Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance—A Randomized, Open-Label, Controlled Phase II Clinical Trial
Abstract
1. Introduction
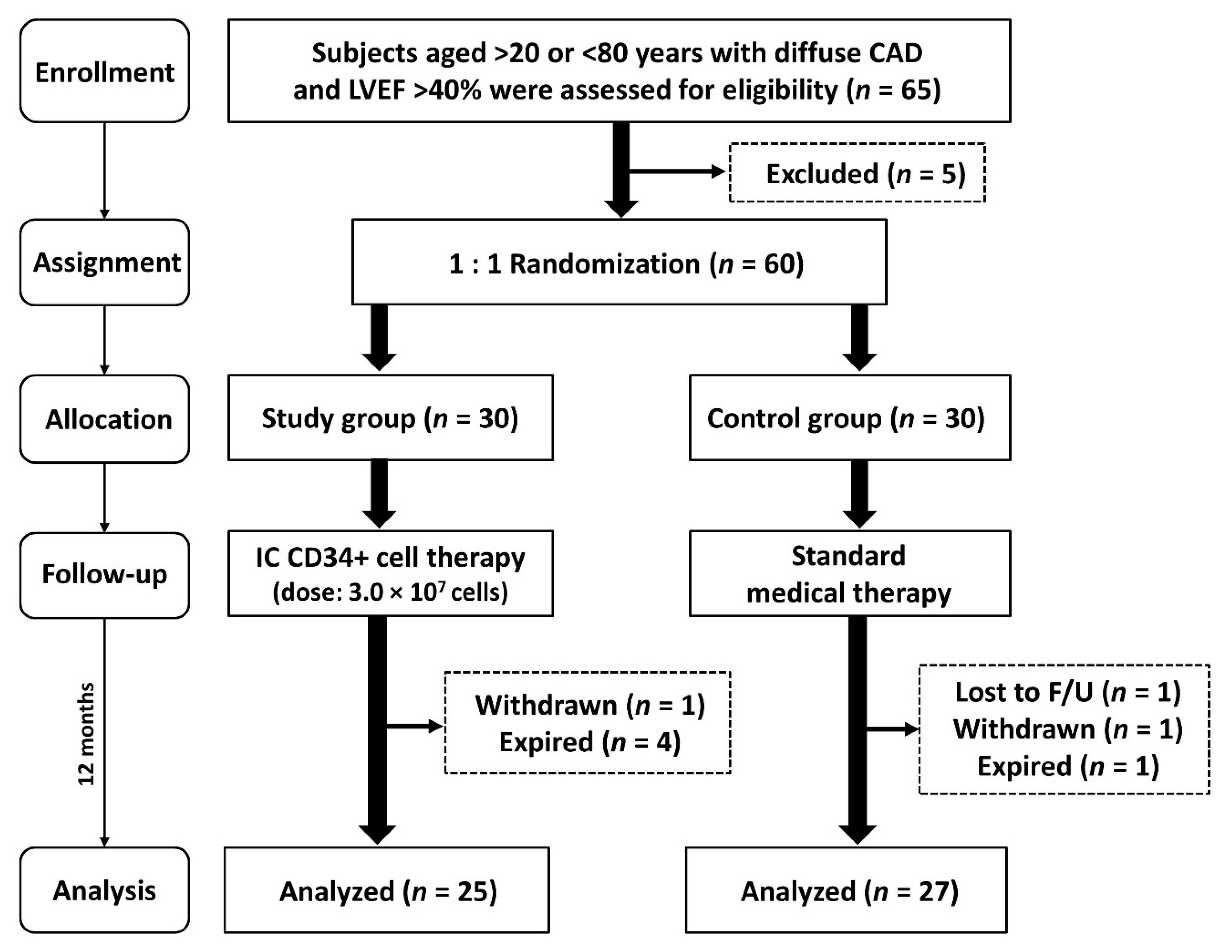
2. Materials and Methods
2.1. Study Design
2.2. Calculation of Rational Sample Size for Endpoints
2.3. Inclusion and Exclusion Criteria
2.4. Procedure and Protocol for Cell Isolation and Intracoronary Autologous CD34+ Cell Therapy
2.5. Flow Cytometric Assessment of Circulating and Coronary Sinus EPC Levels and ELISA Evaluation of Soluble Angiogenesis Factors
2.6. Coronary Angiographic, Imaging and Laboratory Studies
2.7. Definition for Angiographic Angiogenesis Score
2.8. Medications
2.9. One-Year Follow-up for Clinical Outcomes
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Group 1 and Group 2 Patients
3.2. Clinical and Angiographic Findings and Prognostic Outcomes
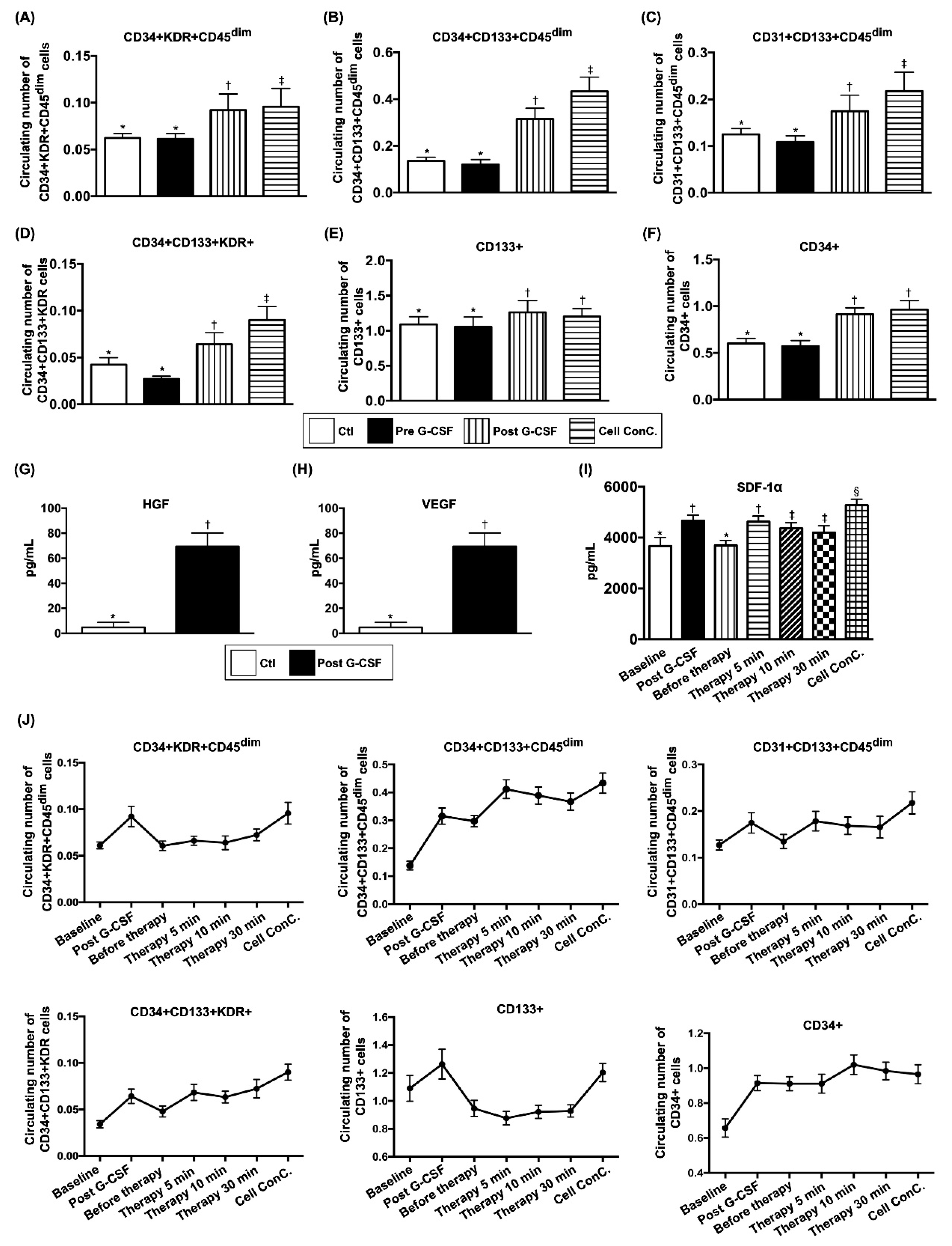
3.3. Comparison of Circulating EPC Surface Markers and Soluble Angiogenesis Factors Between Groups 1 and 2 Before and After G-CSF Treatment in Group 1 and Changes in EPC Population and SDF-1 α Concentration in Coronary Sinus (CS) in Group 1 at Different Time Points
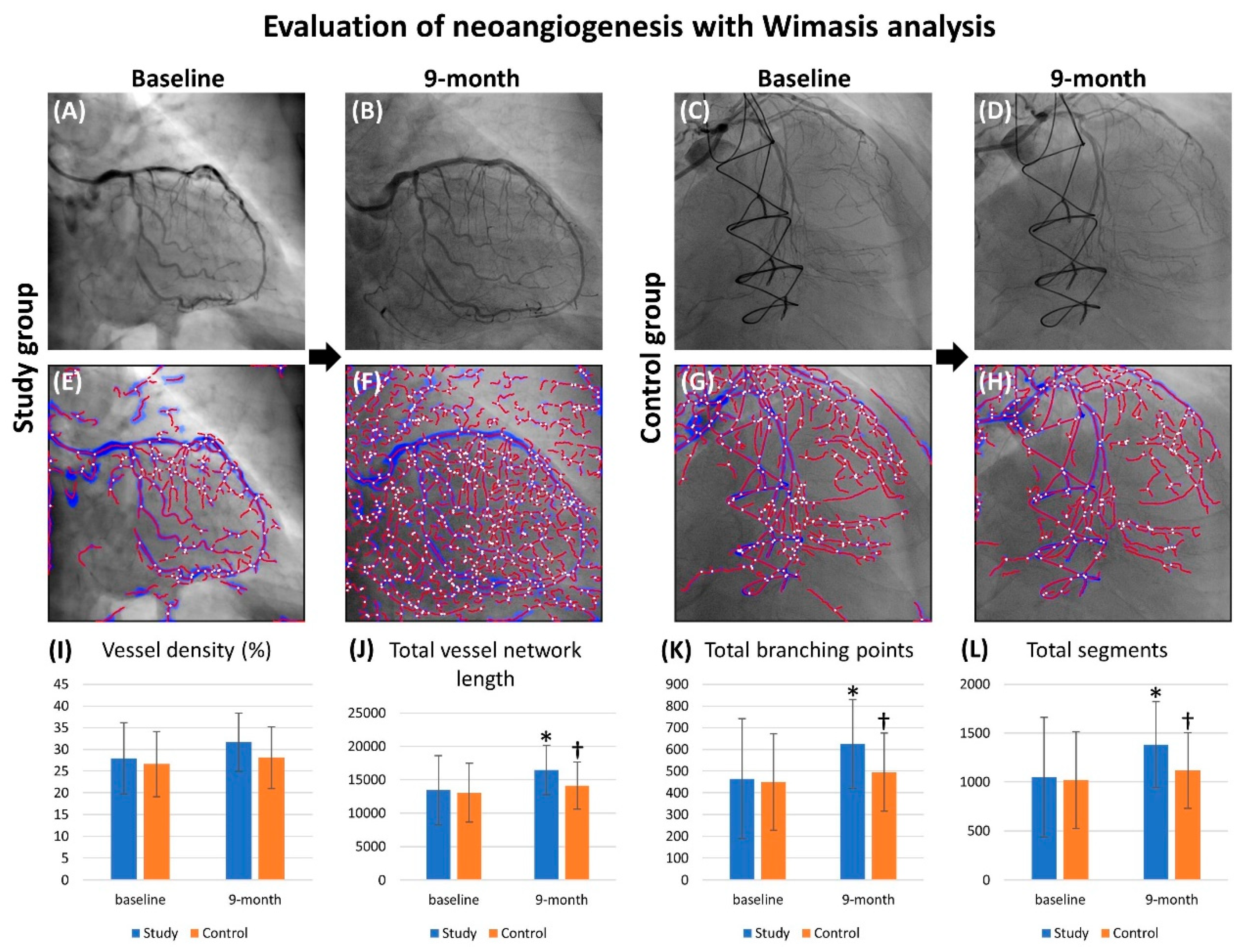
3.4. Objective Evaluation of Angiogenesis with Wimasis Software
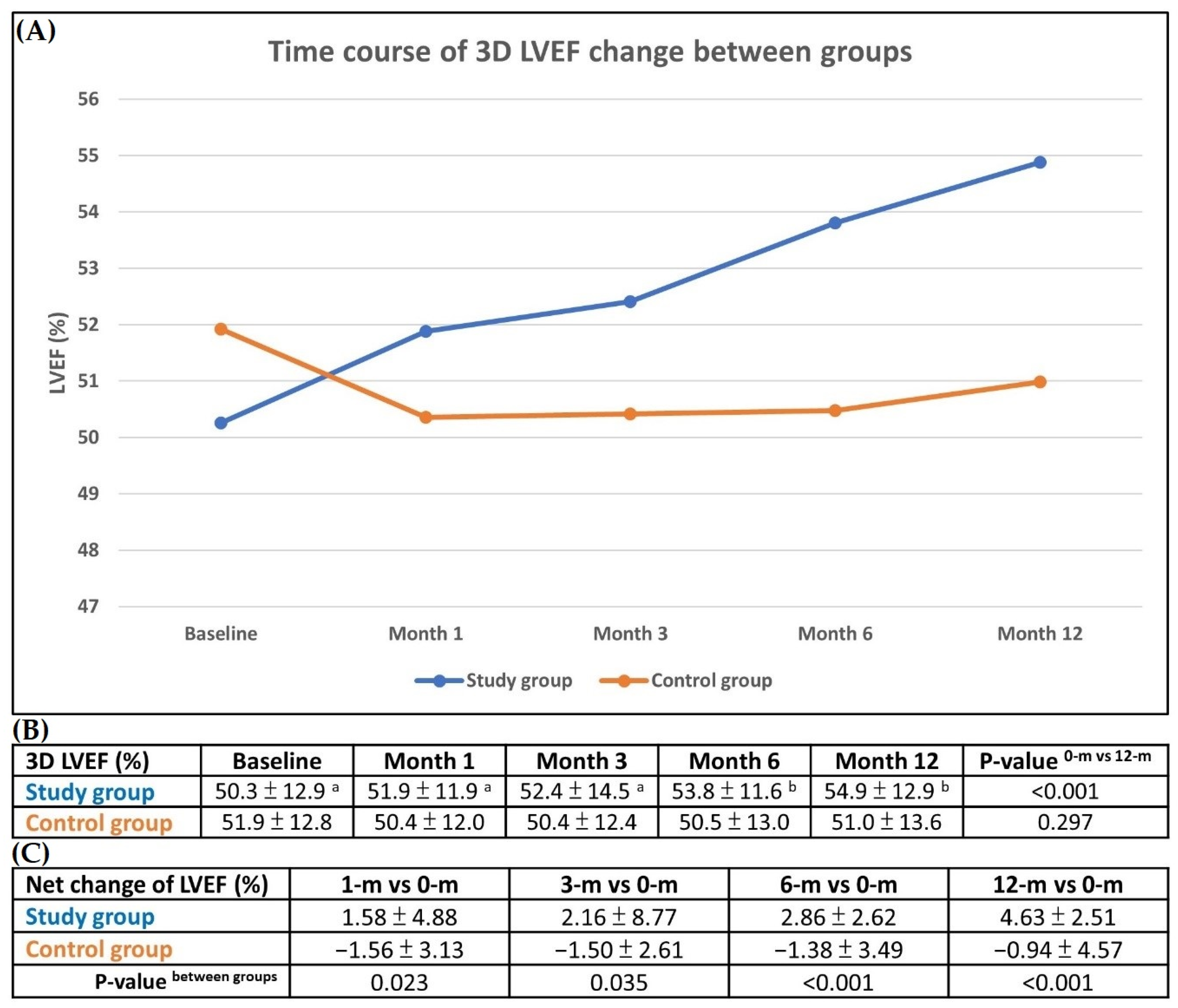
3.5. Changes in LVEF Compared with Baseline and Serial Changes on 3-D Echocardiography During One-Year Follow up
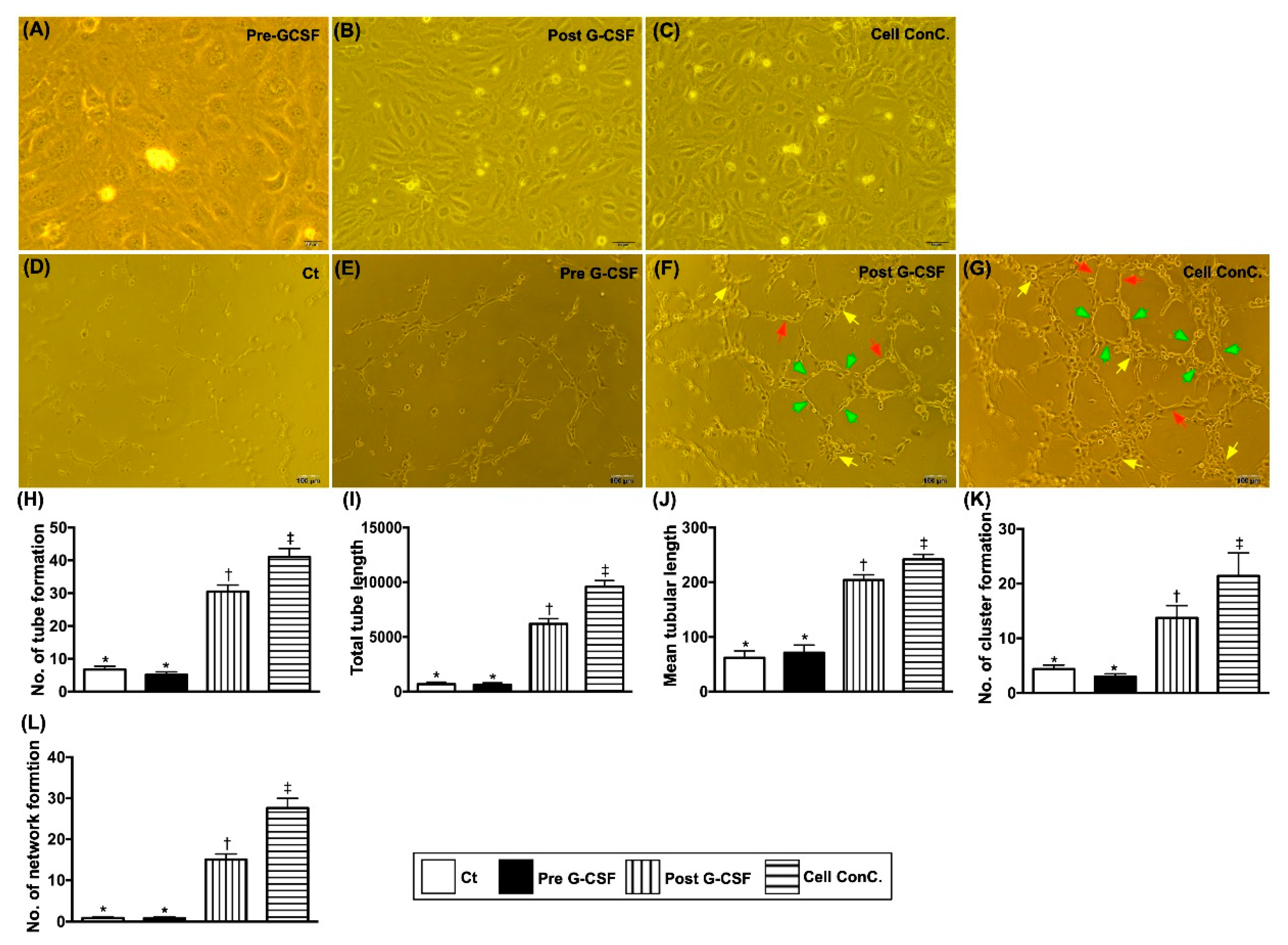
3.6. Matrigel Assay for Assessment of Angiogenesis
3.7. Comparison of Echocardiographic Parameters Between Two Groups at Baseline and 12 Months (Supplementary Table S1)
3.8. Illustrating the Example of Flow Cytometric Analysis how to Gate the EPC Surface Markers (Supplementary Figure S1)
3.9. Illustrating the Presentation of the Backing Gate for EPC Surface Maker (Supplementary Figure S2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | coronary artery disease |
