Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Data Sources and Search Strategy
2.2. Selection Criteria and Quality Assessment
2.3. Endpoint Selection
2.4. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Primary Endpoints
3.2.1. Mortality
3.2.2. Weaning
3.3. Secondary Endpoints
3.3.1. Analysis Stratified by LV Unloading Technique
3.3.2. Sensitivity Analyses
4. Discussion
4.1. IABP
4.2. ECPELLA
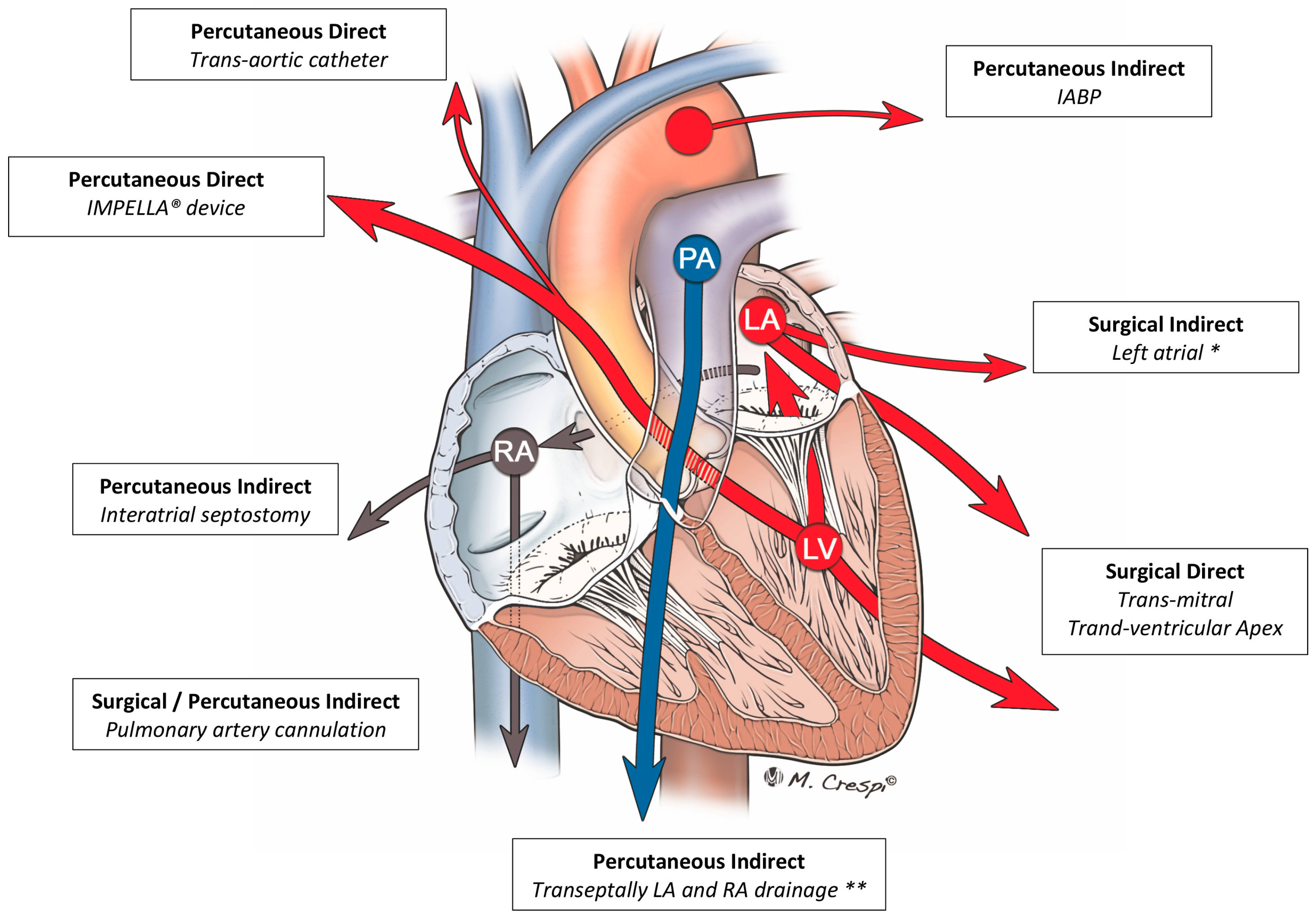
4.3. Other Techniques
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS). | 3 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3–4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 4 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 4 |
| Result | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 5 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 22–24 + Supplementary material |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Supplementary material |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Figure 2, Figure 3 and Figure 4 + Supplementary material |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 5–8 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | Supplementary material |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 5–8 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users and policy makers). | 11–13 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 12 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 11–13 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 13 |
| Study | Setting | Unloading Strategy | Unloading Strategy Usage (%) | N. of pts | Peripheral ECMO (%) | Distal Perfusion (n) | ECMO Duration | Flow Rate | Total Weaning Rate (%) | Bridge to VAD (n) | Bridge to HTx (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acheampong 2016 | PCS | IABP | 58.3 | 24 | NR | NR | 8.4 (0.8–35.4) d | NR | 75 | 1 | 0 |
| Akanni 2018 | mix | Impella | 12.9 | 225 | NR | NR | 3.54 (1.64–5.97) d | NR | NR | 63 | NR |
| Aoyama 2013 | AMI | IABP | 92.1 | 38 | 100 | NR | 126.5 ± 146.4 h | NR | NR | NR | NR |
| Asaumi 2005 | Other | IABP | 42.9 | 14 | 100 | NR | 130 (42–171) h | NR | 71.4 | 1 | 0 |
| Aso 2016 | mix | IABP | 36.6 | 1650 | 100 | NR | 2.31 d | NR | 65.5 | NR | NR |
| Aziz 2010 | mix | IABP | 20 | 10 | 100 | 10 | 5.8 d | 3.5 to 5.0 L/min | 60 | 1 | 1 |
| Beiras-Fernandez 2011 | PCS | IABP | 49.3 | 73 | NR | NR | 4.4 ± 4.0 d | NR | NR | NR | NR |
| Beurtheret 2013 | mix | IABP | 31 | 87 | 100 | NR | NR | NR | 44.8 | 4 | 5 |
| Biancari 2017 | PCS | IABP (47); vent (5) | 25.7 | 148 | 60.1 | 66 | 6.4 ± 5.6 d | NR | 48.6 | 6 | 0 |
| Brechot 2018 | mix | IABP | 40.2 | 259 | 100 | 259 | 2.2 ± 4.3 d | 3.5 to 4.5 L/min | 55.2 | 34 | 21 |
| Carroll 2015 | mix | IABP+Impella | 15.4 | 123 | 75 | NR | NR | NR | 56.1 | 2 | 29 |
| Chen 2005 | Other | IABP | 60 | 10 | 100 | NR | 126.2 ± 56.3 h | NR | 100 | 1 | 0 |
| Chen 2006 | AMI | IABP | 86.1 | 36 | 100 | NR | 108.5 ± 77.5 h | NR | 69.4 | NR | NR |
| Chen 2018 | PCS | IABP | 63.3 | 60 | 100 | 100 | 5.3 ± 2.8 d | NR | 48 | NR | NR |
| Cho 2018 | AMI | IABP | 4.8 | 42 | 100 | NR | NR | initial of 2.2 L/min/m2, which was subsequently regulated to maintain a mean arterial pressure of 65 mmHg | 29.3 | NR | NR |
| Choi 2018 | AMI | IABP | 35.2 | 145 | NR | 21 | 2.0 d [IQR: 1.0–4.0] | 3.3 L/min | 62.8 | NR | 1 |
| Chung 2011 | AMI | IABP | 70 | 20 | NR | NR | 3.8 ± 4.3 d | NR | 70 | NR | NR |
| Czobor 2016 | mix | IABP (10); Impella (1) | 44 | 25 | 100 | 25 | NR | initiated at up to 4.5 L/min and adjusted | NR | NR | NR |
| Elsharkawy 2010 | PCS | IABP | 9.4 | 233 | 33 | NR | NR | NR | NR | 28 | 25 |
| Formica 2010 | PCS | IABP | 69 | 42 | 64.3 | 10 | 7.9 ± 5.3 d | to maintain a cardiac index of 2.5 l/min/m2 | 69 | NR | NR |
| Gass 2014 | mix | IABP | 41.5 | 135 | 100 | NR | 8.5 ± 7.1 d | 2.5 to 4.0 L/min | 40.7 | 20 | 0 |
| Guihaire 2017 | PCS | IABP (25); vent (13) | 27.2 | 92 | 84.8 | NR | 6 d | NR | 48 | 2 | 2 |
| Hei 2011 | PCS | IABP | 16.2 | 68 | 100 | 68 | 4.75 d | 40–220 mL/kg/min | 76.5 | 8 | NR |
| Kagawa 2012 | AMI | IABP | 82.6 | 86 | 100 | NR | 24 (8–65) h | minimum flow of 2.0 L/min | 50 | NR | NR |
| Kim 2014 | AMI | IABP | 75.9 | 58 | NR | NR | 68.7 ± 17.4 h | NR | 41.4 | NR | NR |
| Lee 2016 | mix | IABP | 8.7 | 23 | 100 | NR | 98 (60–192) h | 3.0 to 4.0 L/min | NR | NR | NR |
