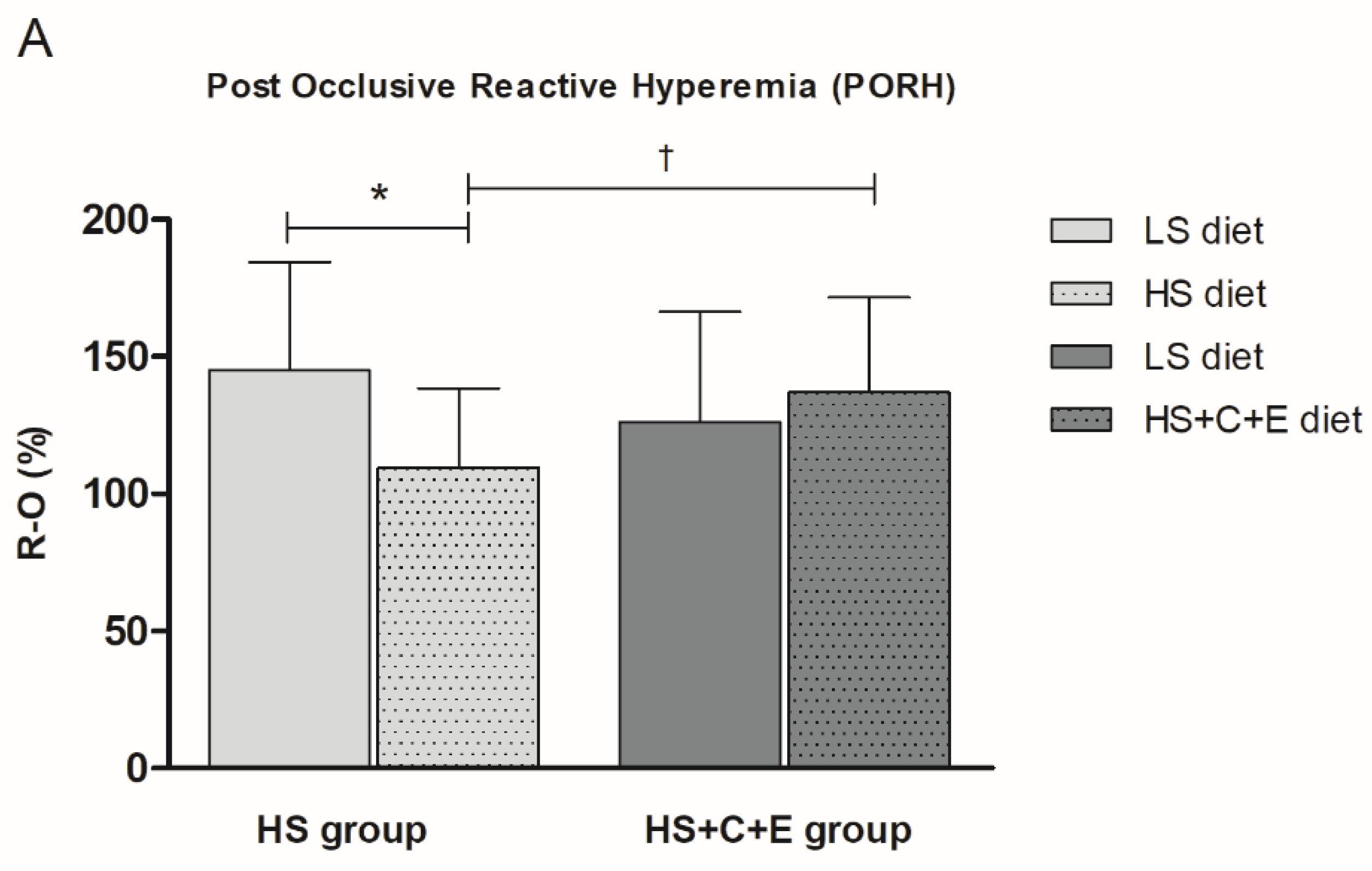





| Parameter | HS Group | HS+C+E Group |
|---|---|---|
| N (W/M) | 24 (11/13) | 27 (15/12) |
| Age (years) | 20 ± 2 | 20 ± 2 |
| BMI (kg/m2) | 23.0 ± 3.4 | 23.3 ± 2.5 |
| WHR | 0.78 ± 0.06 | 0.81 ± 0.05 |
| SBP (mmHg) | 119 ± 12 | 117 ± 12 |
| DBP (mmHg) | 73 ± 7 | 73 ± 10 |
| MAP (mmHg) | 88 ± 8 | 88 ± 9 |
| HR (beats per minute) | 75 ± 10 | 77 ± 11 |
| erythrocytes (× 10e12/L) | 4.9 ± 0.7 | 4.8 ± 0.3 |
| hemoglobin (g/L) | 145 ± 21 | 142 ± 12 |
| hematocrit (%) | 42.96 ± 5.72 | 41.31 ± 2.51 |
| leukocytes (× 10e9/L) | 5.8 ± 0.9 | 6.1 ± 1.6 |
| thrombocytes (× 10e9/L) | 224 ± 40 | 279 ± 66 |
| urea (mmol/L) | 4.7 ± 1.7 | 4.7 ± 1.5 |
| creatinine (µmol/L) | 67 ± 15 | 71 ± 14 |
| sodium (mmol/L) | 138 ± 4 | 139 ± 2 |
| potassium (mmol/L) | 4.2 ± 0.1 | 4.1 ± 0.3 |
| glucose (mmol/L) | 5.0 ± 0.5 | 4.8 ± 0.7 |
| hsCRP (mg/L) | 0.8 ± 0.9 | 1.9 ± 3.2 |
| cholesterol (mmol/L) | 4.6 ± 0.8 | 4.3 ± 0.7 |
| triglycerides (mmol/L) | 1.0 ± 0.3 | 1.1 ± 0.5 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.4 |
| LDL cholesterol (mmol/L) | 2.7 ± 0.7 | 2.5 ± 0.4 |
| Parameter | HS Group | HS+C+E Group | ||
|---|---|---|---|---|
| LS Diet | HS Diet | LS Diet | HS+C+E Diet | |
| BMI (kg/m2) | 22.9 ± 3.5 | 23.0 ± 3.5 | 23.4 ± 2.4 | 23.4 ± 2.4 |
| WHR | 0.78 ± 0.07 | 0.78 ± 0.07 | 0.81 ± 0.05 | 0.81 ± 0.06 |
| SBP (mmHg) | 117 ± 14 | 115 ± 15 | 116 ± 10 | 116 ± 11 |
| DBP (mmHg) | 71 ± 8 | 71 ± 7 | 71 ± 8 | 72 ± 9 |
| MAP (mmHg) | 86 ± 9 | 86 ± 9 | 86 ± 6 | 87 ± 8 |
| HR (beats per minute) | 73 ± 10 | 75 ± 10 | 73 ± 11 | 77 ± 9 |
| sodium (mmol/L) | 137 ± 3 | 138 ± 2 | 137 ± 3 | 138 ± 2 |
| potassium (mmol/L) | 4.2 ± 0.3 | 4.3 ± 0.4 | 4.2 ± 0.3 | 4.1 ± 0.2 |
| hsCRP (mg/L) | 0.6 ± 0.4 | 0.6 ± 0.2 | 1.0 ± 0.9 | 1.2 ± 1.9 |
| PRA (ng/mL/h) | 5.71 ± 3.29 | 1.83 ± 1.0 * | 6.72 ± 4.40 | 2.85 ± 4.12 * |
| aldosterone (pg/mL) | 169 ± 88 | 98 ± 64 * | 214 ± 76 | 152 ± 78 * |
| 24 h urine volume (mL) | 1351 ± 428 | 1491 ± 648 | 1147 ± 485 | 1186 ± 521 |
| 24 h creatinine coefficient (µmol/24h/kg) | 198 ± 57 | 195 ± 58 | 171 ± 50 | 163 ± 63 |