| PCI | percutaneous coronary intervention |
| CABG | coronary artery bypass surgery |
| LV | left ventricular |
| IC | intracoronary |
| HF | heart failure |
| EPC | endothelial progenitor cell |
| LVEF | left ventricular ejection fraction |
| TFDA | Taiwan Food and Drug Administration |
| CCS | Canadian Cardiovascular Society |
| NYHA Fc | New York Heart Association Functional Classification |
| G-CSF | granulocyte-colony stimulating factor |
| ISHAGE | International Society of Hematotherapy and Grafting Engineering |
| PBSC | peripheral-blood stem cell |
| CS | coronary sinus |
| ELISA | enzyme-linked immunosorbent assay |
| VEGF | vascular endothelial growth factor |
| HGF | hepatocyte growth factor |
| SDF-1α | stromal cell-derived growth factor 1 alpha |
| ACEIs | angiotensin converting enzyme inhibitors |
| ARBs | angiotensin II type I receptor blockers |
| RMANOVA | repeated measures analysis of variation |
| FMD | flow-mediated dilatation |
| MACCE | major adverse cardiovascular or cerebrovascular event |
References
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; Åsenblad, N.; Bassand, J.P.; Becker, R.C.; Cannon, C.P.; Claeys, M.J.; Harrington, R.A.; Horrow, J.; Husted, S.; James, S.K. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: Results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J. Am. Coll. Cardiol. 2011, 57, 672–684. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Stahle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.F.; Blumenthal, D.M.; Feyman, Y.; Frakt, A.B.; Turchin, A.; Doros, G.; Gao, Q.; Song, Y.; Joynt Maddox, K.E. Differences in Management of Coronary Artery Disease in Patients with Medicare Advantage vs. Traditional Fee-for-Service Medicare Among Cardiology Practices. JAMA Cardiol. 2019, 4, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hermiller, J.B.; Raizner, A.; Cannon, L.; Gurbel, P.A.; Kutcher, M.A.; Wong, S.C.; Russell, M.E.; Ellis, S.G.; Mehran, R.; Stone, G.W. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: The TAXUS-IV trial. J. Am. Coll. Cardiol. 2005, 45, 1172–1179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheu, J.J.; Tsai, T.H.; Lee, F.Y.; Fang, H.Y.; Sun, C.K.; Leu, S.; Yang, C.H.; Chen, S.M.; Hang, C.L.; Hsieh, Y.K.; et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit. Care Med. 2010, 38, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W.; Muramatsu, T.; Nakatani, S.; van Geuns, R.J.; de Bruyne, B.; Dudek, D.; Christiansen, E.; Smits, P.C.; Chevalier, B.; et al. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb Everolimus-Eluting fully bioresorbable vascular scaffold: Optical coherence tomography assessment in the ABSORB cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc. Interv. 2014, 7, 1400–1411. [Google Scholar] [PubMed]
- Singh, M.; Lennon, R.J.; Holmes, D.R., Jr.; Bell, M.R.; Rihal, C.S. Correlates of procedural complications and a simple integer risk score for percutaneous coronary intervention. J. Am. Coll. Cardiol. 2002, 40, 387–393. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.W.; Hong, M.K.; Lee, C.W.; Lee, J.H.; Kim, J.J.; Jang, Y.S.; Shin, E.K.; Yoshida, Y.; Tamura, T.; et al. Long-term (three-year) outcomes after stenting of unprotected left main coronary artery stenosis in patients with normal left ventricular function. Am. J. Cardiol. 2003, 91, 12–16. [Google Scholar] [CrossRef]
- Tan, W.A.; Tamai, H.; Park, S.J.; Plokker, H.W.; Nobuyoshi, M.; Suzuki, T.; Colombo, A.; Macaya, C.; Holmes, D.R., Jr.; Cohen, D.J.; et al. Long-term clinical outcomes after unprotected left main trunk percutaneous revascularization in 279 patients. Circulation 2001, 104, 1609–1614. [Google Scholar] [CrossRef]