| Lee 2017 | mx | IABP | 16.3 | 135 | 100 | NR | 99.6 ± 103.23h | adjusted to maintain a cardiac index of 2.4 L/min/m2 | 39.3 | NR | NR |
| Li 2015 | PCS | IABP | 59.3 | 123 | 100 | 123 | 4.3 d | 3.0 L/min | 56.1 | NR | NR |
| Lin 2016 | mix | IABP | 57.1 | 529 | 100 | 256 | NR | NR | NR | 2 | 29 |
| Lorusso 2016 | other | IABP (34); vent (13) | 59.6 | 57 | 82.5 | 63.1 | 9.9 ± 19 d | NR | 75.5 | 2 | 3 |
| Luo 2009 | mix | IABP | 24.4 | 45 | 88.9 | NR | 5.48 d | Initially, 2.5 l/min/m2 with the condition improved, 40 mL/kg/min. adjusted the ECMO blood flow rate in time to maintain LVEF | 60 | 5 | NR |
| Mikus 2013 | PCS | IABP | 92.9 | 14 | 42.9 | 14 | 5 d | to maintain cardiac index of 2.6 l/min/m2 | 50 | 0 | 0 |
| Muller 2016 | AMI | IABP (96); Impella (3) | 69.6 | 138 | NR | 132 | 7 d | NR | 35.5 | 13 | 18 |
| Nakamura 2015 | other | IABP | 95.5 | 22 | 100 | 22 | 179 ± 25 h | initial flow rate was 3.0–3.5 L/min; According to the indicators of peripheral circulatory failure (e.g., arterial blood gas analysis, mixed venous oxygen saturation, lactic acid and urinary output), the flow rate of ECMO was decreased | NR | 1 | 0 |
| Negi 2016 | AMI | IABP | 60 | 15 | 100 | NR | 1.875 d | NR | 53.3 | NR | 1 |
| Overtchouk 2018 | AMI | IABP | 59.4 | 106 | NR | 106 | NR | NR | NR | 10 | 2 |
| Papadopoulos 2015 | PCS | IABP | 21.9 | 360 | 90 | NR | 7 ± 1 d | 50-70 mL/kg/min | 58.1 | 6 | 2 |
| Pappalardo 2016 | mix | Impella | 21.7 | 157 | 100 | 39 | 167 (72–286) h * | Maximal speed | 36.3 * | 8 * | 0 * |
| Park 2014 | AMI | IABP | 42.7 | 96 | 100 | NR | NR | initial of 2.2 L/min/ m2 and adjusted to maintain a mean arterial pressure of 65 mm Hg | 60.4 | NR | NR |
| Patel 2018 | mix | Impella | 45.5 | 66 | 100 | NR | NR | NR | 56.1 | 5 | NR |
| Pokersnik 2012 | PCS | IABP | 59.2 | 49 | 65.3 | 32 | 3.8 ± 3.4 d | gradually increased to 2.0 L/min/m2 and adjusted as necessary to maintain adequate hemodynamics and oxygen delivery. | 55.1 | 2 | 0 |
| Poptsov 2014 | PCS | vent | 60.9 | 46 | 100 | 100 | NR | NR | NR | NR | NR |
| Raffa 2017 | PCS | IABP | 26.7 | 86 | 34.9 | NR | 5 d | NR | 49 | NR | NR |
| Rastan 2010 | PCS | IABP | 74.1 | 517 | 39.3 | 121 | 3.28 ± 2.85 d | NR | 63.3 | 15 | 5 |
| Ro 2013 | mix | IABP | 23.7 | 253 | 96.4 | NR | 71.0 h | NR | 46.6 | NR | 3 |
| Russo 2010 | mix | IABP | 85.7 | 14 | 57.1 | 253 | 10.2 d | NR | 78.6 | 2 | 6 |
| Sakamoto 2012 | AMI | IABP | 95.9 | 98 | 100 | NR | 68.9 ± 62.7 h | NR | 55.1 | 0 | 0 |
| Santise 2014 | PCS | IABP | 72.2 | 18 | 17 | NR | 6.7 ± 3.2 d | 4164 ± 679 mL/min | 72.2 | NR | NR |
| Shinn 2009 | mix | IABP | 33.7 | 92 | 100 | 24 | 90.9 ± 126.0 h | NR | 64.1 | NR | NR |
| Shmack 2017 | mix | vent | 41.7 | 48 | 20.1 | NR | 6.10 ± 3.81 d | 2.6 L/min/m2 | NR | 14 | 5 |
| Slottosch 2012 | PCS | IABP | 93.5 | 77 | 100 | 77 | 79 ± 57 h | 4-7 L/min | 62.3 | NR | NR |
| Slottosch 2017 | mix | IABP | 74.8 | 139 | 79.1 | NR | 4.9 d | 4-7 L/min | 43.2 | NR | 15 |
| Smedira 2001 | mix | IABP | 54.5 | 202 | 75.7 | NR | NR | NR | 58.9 | 6 | 42 |
| Tepper 2018 | mix | IABP | 50 | 60 | 0 | NR | NR | 5.2 L/min | 60 | 10 | NR |
| Unosawa 2012 | PCS | IABP | 83 | 47 | 68.1 | NR | 63.5 ± 61.5 h | 2.34 L/min | 61.7 | 0 | 0 |
| van den Brink 2017 | AMI | IABP | 16.7 | 12 | 100 | NR | 5 (1–10) d | NR | 66.7 | 1 | NR |
| Wang 2013 | PCS | IABP | 47.1 | 87 | NR | 37 | 61 ± 37 h | calculated to supply at least adequate total systemic circulatory support (2.2 L/min) and to achieve a SvO2 of 70% | 58.6 | NR | NR |
| Weber 2017 | mix | IABP | 27.3 | 11 | 100 | 11 | 123.8 ± 120.9 h | NR | 0 | NR | NR |
| Wu 2012 | mix | IABP | 73.3 | 60 | NR | NR | NR | NR | 63.3 | NR | NR |
| Xu 2016 | mix | IABP | 68.8 | 16 | NR | NR | 119.3 ± 114.8 h | NR | NR | NR | NR |
| Zhao 2015 | PCS | IABP | 66.7 | 24 | 95.8 | NR | 115.23 ± 70.17 h | 49 mL/ min/kg | 66.7 | 1* | NR |
| Zhong 2017 | PCS | IABP (9); vent (3) | 33.3 | 36 | 80.6 | NR | 77.5 ± 34.5 h | NR | 66.7 | NR | NR |
| Study | Setting | Unloading Strategy | Unloading Strategy Usage (%) | N. of pts | Age (Years) | Male (%) | Diabetes (%) | Hypertension (%) | PCI * (%) | CABG ** (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acheampong 2016 | PCS | IABP | 58.3 | 24 | 41 (IQR: 22–75) | 58.3 | NR | NR | NA | NA |
| Akanni 2018 | mix | Impella | 12.9 | 225 | 57 (46–67) | 69.3 | 29 | 57 | NR | NR |
