Increased Amygdala Activations during the Emotional Experience of Death-Related Pictures in Complicated Grief: An fMRI Study
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Procedure
2.3. Instruments
2.3.1. ICG (Inventory of Complicated Grief)
2.3.2. TRIG (Texas Revised Inventory of Grief)
2.3.3. Symptoms Checklist (SCL-90-R)
2.3.4. Global Assessment of Post-Traumatic Stress Disorder (EGEP in Spanish)
2.3.5. Emotional Experience Task in Functional MRI (fMRI)
2.3.6. Picture Recognition Using the IAPS
2.3.7. Subjective Emotional Evaluation of Affective Pictures via the Self-Assessment Manikin (SAM)
2.4. Data Analysis
2.4.1. Behavioral Analyses
2.4.2. Neuroimaging Analyses
2.4.3. Threshold Criteria
3. Results
3.1. Behavioral Results
3.2. Neuroimaging Results
3.2.1. fMRI Main Task Effects
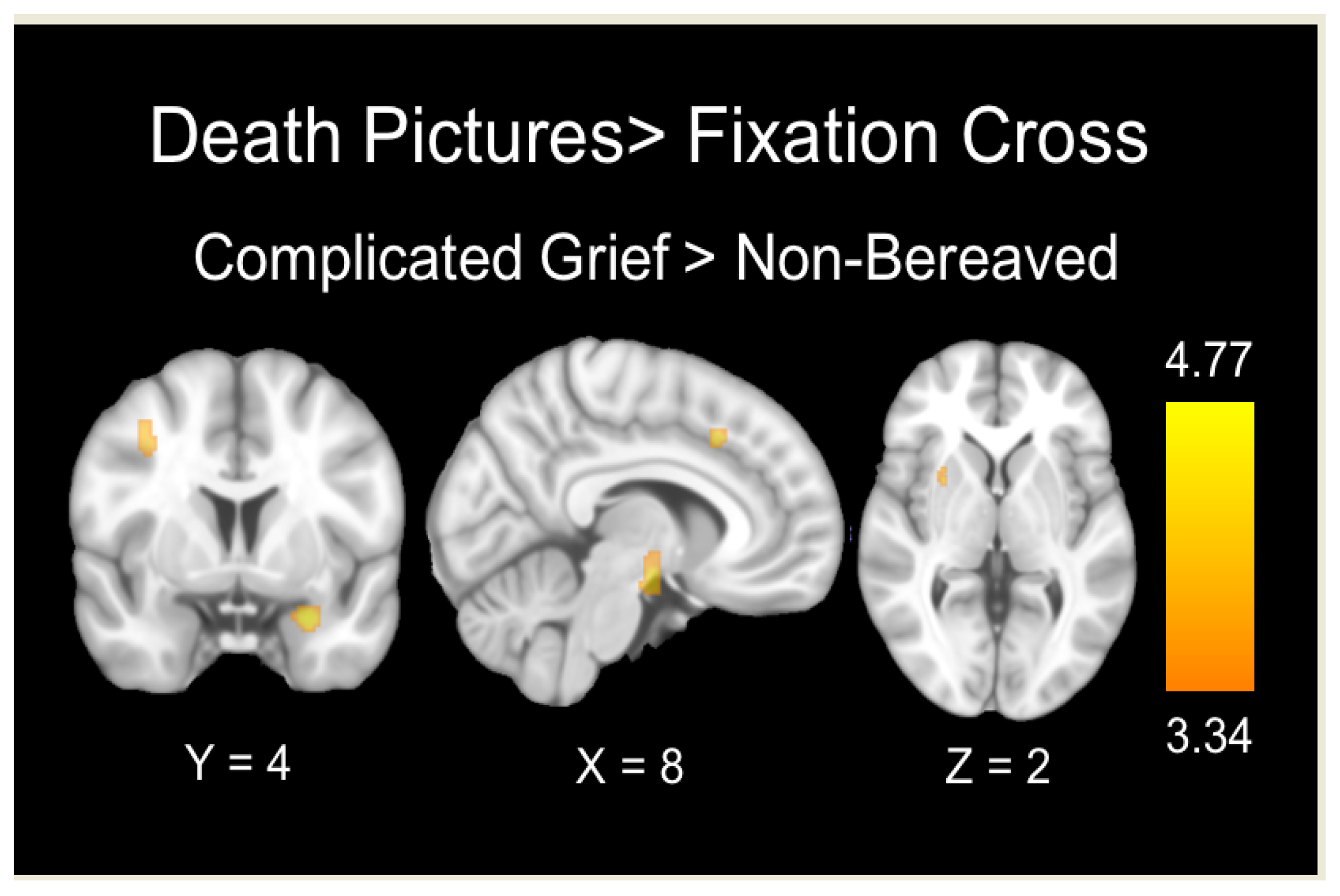
Death Pictures > Fixation Cross
Correlation Analyses
Negative Valence Pictures > Fixation Cross
Positive Valence Pictures > Fxation Cross
3.2.2. Interaction Analysis
Death x Negative Valence
Death x Positive Valence
Positive Valence x Negative Valence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing Statement
References
- Lundorff, M.; Holmgren, H.; Zachariae, R.; Farver-Vestergaard, I.; O’Connor, M. Prevalence of prolonged grief disorder in adult bereavement: A systematic review and meta-analysis. J Affect. Disord. 2017, 212, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Prigerson, H.G.; Vanderwerker, L.C.; Maciejewski, P.K. Prolonged Grief Disorder: A Case for Inclusion in DSM-V. In Handbook of Bereavement Research and Practice: Advances in Theory and Intervention; Stroebe, M.S., Hansson, R.O., Schut, H., Stroebe, W., Eds.; American Psychological Association: Washington, DC, USA, 2008; pp. 165–168. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publications: Washingtong, DC, USA, 2014. [Google Scholar]
- Arizmendi, B.; O′Connor, M.F. What is “normal” in grief? Aust. Crit. Care 2015, 28, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A. Prolonged grief: Where to after Diagnostic and Statistical Manual of Mental Disorders. Curr. Opin. Psychiatry 2014, 27, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Diminich, E.D.; Bonanno, G. Faces, Feelings, Words: Divergence across Channels of Emotional Responding in Complicated Grief. J. Abnorm. Psychol. 2014, 123, 350–361. [Google Scholar] [CrossRef]
- Bonanno, G.A.; Papa, A.; Lalande, K.; Westphal, M.; Coifman, K.A. The importance of being flexible: The ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychol. Sci. 2004, 15, 482–487. [Google Scholar] [CrossRef]
- Gupta, S.; Bonanno, G. Complicated grief and deficits in emotional expressive flexibility. J. Abnorm. Psychol. 2011, 120, 635–643. [Google Scholar] [CrossRef]
- Fernández-Alcántara, M.; Cruz-Quintana, F.; Pérez-Marfil, M.N.; Catena-Martínez, A.; Pérez-García, M.; Turnbull, O.H. Assessment of emotional experience and emotional recognition in complicated grief. Front. Psychol. 2016, 7, 126. [Google Scholar] [CrossRef]