- Lee, M.S.; Yang, T.; Dhoot, J.; Iqbal, Z.; Liao, H. Meta-analysis of studies comparing coronary artery bypass grafting with drug-eluting stenting in patients with diabetes mellitus and multivessel coronary artery disease. Am. J. Cardiol. 2010, 105, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- LaMori, J.C.; Shoheiber, O.; Dudash, K.; Crivera, C.; Mody, S.H. The economic impact of acute coronary syndrome on length of stay: An analysis using the Healthcare Cost and Utilization Project (HCUP) databases. J. Med. Econ. 2014, 17, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Gegouskov, V.; Tochtermann, U.; Badowski-Zyla, D.; Thomas, G.; Hagl, S.; Osswald, B. Long-term results after coronary artery reconstructive surgery. Thorac. Cardiovasc. Surg. 2007, 55, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Jolicoeur, E.M.; Cartier, R.; Henry, T.D.; Barsness, G.W.; Bourassa, M.G.; McGillion, M.; L’Allier, P.L. Patients with coronary artery disease unsuitable for revascularization: Definition, general principles, and a classification. Can. J. Cardiol. 2012, 28, S50–S59. [Google Scholar] [CrossRef]
- Henry, T.D.; Satran, D.; Jolicoeur, E.M. Treatment of refractory angina in patients not suitable for revascularization. Nat. Rev. Cardiol. 2014, 11, 78–95. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Biolè, C.; Raposeiras-Roubin, S.; Gaido, F.; Abu-Assi, E.; Kinnaird, T.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Boccuzzi, G.; et al. Average daily ischemic versus bleeding risk in patients with ACS undergoing PCI: Insights from the BleeMACS and RENAMI registries. Am. Heart J. 2020, 220, 108–115. [Google Scholar] [CrossRef]
- Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N. Engl. J. Med. 1996, 335, 217–225. [Google Scholar] [CrossRef]
- Hill, J.M.; Syed, M.A.; Arai, A.E.; Powell, T.M.; Paul, J.D.; Zalos, G.; Read, E.J.; Khuu, H.M.; Leitman, S.F.; Horne, M.; et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J. Am. Coll. Cardiol. 2005, 46, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Tendera, M.; Wojakowski, W.; Ruzyllo, W.; Chojnowska, L.; Kepka, C.; Tracz, W.; Musialek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar] [PubMed]