| Aoyama 2013 | AMI | IABP | 92.1 | 38 | 59.9 ± 13.5 | 92.1 | NR | NR | 89 | 11 |
| Asaumi 2005 | Other | IABP | 42.9 | 14 | 38.4 ± 15.8 | 50 | NR | NR | NA | NA |
| Aso 2016 | mix | IABP | 36.6 | 1650 | NR | 69.4 | NR | NR | NR | NR |
| Aziz 2010 | mix | IABP | 20 | 10 | 45.3 ± 18.9 | 50 | 10 | 40 | NR | NR |
| Beiras-Fernandez 2011 | PCS | IABP | 49.3 | 73 | 49.3 ± 18.0 | 64.4 | NR | NR | NA | NA |
| Beurtheret 2013 | mix | IABP | 31 | 87 | 46 ± 15 | 67.8 | 15 | 24 | NR | NR |
| Biancari 2017 | PCS | IABP (47); vent (5) | 25.7 | 148 | 65.4 ± 9.4 | 78.4 | 40 | NR | NA | NA |
| Brechot 2018 | mix | IABP | 40.2 | 259 | 50.2 | 69.9 | NR | NR | NR | NR |
| Carroll 2015 | mix | IABP+Impella | 15.4 | 123 | 56 (41–65) | 69 | 20 | 42 | 6 | 4 |
| Chen 2005 | Other | IABP | 60 | 10 | 37.4 ± 14.7 | NR | NR | NR | NA | NA |
| Chen 2006 | AMI | IABP | 86.1 | 36 | 57 ± 10 | 91.7 | 39 | NR | 19 | 78 |
| Chen 2018 | PCS | IABP | 63.3 | 60 | 51.4 ± 12.7 | 75 | 17 | 33 | NA | NA |
| Cho 2018 | AMI | IABP | 4.8 | 42 | 63.48 ± 11.46 | 66.7 | 41 | 48 | 100 [74] | 0 |
| Choi 2018 | AMI | IABP | 35.2 | 145 | 64.6 ± 11.7 | 75.9 | 54 | 53 | 90 [83] | NR |
| Chung 2011 | AMI | IABP | 70 | 20 | 67.7 ± 11.7 | 30 | 35 | 45 | 35 | 55 |
| Czobor 2016 | mix | IABP (10); Impella (1) | 44 | 25 | NR | 80 | 44 | 52 | NR | NR |
| Elsharkawy 2010 | PCS | IABP | 9.4 | 233 | NR | 67.4 | 21 | NR | NA | NA |
| Formica 2010 | PCS | IABP | 69 | 42 | 64.3 ± 11.3 | 66.7 | 33 | 67 | NA | NA |
| Gass 2014 | mix | IABP | 41.5 | 135 | 57.3 ± 15.3 | 64.4 | 31 | 48 | NR | NR |
| Guihaire 2017 | PCS | IABP (25); vent (13) | 27.2 | 92 | 64.5 (18-83) | 59 | NR | NR | NA | NA |
| Hei 2011 | PCS | IABP | 16.2 | 68 | 49.2 ± 13.3 | 76.5 | NR | NR | NA | NA |
| Kagawa 2012 | AMI | IABP | 82.6 | 86 | 63 (56–72) | 81 | 31 | 63 | 71 | 0 |
| Kim 2014 | AMI | IABP | 75.9 | 58 | 61.2 ± 11.3 | 82.8 | NR | NR | NR | NR |
| Lee 2016 | mix | IABP | 8.7 | 23 | 55 (40, 68) | 90 | 52 | 52 | 65 | NR |
| Lee 2017 | mx | IABP | 16.3 | 135 | 59.44 ± 16.55 | 69.6 | 38 | 42 | NR | NR |
| Li 2015 | PCS | IABP | 59.3 | 123 | 56.2 ± 11.8 | 65.9 | NR | NR | NA | NA |
| Lin 2016 | mix | IABP | 57.1 | 529 | 55.1 ± 15.3 | 75.4 | 32 | 35 | NR | NR |
| Lorusso 2016 | other | IABP (34); vent (13) | 59.6 | 57 | 37.6 ± 11.8 | 35.1 | NR | NR | NR | NR |
| Luo 2009 | mix | IABP | 24.4 | 45 | 49.0 ± 14.1 | 76 | NR | NR | NA | NA |
| Mikus 2013 | PCS | IABP | 92.9 | 14 | 53.1 ± 14.3 | 64.3 | 29 | 64 | NA | NA |
| Muller 2016 | AMI | IABP (96); Impella (3) | 69.6 | 138 | 55 (46–63) | 80 | NR | NR | 81 [72] | NR |
| Nakamura 2015 | other | IABP | 95.5 | 22 | 46.2 ± 18.7 | 45.5 | NR | NR | NA | NA |
| Negi 2016 | AMI | IABP | 60 | 15 | 57 ± 13 | 60 | 20 | 87 | NR | NR |
| Overtchouk 2018 | AMI | IABP | 59.4 | 106 | 52.7 ± 10.4 | 84 | 21 | 37 | 75 [72] | 4 |
| Papadopoulos 2015 | PCS | IABP | 21.9 | 360 | 62 ± 17 | 76.1 | 42 | 63 | NA | NA |
| Pappalardo 2016 | mix | Impella | 21.7 | 157 | 53 (46–65) | 83 | NR | NR | 36 | NR |
| Park 2014 | AMI | IABP | 42.7 | 96 | NR | 77.1 | 61 | 48 | 81 [63] | 10 |
| Patel 2018 | mix | Impella | 45.5 | 66 | NR | 68.2 | NR | NR | 15 | 29 |
| Pokersnik 2012 | PCS | IABP | 59.2 | 49 | 65 ± 13 | 67.3 | 39 | 90 | NA | NA |
| Poptsov 2014 | PCS | vent | 60.9 | 46 | 42.1 ± 4.1 | 76.1 | NR | NR | NA | NA |
| Raffa 2017 | PCS | IABP | 26.7 | 86 | 65 ± 11.2 | 65.1 | 17 | 94 | NA | NA |
| Rastan 2010 | PCS | IABP | 74.1 | 517 | 63.5 ± 11.2 | 71.5 | 33 | 70 | NA | NA |
| Ro 2013 | mix | IABP | 23.7 | 253 | 58.8 ± 15.3 | 60.9 | 23 | 39 | NR | NR |
| Russo 2010 | mix | IABP | 85.7 | 14 | 47.8 ± 16.8 | 71.4 | NR | NR | NR | NR |
| Sakamoto 2012 | AMI | IABP | 95.9 | 98 | 72 ± 12 | 66.3 | 35 | 45 | 94 [66] | 2 |
| Santise 2014 | PCS | IABP | 72.2 | 18 | 49 ± 11 | 77.8 | 17 | 22 | NA | NA |
| Shinn 2009 | mix | IABP | 33.7 | 92 | 56 ± 18 | 64.1 | 24 | 29 | NR | NR |
| Shmack 2017 | mix | vent | 41.7 | 48 | 49.7 ± 19.5 | 47.9 | NR | NR | NR | NR |
| Slottosch 2012 | PCS | IABP | 93.5 | 77 | 60 ± 13 | 76.6 | 18 | 50 | NA | NA |
| Slottosch 2017 | mix | IABP | 74.8 | 139 | 58 ± 15 | 76.3 | 27 | NR | NR | NR |
| Smedira 2001 | mix | IABP | 54.5 | 202 | 55 ± 14 | 72 | 21 | NR | NR | NR |
| Tepper 2018 | mix | IABP | 50 | 60 | 53.9 ± 14.9 | 53.3 | 38 | 53 | NR | NR |