- Maccallum, F.; Bryant, R.A. A cognitive attachment model of prolonged grief: Integrating attachments, memory, and identity. Clin. Psychol. Rev. 2013, 33, 713–727. [Google Scholar] [CrossRef]
- O′Connor, M.F.; Arizmendi, B. Neuropsychological correlates of complicated grief in older spousally bereaved adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 12–18. [Google Scholar] [CrossRef]
- Maccallum, F.; Sawday, S.; Rinck, M.; Bryant, R.A. The push and pull of grief: Approach and avoidance in bereavement. J. Behav. Ther. Exp. Psychiatry 2015, 48, 105–109. [Google Scholar] [CrossRef]
- Eisma, M.C.; Shut, H.A.W.; Stroebe, M.S.; Van den Bout, J.; Stroebe, W.; Boelen, P.A. Is Rumination after Bereavement Linked with Loss Avoidance? Evidence from Eye-Tracking. PLoS ONE 2014, 9, e104980. [Google Scholar] [CrossRef] [PubMed]
- Eisma, M.C.; Rinck, M.; Stroebe, M.S.; Schut, H.A.W.; Boelen, P.A.; Stroebe, W.; Van den Bout, J. Rumination and implicit avoidance following bereavement: An approach avoidance task investigation. J. Behav. Ther. Exp. Psychiatry 2015, 47, 84–91. [Google Scholar] [CrossRef]
- Delespaux, E.; Zech, E. Why do bereaved partners experience interfering rumination? Evidence for deficits in cognitive inhibition. Death Stud. 2015, 39, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Delespaux, E.; Zech, E. Deficits in cognitive inhibition and post-loss rumination: Evidence from a thought suppression task/Déficits de inhibición cognitiva y rumiación posterior a una pérdida: Evidencia a partir de una tarea de supresión de pensamientos. Estud. Psicol. Stud. Psychol. 2017, 38, 608–638. [Google Scholar] [CrossRef]
- Freed, P.J.; Yanagihara, T.K.; Hirsch, J.; Mann, J.J. Neural mechanisms of grief regulation. Biol. Psychiatry 2009, 66, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gündel, H.; O’Connor, M.F.; Littrell, L.; Fort, C.; Lane, R.D. Functional neuroanatomy of grief: An FMRI study. Am. J. Psychiatry 2003, 160, 1946–1953. [Google Scholar] [CrossRef]
- O’Connor, M.F.; Wellisch, D.K.; Stanton, A.L.; Eisenberger, N.I.; Irwin, M.R.; Lieberman, M.D. Craving love? Enduring grief activates brain’s reward center. NeuroImage 2008, 42, 969–972. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Arizmendi, B.; Kaszniak, A.W.; O′Connor, M.F. Disrupted prefrontal activity during emotion processing in complicated grief: An fMRI investigation. NeuroImage 2016, 124, 968–976. [Google Scholar] [CrossRef]
- O’Connor, M.F.; Gündel, H.; McRae, K.; D’Lane, R. Baseline Vagal Tone Predicts BOLD Response during Elicitation of Grief. Neuropsychopharmacology 2007, 32, 2184–2189. [Google Scholar] [CrossRef]