- Wang, S.; Cui, J.; Peng, W.; Lu, M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology 2010, 117, 140–147. [Google Scholar] [CrossRef]
- Lee, F.-Y.; Chen, Y.-L.; Sung, P.-H.; Ma, M.-C.; Pei, S.-N.; Wu, C.-J.; Yang, C.-H.; Fu, M.; Ko, S.-F.; Leu, S. Intracoronary transfusion of circulation-derived CD34+ cells improves left ventricular function in patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention. Crit. Care Med. 2015, 43, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-H.; Lee, F.-Y.; Tong, M.-S.; Chiang, J.Y.; Pei, S.-N.; Ma, M.-C.; Li, Y.-C.; Chen, Y.-L.; Wu, C.-J.; Sheu, J.-J. The Five-Year Clinical and Angiographic Follow-Up Outcomes of Intracoronary Transfusion of Circulation-Derived CD34+ Cells for Patients with End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention—Phase I Clinical Trial. Crit. Care Med. 2018, 46, e411–e418. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Sup Lee, J.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, S.; Kearney, M.T.; Siva, A.; Gall, N.; Cooklin, M.; Monaghan, M.J. Real-time three-dimensional echocardiography: A novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005, 112, 992–1000. [Google Scholar] [CrossRef] [PubMed]
| Variables | Study Group (n = 30) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| Clinical information | |||
| Age, year | 64.57 ± 8.00 | 65.77 ± 7.29 | 0.546 |
| Male sex, n (%) | 28 (93.3%) | 22 (73.3%) | 0.038 |
| Body height, cm | 163.87 ± 12.53 | 160.37 ± 7.48 | 0.036 |
| Body weight, kg | 70.71 ± 10.71 | 72.65 ± 15.56 | 0.576 |
| Body mass index, kg/m2 | 26.58 ± 5.11 | 28.18 ± 5.47 | 0.114 |
| Smoker, n (%) | 11 (36.7%) | 12 (40.0%) | 0.791 |
| Hypertension, n (%) | 28 (93.3%) | 28 (93.3%) | 1.000 |