| Unosawa 2012 | PCS | IABP | 83 | 47 | 64.4 ± 12.5 | 74.4 | 38 | 43 | NA | NA |
| van den Brink 2017 | AMI | IABP | 16.7 | 12 | 63 (47–75) | 83 | 17 | 42 | 100 | 0 |
| Wang 2013 | PCS | IABP | 47.1 | 87 | 65 ± 7 | 58.6 | 11 | 19 | NA | NA |
| Weber 2017 | mix | IABP | 27.3 | 11 | 52.5 ± 16.4 | 81.8 | NR | NR | NR | NR |
| Wu 2012 | mix | IABP | 73.3 | 60 | 49 | 66.7 | 43 *** | NR | 48 *** | 48 *** |
| Xu 2016 | mix | IABP | 68.8 | 16 | 62.3 ± 11.1 | 62.5 | 38 | NR | NR | NR |
| Zhao 2015 | PCS | IABP | 66.7 | 24 | 59.3 ± 11.9 | 79.2 | 25 | 42 | NA | NA |
| Zhong 2017 | PCS | IABP (9); vent (3) | 33.3 | 36 | 50.4 ± 12.2 | 91.7 | 25 | 81 | NA | NA |
| Study | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Measurement of Interventions | Bias Due to Departures from Intended Interventions | Bias Due to Missing Data * | Bias in Measurement of Outcomes * | Bias in Selection of Reported Result * | Overall Bias | Cohen’s Kappa |
|---|---|---|---|---|---|---|---|---|---|
| Acheampong 2016 | Serious | Critical | Serious | NA | Moderate | Moderate | Low | Serious | 0.83 |
| Akanni 2018 | Moderate | Low | Low | NA | Low | Moderate | Moderate | Moderate | 1 |
| Aoyama 2013 | Serious | Low | Moderate | NA | Moderate | Serious | Serious | Serious | 1 |
| Asaumi 2005 | Serious | Moderate | Serious | NA | Low | Critical | Critical | Critical | 0.67 |
| Aso 2016 | Moderate | Low | Critical | NA | Low | Serious | Moderate | Moderate | 0.83 |
| Aziz 2010 | Serious | Low | Low | NA | Low | Moderate | Moderate | Low | 0.83 |
| Beiras-Fernandez 2011 | Moderate | Low | Low | NA | Moderate | Critical | Critical | Critical | 0.83 |
| Beurtheret 2013 | Serious | Moderate | Low | NA | Moderate | Low | Low | Low | 1 |
| Biancari 2017 | Low | Low | Serious | NA | Moderate | Low | Low | Low | 0.83 |
| Brechot 2018 | Moderate | Low | Critical | NA | Low | Critical | Critical | Critical | 1 |
| Carroll 2015 | Moderate | Low | Moderate | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Chen 2005 | Moderate | Moderate | Low | NA | Low | Serious | Serious | Serious | 0.83 |
| Chen 2006 | Serious | Low | Low | NA | Moderate | Serious | Serious | Serious | 0.83 |
| Chen 2018 | Moderate | Low | Critical | NA | Moderate | Serious | Serious | Serious | 0.67 |
| Cho 2018 | Serious | Moderate | Moderate | NA | Moderate | Serious | Serious | Serious | 1 |
| Choi 2018 | Serious | Low | Serious | NA | Moderate | Serious | Serious | Serious | 0.83 |
| Chung 2011 | Moderate | Low | Low | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Czobor 2016 | Serious | Low | Moderate | NA | Moderate | Serious | Serious | Serious | 1 |
| Elsharkawy 2010 | Serious | Low | Low | NA | Moderate | Moderate | Moderate | Moderate | 0.83 |
| Formica 2010 | Moderate | Moderate | Serious | NA | Moderate | Moderate | Moderate | Moderate | 0.67 |
| Gass 2014 | Moderate | Low | Critical | NA | Low | Moderate | Moderate | Moderate | 1 |
| Guihaire 2017 | Low | Serious | Low | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Hei 2011 | Serious | Low | Low | NA | Moderate | Moderate | Low | Low | 0.83 |
| Kagawa 2012 | Moderate | Serious | Serious | NA | Moderate | Serious | Serious | Serious | 0.83 |
| Kim 2014 | Moderate | Moderate | Critical | NA | Moderate | Critical | Critical | Critical | 0.50 |
| Lee 2016 | Serious | Moderate | Serious | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Lee 2017 | Moderate | Low | Moderate | NA | Moderate | Serious | Serious | Serious | 1 |
| Li 2015 | Moderate | Low | Low | NA | Moderate | Moderate | Low | Moderate | 0.83 |
| Lin 2016 | Moderate | Low | Critical | NA | Low | Serious | Serious | Serious | 0.831 |
| Lorusso 2016 | Low | Moderate | Critical | NA | Moderate | Moderate | Low | Moderate | 0.83 |
| Luo 2009 | Moderate | Low | Low | NA | Moderate | Moderate | Low | Moderate | 0.67 |
| Mikus 2013 | Serious | Low | Low | NA | Low | Moderate | Moderate | Low | 0.67 |
| Muller 2016 | Serious | Low | Low | NA | Moderate | Moderate | Moderate | Moderate | 0.83 |
| Nakamura 2015 | Serious | Moderate | Serious | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Negi 2016 | Moderate | Low | Low | NA | Low | Moderate | Moderate | Moderate | 0.83 |
| Overtchouk 2018 | Moderate | Serious | Low | NA | Moderate | Critical | Critical | Critical | 1 |