- Schneck, N.; Haufe, S.; Tu, T.; Bonanno, G.A.; Ochsner, K.; Sajda, P.; Mann, J.J. Tracking Deceased-Related Thinking with Neural Pattern Decoding of a Cortical-Basal Ganglia Circuit. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.E.; Radovic, D.; Turnbull, O.H. Inside-out: Comparing internally generated and externally generated basic emotions. Emotion 2012, 12, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Qin, J.; Ma, Y. Neurocognitive processes of linguistic cues related to death. Neuropsychologia 2010, 48, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Martí-García, C.; García-Caro, M.P.; Cruz-Quintana, F.; Schmidt-RioValle, J.; Perez-Garcia, M. Emotional Responses to Images of Death A New Category of Emotional Processing? OMEGA J. Death Dying 2016, 72, 191–209. [Google Scholar] [CrossRef]
- Martí-García, C.; Fernández-Alcántara, M.; Schmidt-RioValle, J.; Cruz-Quintana, F.; García-Caro, M.P.; Perez-Garcia, M. Specific emotional schema of death-related images vs unpleasant images/Esquema emocional específico de imágenes relacionadas con la muerte frente a imágenes desagradables. Stud. Psychol. Estud. Psicol. 2017, 38, 689–706. [Google Scholar] [CrossRef]
- Prigerson, H.G.; Maciejewski, P.K.; Reynolds, C.F.; Bierhals, A.J.; Newsom, J.T.; Fasiczka, A.; Miller, M. Inventory of Complicated Grief: A scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995, 59, 65–79. [Google Scholar] [CrossRef]
- Limonero-García, J.; Lacasta-Reverte, M.; García, J.; Maté-Méndez, J.; Prigerson, H.G. Adaptación al castellano del inventario de duelo complicado. [Spanish adaptation of the inventory of complicated grief]. Med. Paliativa 2009, 16, 291–297. [Google Scholar]
- García-García, J.; Landa-Petralanda, V.; Trigueros-Manzano, M.; Gaminde-Inda, I. Inventario Texas Revisado de Duelo (ITRD): Adaptación al castellano, fiabilidad y validez [Texas Revised Inventory of Grief (TRIG): Adaptation to Spanish, reliability and validity]. Aten. Primaria 2005, 35, 353–358. [Google Scholar] [CrossRef][Green Version]
- Derogatis, L.R. SCL-90-R: Cuestionario de 90 Síntomas. [SCL-90-R: Symptom Checklist-90-R: Administration, Scoring, and Procedures Manual]; TEA Ediciones: Madrid, Spain, 2002. [Google Scholar]
- Crespo, M.; Gómez, M. La evaluación del estrés postraumático: Presentación de la escala de Evaluación Global de Estrés Postraumático (EGEP). [Posttraumatic Stress Assessment: Introducing the Global Assessment of Posttraumatic Stress Questionnaire]. Clínica Salud 2012, 23, 25–41. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Emotion, attention, and the startle reflex. Psychol. Rev. 1990, 97, 377–395. [Google Scholar] [CrossRef]
- Vila, J.; Sánchez, M.; Ramírez, I.; Fernández, M.C.; Cobos, P.; Rodríguez, S.; Moltó, J. El sistema internacional de imágenes afectivas (IAPS): Adaptación española. Segunda parte. [The international affective picture system (IAPS): Spanish validation. Second part]. Rev. Psicol. Gen. Apl. 2001, 54, 635–657. [Google Scholar]