| Diabetes mellitus, n (%) | 22 (73.3%) | 19 (63.3%) | 0.405 |
| Dyslipidemia, n (%) | 28 (93.3%) | 21 (70.0%) | 0.020 |
| Old stroke, n (%) | 8 (26.7%) | 6 (20.0%) | 0.542 |
| Old myocardial infarction, n (%) | 5 (16.7%) | 5 (16.7%) | 1.000 |
| LM involvement, n (%) | 14 (46.7%) | 12 (40.0%) | 0.602 |
| Triple vessel CAD, n (%) | 29 (96.7%) | 28 (93.3%) | 1.000 |
| In-stent restenosis, n (%) | 26 (86.7%) | 17 (56.7%) | 0.010 |
| History of CABG, n (%) | 10 (33.3%) | 13 (43.3%) | 0.426 |
| History of PCI, n (%) | 28 (93.3%) | 22 (73.3%) | 0.038 |
| Laboratory data | |||
| Leukocyte, 1000/μL | 7.31 ± 2.42 | 6.80 ± 1.83 | 0.549 |
| Hemoglobin, g/dL | 13.73 ± 1.75 | 13.51 ± 1.82 | 0.624 |
| Platelet, 1000/μL | 210.27 ± 60.08 | 203.80 ± 55.81 | 0.673 |
| Serum creatinine, mg/dL | 1.25 ± 0.49 | 1.06 ± 0.30 | 0.178 |
| eGFR, mL/min | 65.18 ± 20.85 | 70.80 ± 21.36 | 0.451 |
| Alanine aminotransferase, U/L | 22.67 ± 14.17 | 25.41 ± 13.08 | 0.255 |
| Total cholesterol, mg/dL | 156.50 ± 41.41 | 151.72 ± 30.35 | 0.616 |
| Low density lipoprotein | 88.90 ± 35.77 | 81.69 ± 27.61 | 0.399 |
| High density lipoprotein | 42.37 ± 8.85 | 43.28 ± 8.06 | 0.682 |
| Triglyceride | 137.50 ± 84.27 | 134.86 ± 73.52 | 0.891 |
| Endothelial dysfunction *, n (%) | 17 (56.7%) | 16 (53.3%) | 0.795 |
| Medications | |||
| Antiplatelet, n (%) | 30 (100.0%) | 30 (100.0%) | 1.000 |
| Beta blocker, n (%) | 28 (93.3%) | 28 (93.3%) | 1.000 |
| RAAS blocker, n (%) | 27 (90.0%) | 26 (86.7%) | 1.000 |
| Calcium channel blocker, n (%) | 13 (43.3%) | 12 (40.0%) | 0.793 |
| Diuretic, n (%) | 9 (30.0%) | 8 (26.7%) | 0.774 |
| Lipid lowering agent, n (%) | 22 (73.3%) | 23 (76.7%) | 0.776 |
| Vasodilator, n (%) | 17 (56.7%) | 22 (73.3%) | 0.176 |
| Variables | Study Group (n = 30) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| No. of vessel treated by CD34+ cells | 1.72 ± 0.53 | ||
| 1 vessel, n (%) | 9 (31.0%) | ||