| Papadopoulos 2015 | Serious | Low | Low | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Pappalardo 2016 | Moderate | Low | Critical | NA | Low | Serious | Serious | Serious | 0.83 |
| Park 2014 | Moderate | Moderate | Low | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Patel 2018 | Serious | Low | Low | NA | Low | Moderate | Moderate | Low | 0.83 |
| Pokersnik 2012 | Serious | Serious | Critical | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Poptsov 2014 | Moderate | Serious | Critical | NA | Moderate | Critical | Critical | Critical | 1 |
| Raffa 2017 | Moderate | Low | Serious | NA | Moderate | Moderate | Low | Moderate | 0.50 |
| Rastan 2010 | Moderate | Low | Low | NA | Moderate | Moderate | Moderate | Moderate | 0.83 |
| Ro 2013 | Serious | Low | Critical | NA | Low | Critical | Critical | Critical | 0.83 |
| Russo 2010 | Serious | Low | Low | NA | Low | Critical | Serious | Low | 0.83 |
| Sakamoto 2012 | Moderate | Low | Moderate | NA | Moderate | Serious | Serious | Moderate | 0.67 |
| Santise 2014 | Moderate | Serious | Serious | NA | Moderate | Moderate | Moderate | Moderate | 0.83 |
| Shinn 2009 | Moderate | Low | Critical | NA | Moderate | Serious | Serious | Serious | 0.83 |
| Shmack 2017 | Serious | Low | Serious | NA | Low | Critical | Critical | Critical | 1 |
| Slottosch 2012 | Low | Low | Low | NA | Moderate | Low | Low | Low | 1 |
| Slottosch 2017 | Low | Low | Low | NA | Moderate | Moderate | Low | Low | 0.67 |
| Smedira 2001 | Moderate | Low | Serious | NA | Moderate | Serious | Moderate | Moderate | 0.83 |
| Tepper 2018 | Moderate | Low | Critical | NA | Low | Moderate | Moderate | Moderate | 1 |
| Unosawa 2012 | Serious | Low | Low | NA | Moderate | Moderate | Low | Low | 1 |
| van den Brink 2017 | Moderate | Low | Critical | NA | Moderate | Serious | Serious | Serious | 1 |
| Wang 2013 | Moderate | Critical | Low | NA | Moderate | Low | Low | Low | 0.67 |
| Weber 2017 | Low | Critical | Critical | NA | Low | Critical | Critical | Critical | 0.83 |
| Wu 2012 | Serious | Low | Moderate | NA | Moderate | Moderate | Moderate | Moderate | 1 |
| Xu 2016 | Moderate | Low | Critical | NA | Moderate | Serious | Serious | Serious | 0.83 |
| Zhao 2015 | Serious | Critical | Critical | NA | Moderate | Moderate | Moderate | Moderate | 0.83 |
| Zhong 2017 | Low | Critical | Low | NA | Moderate | Serious | Low | Low | 0.50 |
| LA | RSPV | Direct LV Apex | LV by RSPV | PA | |
|---|---|---|---|---|---|
| Guihaire 2017 | 13 patients | ||||
| Biancari 2017 | 3 patients | 1 patient | 1 patient | ||
| Poptsov 2014 | 19 patients (percutaneous) | ||||
| Shmack 2017 | 29 patients | ||||
| Lorusso 2016 | 4 patients | 4 patients | 2 patients |













References
- Bellumkonda, L.; Gul, B.; Masri, S.C. Evolving concepts in diagnosis and management of cardiogenic shock. Am. J. Cardiol. 2018, 122, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.; Raffa, G.M.; Zielinski, K.; Alanazi, M.; Gilbers, M.; Heuts, S.; Natour, E.; Bidar, E.; Schreurs, R.; Delnoij, T.; et al. The impact of Centre’s heart transplant status and volume on in-hospital outcomes following extracorporeal membrane oxygenation for refractory post-cardiotomy cardiogenic shock: A meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Matteucci, M.; Jiritano, F.; Fina, D.; Panzeri, F.; Raffa, G.M.; Kowalewski, M.; Morici, N.; Viola, G.; Sacco, A.; et al. Long-term survival and major outcomes in post-cardiotomy extracorporeal membrane oxygenation for adult patients in cardiogenic shock. Ann. Cardiothorac. Surg. 2019, 8, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, F.H.; McDermott, K.M.; Kini, V.; Gutsche, J.T.; Wald, J.W.; Xie, D.; Szeto, W.Y.; Bermudez, C.A.; Atluri, P.; Acker, M.A.; et al. Trends in U.S. Extracorporeal Membrane Oxygenation Use and Outcomes: 2002–2012. Semin. Thorac. Cardiovasc. Surg. 2015, 27, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, C.M.; Yuh, D.D.; Bonde, P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015, 61, 31–36. [Google Scholar] [CrossRef]