- Schneider, W.; Eschman, A.; Zuccolotto, A. E-Prime: User’s Guide; Psychology Software Incorporated: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Lang, P.J. The emotion probe: Studies of motivation and attention. Am. Psychol. 1995, 50, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6, e25031. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.L.; Gotlib, I.H.; Thompson, P.M.; Thomason, M.E. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connect. 2011, 1, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Hsu, A.L.; Hsu, L.M.; Tsai, J.S.; Huang, C.M.; Chao, Y.P.; Hwang, T.J.; Wu, C.W. Mindfulness improves emotion regulation and executive control on bereaved individuals: An fMRI study. Front. Hum. Neurosci. 2019, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Bueso-Izquierdo, N.; Verdejo-Román, J.; Contreras-Rodríguez, O.; Carmona-Perera, M.; Pérez-García, M.; Hidalgo-Ruzzante, N. Are batterers different from other criminals? An fMRI study. Soc. Cogn. Affect. Neurosci. 2016, 11, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.D. AFNI and NIFTI Server at NIMH in Bethesda, MD, USA. Simultaneous Inference for FMRI Data. 2013. Available online: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf (accessed on 17 July 2017).
- Quirin, M.; Loktyushin, A.; Arndt, J.; Küstermann, E.; Lo, Y.Y.; Kuhl, J.; Eggert, L. Existential neuroscience: A functional magnetic resonance imaging investigation of neural responses to reminders of one’s mortality. Soc. Cogn. Affect. Neurosci. 2012, 7, 193–198. [Google Scholar] [CrossRef]
- Banks, S.J.; Eddy, K.T.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007, 2, 303–312. [Google Scholar] [CrossRef]
- Kim, M.J.; Loucks, R.A.; Palmer, A.L.; Brown, A.C.; Solomon, K.M.; Marchante, A.N.; Whalen, P.J. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav. Brain. Res. 2011, 223, 403–410. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Abe, N.; Kashima, E.S.; Nomura, M. Self-esteem modulates amygdala-ventrolateral prefrontal cortex connectivity in response to mortality threats. J. Exp. Psychol. Gen. 2016, 145, 273. [Google Scholar] [CrossRef]
- Shi, Z.; Han, S. Transient and sustained neural responses to death-related linguistic cues. Soc. Cogn. Affect. Neurosci. 2012, 8, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Futterman, A.; Holland, J.M.; Brown, P.J.; Thompson, L.W.; Gallagher-Thompson, D. Factorial validity of the Texas Revised Inventory of Grief—Present scale among bereaved older adults. Psychol. Assess. 2010, 22, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.E.; Brown, L.L.; Aron, A.; Strong, G.; Mashek, D. Reward, Addiction, and Emotion Regulation Systems Associated With Rejection in Love. J. Neurophysiol. 2010, 104, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Najib, A.; Lorberbaum, J.P.; Kose, S.; Bohning, D.E.; George, M.S. Regional brain activity in women greiving a romantic relationship breakup. Am. J. Psychiatry 2004, 161, 2245–2256. [Google Scholar] [CrossRef]
- Kersting, A.; Ohrmann, P.; Pedersen, A.; Kroker, K.; Samberg, D.; Bauer, J.; Kugel, H.; Koelkebeck, K.; Steinhard, J.; Heindel, W.; et al. Neural activation underlying acute grief in women after the loss of an unborn child. Am. J. Psychiatry 2009, 166, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.S.; Noonan, M.P.; Boorman, E.D.; Walton, M.E.; Behrens, T.E. Frontal cortex and reward-guided learning and decision-making. Neuron 2011, 70, 1054–1069. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring Emotion: Behavior, Feeling and Physiology. In Cognitive Neuroscience of Emotion; Lane, R.D., Nadel, R., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 106–128. [Google Scholar]