| 24 h urine urea (mmol/dU) | 294 ± 127 | 298 ± 114 | 289 ± 98 | 251 ± 94 |
| 24 h sodium (mmol/dU) | 118 ± 40 | 244 ± 97 * | 118 ± 50 | 219 ± 99 * |
| 24 h potassium (mmol/dU) | 48 ± 20 | 50 ± 24 | 43 ± 14 | 41 ± 22 |
| calculated salt intake (g/day) | 6.9 ± 2.4 | 14.3 ± 5.7 * | 6.9 ± 2.9 | 13.0 ± 5.6 * |
| Parameter | HS Group | HS+C+E Group | ||
|---|---|---|---|---|
| LS Diet | HS Diet | LS Diet | HS+C+E Diet | |
| FRAP (mM/L TE) | 0.45 ± 0.08 | 0.38 ± 0.08 * | 0.48 ± 0.11 | 0.46 ± 0.11 † |
| TBARS (µm/MDA) | 0.45 ± 0.07 | 0.60 ± 0.22 * | 0.42 ± 0.21 | 0.42 ± 0.15 † |
| 8-iso-PGF2α (pg/mL) | 1232 ± 184 | 1442 ± 114 * | 1131 ± 142 | 1146 ± 191 † |
| CuZn SOD (ng/mL) | 82.9 ± 34.0 | 82.8 ± 45.6 | 97.3 ± 39.8 | 86.0 ± 38.6 |
| GPx1 (ng/mL) | 5.99 ± 3.80 | 6.29 ± 4.16 | 4.71 ± 3.19 | 3.76 ± 2.59 |
| catalase (pg/mL) | 347 ± 495 | 261 ± 462 * | 249 ± 375 | 186 ± 349 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barić, L.; Drenjančević, I.; Mihalj, M.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Mrakovčić-Šutić, I.; Šerić, V.; Stupin, A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. J. Clin. Med. 2020, 9, 843. https://doi.org/10.3390/jcm9030843
Barić L, Drenjančević I, Mihalj M, Matić A, Stupin M, Kolar L, Mihaljević Z, Mrakovčić-Šutić I, Šerić V, Stupin A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. Journal of Clinical Medicine. 2020; 9(3):843. https://doi.org/10.3390/jcm9030843
Chicago/Turabian StyleBarić, Lidija, Ines Drenjančević, Martina Mihalj, Anita Matić, Marko Stupin, Luka Kolar, Zrinka Mihaljević, Ines Mrakovčić-Šutić, Vatroslav Šerić, and Ana Stupin. 2020. "Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals" Journal of Clinical Medicine 9, no. 3: 843. https://doi.org/10.3390/jcm9030843
APA StyleBarić, L., Drenjančević, I., Mihalj, M., Matić, A., Stupin, M., Kolar, L., Mihaljević, Z., Mrakovčić-Šutić, I., Šerić, V., & Stupin, A. (2020). Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. Journal of Clinical Medicine, 9(3), 843. https://doi.org/10.3390/jcm9030843