- Maxwell, B.G.; Powers, A.J.; Sheikh, A.Y.; Lee, P.H.; Lobato, R.L.; Wong, J.K. Resource use trends in extracorporeal membrane oxygenation in adults: An analysis of the Nationwide Inpatient Sample 1998–2009. J. Thorac. Cardiovasc. Surg. 2014, 148, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Whitman, G.J. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J. Thorac. Cardiovasc. Surg. 2017, 153, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Chung, M.; Shiloh, A.L.; Carlese, A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. Sci. World J. 2014, 2014, 393258. [Google Scholar] [CrossRef]
- Lorusso, R.; Raffa, G.M.; Heuts, S.; Lo Coco, V.; Meani, P.; Natour, E.; Bidar, E.; Delnoij, T.; Loforte, A. Pulmonary artery cannulation to enhance extracorporeal membrane oxygenation management in acute cardiac failure. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Burkhoff, D.; Kloner, R.A. Beyond Reperfusion: Acute Ventricular Unloading and Cardioprotection During Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, R.E.; Doursout, M.F. Pulmonary edema and elevated left atrial pressure: Four hours and beyond. Physiology 2002, 17, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.; Forrest, P.; Loforte, A. Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019, 8, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevasco, M.; Takayama, H.; Ando, M.; Garan, A.R.; Naka, Y.; Takeda, K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J. Thorac. Dis. 2019, 11, 1676–1683. [Google Scholar] [CrossRef]
- Garan, A.R.; Takeda, K.; Salna, M.; Vandenberge, J.; Doshi, D.; Karmpaliotis, D.; Kirtane, A.J.; Takayama, H.; Kurlansky, P. Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J. Am. Heart Assoc. 2019, 8, e012171. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Smith, A.R.; Bogdanova, Y.; Roydhouse, S.; Phan, K.; Tian, D.H.; Yan, T.D.; Loforte, A. Outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock: Systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2019, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Romeo, F.; Acconcia, M.C.; Sergi, D.; Romeo, A.; Francioni, S.; Chiarotti, F.; Caretta, Q. Percutaneous assist devices in acute myocardial infarction with cardiogenic shock: Review, meta-analysis. World J. Cardiol. 2016, 8, 98–111. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Wongpakaran, N.; Wongpakaran, T.; Wedding, D.; Gwet, K.L. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: A study conducted with personality disorder samples. BMC Med. Res. Methodol. 2013, 13, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martucci, G.; Panarello, G.; Occhipinti, G.; Raffa, G.; Tuzzolino, F.; Capitanio, G.; Carollo, T.; Lino, G.; Bertani, A.; Vitulo, P.; et al. Impact of cannula design on packed red blood cell transfusions: Technical advancement to improve outcomes in extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, 5813–5821. [Google Scholar] [CrossRef]
- Raffa, G.M.; Kowalewski, M.; Brodie, D.; Ogino, M.; Whitman, G.; Meani, P.; Pilato, M.; Arcadipane, A.; Delnoij, T.; Natour, E.; et al. Meta-analysis of peripheral or central extracorporeal membrane oxygenation in postcardiotomy and non-postcardiotomy shock. Ann. Thorac. Surg. 2019, 107, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef] [Green Version]
- Ostadal, P.; Mlcek, M.; Kruger, A.; Hala, P.; Lacko, S.; Mates, M.; Vondrakova, D.; Svoboda, T.; Hrachovina, M.; Janotka, M.; et al. Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J. Transl. Med. 2015, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- Dickstein, M.L. The starling relationship and veno-arterial ecmo: Ventricular distension explained. ASAIO J. 2018, 64, 497–501. [Google Scholar] [CrossRef]
- Turner, D.A.; Cheifetz, I.M. Extracorporeal membrane oxygenation for adult respiratory failure. Respir. Care 2013, 58, 1038–1052. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Hachamovitch, R.; Makkar, R.; Ramzy, D.; Moriguchi, J.D.; Arabia, F.A.; Esmailian, F.; Azarbal, B. Lack of survival benefit found with use of intraaortic balloon pump in extracorporeal membrane oxygenation: A pooled experience of 1517 patients. J. Invasive Cardiol. 2015, 27, 453–458. [Google Scholar]