| 2 vessels, n (%) | 19 (65.5%) | ||
| 3 vessels, n (%) | 1 (3.40%) | ||
| Troponin-I after CD34+cell therapy | 1.37 ± 4.09 | ||
| Scores of angina and HF | |||
| CCS angina score at baseline | 2.81 ± 0.54 | 2.52 ± 0.75 | 0.325 |
| CCS angina score at 1 months | 1.33 ± 0.88 | 2.53 ± 0.57 | <0.001 |
| CCS angina score at 3 months | 0.78 ± 0.79 | 2.25 ± 0.66 | <0.001 |
| CCS angina score at 6 months | 0.56 ± 0.80 | 2.26 ± 0.94 | <0.001 |
| CCS angina score at 12 months | 0.44 ± 0.75 | 1.81 ± 0.88 | <0.001 |
| p-value 12 M vs. 0 M | <0.001 | 0.009 | |
| NYHA Fc at baseline | 2.07 ± 0.87 | 1.93 ± 0.83 | 0.189 |
| NYHA Fc at 1 months | 1.32 ± 0.82 | 1.97 ± 0.67 | 0.002 |
| NYHA Fc at 3 months | 1.00 ± 0.77 | 2.00 ± 0.62 | <0.001 |
| NYHA Fc at 6 months | 0.59 ± 0.75 | 2.07 ± 0.62 | <0.001 |
| NYHA Fc at 12 months | 0.67 ± 0.83 | 1.78 ± 0.64 | <0.001 |
| p-value 12 M vs. 0 M | < 0.001 | 0.377 | |
| Angiogenesis score on 9-month by coronary angiographic study | 2.83 ± 0.87 | 1.32 ± 1.10 | <0.001 |
| Clinical outcomes at 1 year | |||
| All-cause mortality, n (%) | 4 (13.8%) | 1 (3.4%) | 0.352 |
| MACCE, n (%) | 3 (10.3%) | 3 (10.3%) | 1.000 |
| Cardiovascular death | 1 (3.4%) | 0 (0.0%) | 1.000 |
| Acute myocardial infarction | 0 (0.0%) | 1 (3.4%) | 1.000 |
| Acute stroke | 2 (6.9%) | 2 (6.9%) | 1.000 |
| Hospitalization for HF, n (%) | 3 (10.3%) | 0 (0.0%) | 0.237 |
| Revascularization, n (%) | 4 (13.8%) | 7 (24.1%) | 0.315 |
| Sepsis, n (%) | 3 (10.3%) | 1 (3.4%) | 0.611 |
| Variables | Study Group (n = 25) | Control Group (n = 27) | p-Value |
|---|---|---|---|
| Baseline CAG | |||
| Global metrics | |||
| Vessel density, % | 27.92 ± 8.20 | 26.63 ± 7.54 | 0.530 |
| Total vessel network length, pixel | 13438 ± 5200 | 13064 ± 4377 | 0.765 |
| Total branching points | 465.6 ± 276.9 | 450.4 ± 221.2 | 0.815 |
| Total nets | 45.77 ± 22.79 | 46.46 ± 21.32 | 0.796 |
| Segment characteristics | |||