- Li, J.; Xu, C.; Cao, X.; Gao, Q.; Wang, Y.; Wang, Y.; Peng, J.; Zhang, K. Abnormal activation of the occipital lobes during emotion picture processing in major depressive disorder patients. Neural Regen. Res. 2013, 8, 1693–1701. [Google Scholar]
- Fernández-Alcántara, M.; Pérez-García, M.; Pérez-Marfil, M.N.; Catena-Martínez, A.; Hueso-Montoro, C.; Cruz-Quintana, F. Assessment of different components of executive function in grief. Psicothema 2016, 28, 260–265. [Google Scholar]
- Maccallum, F.; Bonanno, G.A. The economics of losing a loved one: Delayed reward discounting in prolonged Grief. Clin. Psychol. Sci. 2016, 4, 683–690. [Google Scholar] [CrossRef]
- Maccallum, F.; Bryant, R.A. Imagining the future in complicated grief. Depress. Anxiety 2011, 28, 658–665. [Google Scholar] [CrossRef]
- Boelen, P.A.; van den Bout, J.; van den Hout, M.A. Negative cognitions and avoidance in emotional problems after bereavement: A prospective study. Behav. Res. Ther. 2006, 44, 1657–1672. [Google Scholar] [CrossRef] [PubMed]



| Variables | CG (n = 19) | NB (n = 19) | t or χ2 | p |
|---|---|---|---|---|
| Mean (SE) or n (%) | Mean (SE) or n (%) | |||
| Age | 40.42 (2.74) | 39.42 (2.85) | –0.25 | 0.802 |
| Sex | 0 | 1 | ||
| Men | 2 (10.5%) | 2 (10.5%) | ||
| Women | 17 (89.5%) | 17 (89.5%) | ||
| Marital status | 1.56 | 0.670 | ||
| Single | 8(42.1%) | 8 (42.1%) | ||
| Married | 8 (42.1%) | 10 (52.6%) | ||
| Widow/widower | 1 (5.3%) | 0 (0%) | ||
| Separated | 2 (10.5%) | 1 (5. 3%) | ||
| Education | 0 | 1 | ||
| Basic | 2 (10.5%) | 2 (10.5%) | ||
| Bachelor/FP | 9 (47.4%) | 9 (47.4%) | ||
| University student | 8 (42.1%) | 8 (42.1%) | ||
| Time since loss (months) | 41.58 (5.51) | |||
| Drug consumption | 0.17 | 0.676 | ||
| Yes | 3 (15.8%) | 4(21.1%) | ||
| No | 16 (84.2%) | 15 (79%) | ||
| Relationship to deceased | ||||
| Son | 4 (21.1%) | |||
| Spouse | 1 (100%) | |||
| Mother/father | 12 (63.2%) | |||
| Brother | 1 (5.3%) | |||
| Grandparent | 1 (5.3%) | |||
| Another relative | 0 (0%) | |||
| Friend | 0 (0%) | |||
| Inventory of Complicated Grief (ICG) | 36.32 (1.67) | 6.89 (1.58) | –12.76 | < 0.001 |
| Texas Revised Inventory of Grief (TRIG) | ||||
| Past Behavior | 19.63 (1.29) | 31.68 (1.65) | 5.74 | < 0.001 |
| Present Feelings | 24 (1.15) | 53.26 (1.96) | 12.86 | < 0.001 |
| Depression (SCL-90-R) | 20.68 (2.13) | 4.37 (0.95) | –6.99 | < 0.001 |
| Anxiety (SCL-90-R) | 10.8 (1.30) | 2.32 (0.59) | -5.94 | < 0.001 |
| Post-traumatic stress (EGEP) | ||||
| Symptoms of re-experimentation | 3.68 (0.23) | 0.32 (0.17) | –11.73 | < 0.001 |
| Intensity of re-experimentation symptoms | 7.16 (0.75) | 0.53 (0.31) | -8.20 | < 0.001 |