- Weber, C.; Deppe, A.C.; Sabashnikov, A.; Slottosch, I.; Kuhn, E.; Eghbalzadeh, K.; Scherner, M.; Choi, Y.H.; Madershahian, N.; Wahlers, T. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion 2018, 33, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Gelsomino, S.; Natour, E.; Johnson, D.M.; Rocca, H.B.; Pappalardo, F.; Bidar, E.; Makhoul, M.; Raffa, G.; Heuts, S.; et al. Modalities and effects of left ventricle unloading on extracorporeal life support: A review of the current literature. Eur. J. Heart Fail. 2017, 19, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truby, L.K.; Takeda, K.; Mauro, C.; Yuzefpolskaya, M.; Garan, A.R.; Kirtane, A.J.; Topkara, V.K.; Abrams, D.; Brodie, D.; Colombo, P.C.; et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017, 63, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. shock investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camboni, D.; Schmid, C. To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome? J. Thorac. Dis. 2017, 9, 4915–4918. [Google Scholar] [CrossRef] [Green Version]
- Meani, P.; Delnoij, T.; Raffa, G.M.; Morici, N.; Viola, G.; Sacco, A.; Oliva, F.; Heuts, S.; Sels, J.W.; Driessen, R.; et al. Protracted aortic valve closure during peripheral veno-arterial extracorporeal life support: Is intra-aortic balloon pump an effective solution? Perfusion 2019, 34, 35–41. [Google Scholar] [CrossRef]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef]
- Williams, D.O.; Korr, K.S.; Gewirtz, H.; Most, A.S. The effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis in patients with unstable angina. Circulation 1982, 66, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Werdan, K.; Gielen, S.; Ebelt, H.; Hochman, J.S. Mechanical circulatory support in cardiogenic shock. Eur. Heart J. 2014, 35, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Zobel, G.; Dacar, D.; Kuttnig, M.; Rodl, S.; Rigler, B. Mechanical support of the left ventricle in ischemia induced left ventricular failure: An experimental study. Int. J. Artif. Organs 1992, 15, 114–119. [Google Scholar] [CrossRef]
- Sauren, L.D.; Reesink, K.D.; Selder, J.L.; Beghi, C.; van der Veen, F.H.; Maessen, J.G. The acute effect of intra-aortic balloon counterpulsation during extracorporeal life support: An experimental study. Artif. Organs 2007, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Belohlavek, J.; Mlcek, M.; Huptych, M.; Svoboda, T.; Havranek, S.; Ost’adal, P.; Boucek, T.; Kovarnik, T.; Mlejnsky, F.; Mrazek, V.; et al. Coronary versus carotid blood flow and coronary perfusion pressure in a pig model of prolonged cardiac arrest treated by different modes of venoarterial ECMO and intraaortic balloon counterpulsation. Crit. Care 2012, 16, R50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Zhang, Z.; Song, T.; Yang, Y.; Meng, G.; Zhao, J.; Wang, C.; Gu, K.; Peng, J.; Jiang, B.; et al. Combining ECMO with IABP for the treatment of critically Ill adult heart failure patients. Heart Lung Circ. 2014, 23, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Brechot, N.; Mastroianni, C.; Schmidt, M.; Santi, F.; Lebreton, G.; Hoareau, A.M.; Luyt, C.E.; Chommeloux, J.; Rigolet, M.; Lebbah, S.; et al. Retrieval of severe acute respiratory failure patients on extracorporeal membrane oxygenation: Any impact on their outcomes? J. Thorac. Cardiovasc. Surg. 2018, 155, 1621–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santise, G.; Panarello, G.; Ruperto, C.; Turrisi, M.; Pilato, G.; Giunta, A.; Sciacca, S.; Pilato, M. Extracorporeal membrane oxygenation for graft failure after heart transplantation: A multidisciplinary approach to maximize weaning rate. Int. J. Artif. Organs 2014, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Doll, J.A.; Sketch, M.H., Jr. ECMO and the intraaortic balloon pump: In search of the ideal mechanical circulatory support device. J. Invasive Cardiol. 2015, 27, 459–460. [Google Scholar]
- Patel, S.M.; Lipinski, J.; Al-Kindi, S.G.; Patel, T.; Saric, P.; Li, J.; Nadeem, F.; Ladas, T.; Alaiti, A.; Phillips, A.; et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019, 65, 21–28. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; Greco, T.; Lembo, R.; Mullerleile, K.; Colombo, A.; et al. Concomitant implantation of Impella ((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2017, 19, 404–412. [Google Scholar] [CrossRef]
- Fiedler, A.G.; Dalia, A.; Axtell, A.L.; Ortoleva, J.; Thomas, S.M.; Roy, N.; Villavicencio, M.A.; D’Alessandro, D.A.; Cudemus, G. Impella placement guided by echocardiography can be used as a strategy to unload the left ventricle during peripheral venoarterial extracorporeal membrane oxygenation. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2585–2591. [Google Scholar] [CrossRef]