| Total segments | 1049.8 ± 612.9 | 1019.5 ± 494.8 | 0.834 |
| Segment length, pixel | 15.80 ± 5.42 | 15.80 ± 4.34 | 1.000 |
| Follow-up CAG at 9 months | |||
| Global metrics | |||
| Vessel density, % | 31.66 ± 6.69 | 28.13 ± 7.13 | 0.116 |
| Total vessel network length, pixel | 16466 ± 3720 | 14104 ± 3523 | 0.033 |
| Total branching points | 625.4 ± 204.4 | 495.2 ± 180.2 | 0.027 |
| Total nets | 49.68 ± 13.51 | 49.29 ± 17.15 | 0.524 |
| Segment characteristics | |||
| Total segments | 1383.5 ± 439.8 | 1117.7 ± 385.9 | 0.035 |
| Segment length, pixel | 13.38 ± 1.67 | 12.71 ± 1.79 | 0.240 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-H.; Li, Y.-C.; Lee, M.S.; Hsiao, H.-Y.; Ma, M.-C.; Pei, S.-N.; Chiang, H.-J.; Lee, F.-Y.; Yip, H.-K. Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance—A Randomized, Open-Label, Controlled Phase II Clinical Trial. J. Clin. Med. 2020, 9, 1043. https://doi.org/10.3390/jcm9041043
Sung P-H, Li Y-C, Lee MS, Hsiao H-Y, Ma M-C, Pei S-N, Chiang H-J, Lee F-Y, Yip H-K. Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance—A Randomized, Open-Label, Controlled Phase II Clinical Trial. Journal of Clinical Medicine. 2020; 9(4):1043. https://doi.org/10.3390/jcm9041043
Chicago/Turabian StyleSung, Pei-Hsun, Yi-Chen Li, Mel S. Lee, Hao-Yi Hsiao, Ming-Chun Ma, Sung-Nan Pei, Hsin-Ju Chiang, Fan-Yen Lee, and Hon-Kan Yip. 2020. "Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance—A Randomized, Open-Label, Controlled Phase II Clinical Trial" Journal of Clinical Medicine 9, no. 4: 1043. https://doi.org/10.3390/jcm9041043
APA StyleSung, P.-H., Li, Y.-C., Lee, M. S., Hsiao, H.-Y., Ma, M.-C., Pei, S.-N., Chiang, H.-J., Lee, F.-Y., & Yip, H.-K. (2020). Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance—A Randomized, Open-Label, Controlled Phase II Clinical Trial. Journal of Clinical Medicine, 9(4), 1043. https://doi.org/10.3390/jcm9041043