| Symptoms of avoidance—Affective bewilderment | 2.95 (0.43) | 0.05 (0.05) | –6.60 | < 0.001 |
| Intensity of avoidance symptoms—affective bewilderment | 6.58 (1.23) | 0.05 (0.05) | –5.31 | < 0.001 |
| Symptoms of hyper-activation | 3.37 (0.31) | 0.21 (0.14) | –9.30 | < 0.001 |
| Intensity of hyper-activation symptoms | 6.63 (0.99) | 0.37 (0.28) | –6.06 | < 0.001 |
| Brain Region | X | Y | Z | kE | t-Value |
|---|---|---|---|---|---|
| CG > NB | |||||
| Amygdala | −26 | 2 | −22 | 55 | 4.77 |
| Hypothalamus | −2 | −8 | −8 | 337 | 4.16 |
| Lateral middle frontal gyrus | 38 | 6 | 44 | 52 | 4.33 |
| Putamen | 28 | 8 | 2 | 64 | 3.45 |
| Dorsal anterior cingulate | 4 | 22 | 40 | 69 | 3.77 |
| Brain Region | X | Y | Z | kE | t-Value |
|---|---|---|---|---|---|
| CG > NB | |||||
| Inferior temporal cortex | 44 | 0 | 26 | 107 | 4.94 |
| NB > CG | |||||
| Visual association cortex | −40 | −78 | 2 | 81 | 4.23 |
| Primary visual cortex | 18 | −90 | −2 | 127 | 4.06 |
| Brain Region | X | Y | Z | kE | t Value |
|---|---|---|---|---|---|
| Death x positive interactions | |||||
| Amygdala | 24 | −6 | −16 | 1 916 a | 3.92 |
| Amygdala | −28 | −6 | −20 | 1 916 a | 4.35 |
| Midbrain | 12 | −20 | −22 | 1 916 a | 4.71 |
| Hippocampus | 24 | −22 | −20 | 1 916 a | 3.60 |
| Periaqueductal gray | 2 | −34 | −10 | 1 916 a | 4.21 |
| Cerebellum | 4 | −44 | −12 | 1 916 a | 4.54 |
| Negative x positive interactions | |||||
| Midbrain | 10 | −24 | −20 | 423 b | 4.17 |
| Periaqueductal gray | 2 | −30 | −12 | 423 b | 4.11 |
| Hippocampus | 20 | −24 | −20 | 423 b | 4.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Alcántara, M.; Verdejo-Román, J.; Cruz-Quintana, F.; Pérez-García, M.; Catena-Martínez, A.; Fernández-Ávalos, M.I.; Pérez-Marfil, M.N. Increased Amygdala Activations during the Emotional Experience of Death-Related Pictures in Complicated Grief: An fMRI Study. J. Clin. Med. 2020, 9, 851. https://doi.org/10.3390/jcm9030851
Fernández-Alcántara M, Verdejo-Román J, Cruz-Quintana F, Pérez-García M, Catena-Martínez A, Fernández-Ávalos MI, Pérez-Marfil MN. Increased Amygdala Activations during the Emotional Experience of Death-Related Pictures in Complicated Grief: An fMRI Study. Journal of Clinical Medicine. 2020; 9(3):851. https://doi.org/10.3390/jcm9030851
Chicago/Turabian StyleFernández-Alcántara, Manuel, Juan Verdejo-Román, Francisco Cruz-Quintana, Miguel Pérez-García, Andrés Catena-Martínez, María Inmaculada Fernández-Ávalos, and María Nieves Pérez-Marfil. 2020. "Increased Amygdala Activations during the Emotional Experience of Death-Related Pictures in Complicated Grief: An fMRI Study" Journal of Clinical Medicine 9, no. 3: 851. https://doi.org/10.3390/jcm9030851
APA StyleFernández-Alcántara, M., Verdejo-Román, J., Cruz-Quintana, F., Pérez-García, M., Catena-Martínez, A., Fernández-Ávalos, M. I., & Pérez-Marfil, M. N. (2020). Increased Amygdala Activations during the Emotional Experience of Death-Related Pictures in Complicated Grief: An fMRI Study. Journal of Clinical Medicine, 9(3), 851. https://doi.org/10.3390/jcm9030851