- Schrage, B.; Burkhoff, D.; Rubsamen, N.; Becher, P.M.; Schwarzl, M.; Bernhardt, A.; Grahn, H.; Lubos, E.; Soffker, G.; Clemmensen, P.; et al. Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. JACC. Heart Fail. 2018, 6, 1035–1043. [Google Scholar] [CrossRef]
- Eliet, J.; Gaudard, P.; Zeroual, N.; Rouviere, P.; Albat, B.; Mourad, M.; Colson, P.H. Effect of impella during veno-arterial extracorporeal membrane oxygenation on pulmonary artery flow as assessed by end-tidal carbon dioxide. ASAIO J. 2018, 64, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S. The effect of impella cp on cardiopulmonary physiology during venoarterial extracorporeal membrane oxygenation support. Artif. Organs 2017, 41, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, D.M.; de Brabander, J.; Karami, M.; Sjauw, K.D.; Engstrom, A.E.; Vis, M.M.; Wykrzykowska, J.J.; Beijk, M.A.; Koch, K.T.; Baan, J.; et al. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 338–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Poptsov, V.; Spirina, E.; Dogonasheva, A.; Zolotova, E. Five years’ experience with a peripheral veno-arterial ECMO for mechanical bridge to heart transplantation. J. Thorac. Dis. 2019, 11, S889–S901. [Google Scholar] [CrossRef]
- Biancari, F.; Dalen, M.; Perrotti, A.; Fiore, A.; Reichart, D.; Khodabandeh, S.; Gulbins, H.; Zipfel, S.; Al Shakaki, M.; Welp, H.; et al. Venoarterial extracorporeal membrane oxygenation after coronary artery bypass grafting: Results of a multicenter study. Int. J. Cardiol. 2017, 241, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, R.; Centofanti, P.; Gelsomino, S.; Barili, F.; Di Mauro, M.; Orlando, P.; Botta, L.; Milazzo, F.; Actis Dato, G.; Casabona, R.; et al. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: A 5-year multi-institutional experience. Ann. Thorac. Surg. 2016, 101, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Schmack, B.; Seppelt, P.; Weymann, A.; Alt, C.; Farag, M.; Arif, R.; Doesch, A.O.; Raake, P.W.; Kallenbach, K.; Mansur, A.; et al. Extracorporeal life support with left ventricular decompression-improved survival in severe cardiogenic shock: Results from a retrospective study. PeerJ 2017, 5, e3813. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, R.; Raffa, G.M.; Alenizy, K.; Sluijpers, N.; Makhoul, M.; Brodie, D.; McMullan, M.; Wang, I.W.; Meani, P.; MacLaren, G.; et al. Structured review of post-cardiotomy extracorporeal membrane oxygenation: Part 1-Adult patients. J. Heart Lung Transpl. 2019, 38, 1125–1143. [Google Scholar] [CrossRef]
- Kowalewski, M.; Raffa, G.; Zielinski, K.; Meani, P.; Alanazi, M.; Gilbers, M.; Heuts, S.; Natour, E.; Bidar, E.; Schreurs, R.; et al. Baseline surgical status and short-term mortality after extracorporeal membrane oxygenation for post-cardiotomy shock: A meta-analysis. Perfusion 2019. [Google Scholar] [CrossRef]
- Lorusso, R.; Raffa, G.M.; Kowalewski, M.; Alenizy, K.; Sluijpers, N.; Makhoul, M.; Brodie, D.; McMullan, M.; Wang, I.W.; Meani, P.; et al. Structured review of post-cardiotomy extracorporeal membrane oxygenation: Part 2-pediatric patients. J. Heart Lung Transpl. 2019, 38, 1144–1161. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalewski, M.; Malvindi, P.G.; Zieliński, K.; Martucci, G.; Słomka, A.; Suwalski, P.; Lorusso, R.; Meani, P.; Arcadipane, A.; Pilato, M.; et al. Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1039. https://doi.org/10.3390/jcm9041039
Kowalewski M, Malvindi PG, Zieliński K, Martucci G, Słomka A, Suwalski P, Lorusso R, Meani P, Arcadipane A, Pilato M, et al. Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(4):1039. https://doi.org/10.3390/jcm9041039
Chicago/Turabian StyleKowalewski, Mariusz, Pietro Giorgio Malvindi, Kamil Zieliński, Gennaro Martucci, Artur Słomka, Piotr Suwalski, Roberto Lorusso, Paolo Meani, Antonio Arcadipane, Michele Pilato, and et al. 2020. "Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 4: 1039. https://doi.org/10.3390/jcm9041039
APA StyleKowalewski, M., Malvindi, P. G., Zieliński, K., Martucci, G., Słomka, A., Suwalski, P., Lorusso, R., Meani, P., Arcadipane, A., Pilato, M., & Raffa, G. M. (2020). Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(4), 1039. https://doi.org/10.3390/jcm9041039








