Modifiable Risk Factor Possession Patterns of Dementia in Elderly with MCI: A 4-Year Repeated Measures Study
Abstract
:1. Introduction
2. Experimental Section
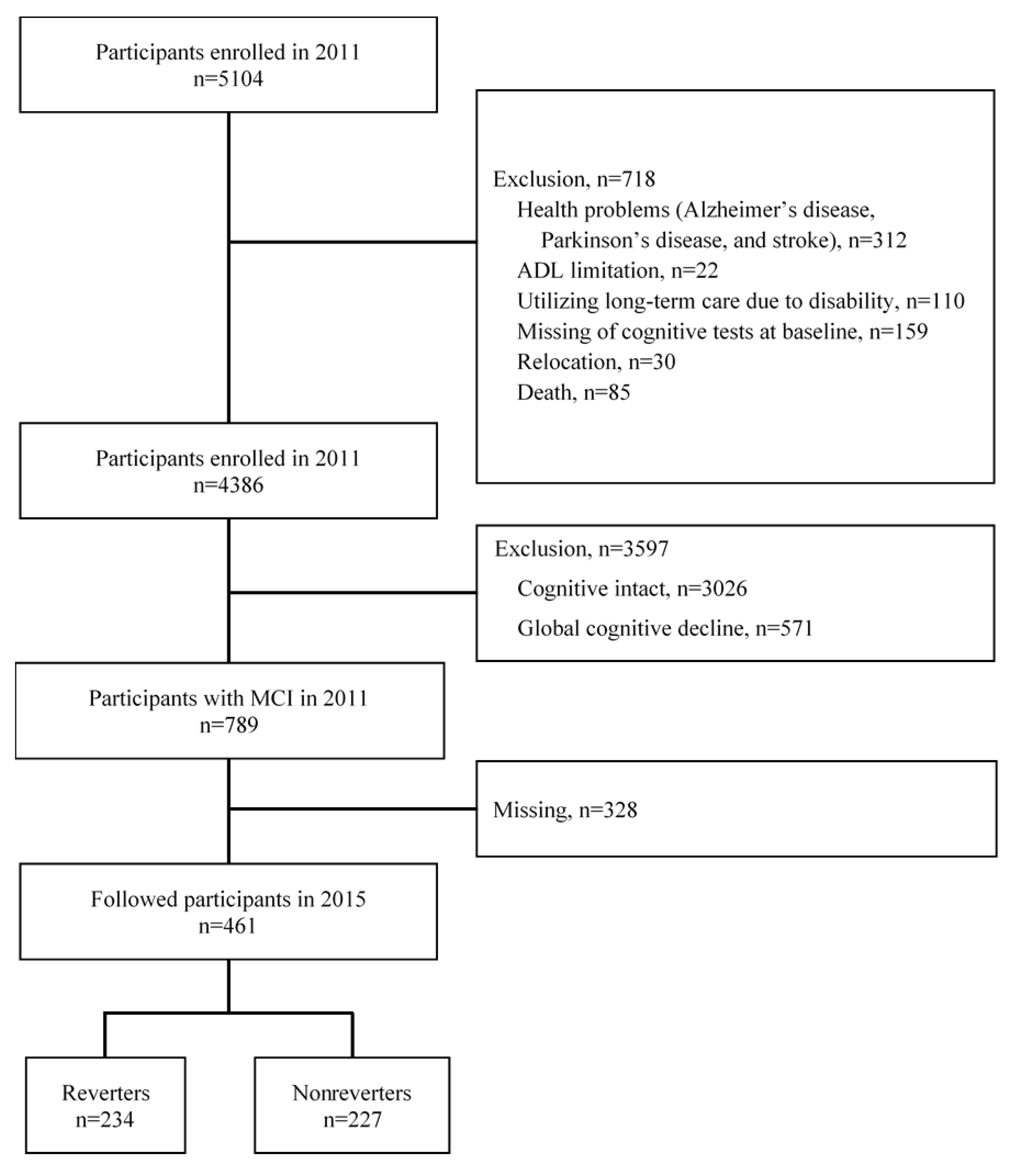
2.1. Study Participants, Design, and Setting
2.2. Measurements of Modifiable Risk Factors
2.3. Measurement of Cognitive Functions and Incident AD
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
3.2. LCA of Modifiable Risk Factor Possession Patterns
3.3. Demographical and Modifiable Risk Factor Cluster Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med 2011, 364, 2227–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.F.; Snitz, B.E.; Ganguli, M. Should mild cognitive impairment be subtyped? Curr. Opin. Psychiatry 2011, 24, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Knopman, D.S.; Mielke, M.M.; Cha, R.H.; Pankratz, V.S.; Christianson, T.J.; Geda, Y.E.; Boeve, B.F.; Ivnik, R.J.; Tangalos, E.G.; et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014, 82, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Moore, A.; Mpofu, E.; Dorstyn, D.; Li, Q.; Yin, C. Effectiveness of Combined Cognitive and Physical Interventions to Enhance Functioning in Older Adults With Mild Cognitive Impairment: A Systematic Review of Randomized Controlled Trials. Gerontologist 2019. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.; Booth, A.; Rockwood, K.; Peters, J.; D’Este, C.; Anstey, K.J. Combining modifiable risk factors and risk of dementia: A systematic review and meta-analysis. BMJ Open 2019, 9, e022846. [Google Scholar] [CrossRef]
- Kivipelto, M.; Solomon, A.; Ahtiluoto, S.; Ngandu, T.; Lehtisalo, J.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimers Dement. 2013, 9, 657–665. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Lee, S.; Lee, S. Conversion and Reversion Rates in Japanese Older People With Mild Cognitive Impairment. J. Am. Med. Dir. Assoc. 2017, 18, 808.e1–808.e6. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Jia, Y.; Hughes, T.F.; Snitz, B.E.; Chang, C.H.; Berman, S.B.; Sullivan, K.J.; Kamboh, M.I. Mild Cognitive Impairment that Does Not Progress to Dementia: A Population-Based Study. J. Am. Geriatr. Soc. 2019, 67, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Han, C. Is there an MCI reversion to cognitively normal? Analysis of Alzheimer’s disease biomarkers profiles. Int. Psychogeriatr. 2014, 27, 429–437. [Google Scholar] [CrossRef]
- Pandya, S.Y.; Clem, M.A.; Silva, L.M.; Woon, F.L. Does mild cognitive impairment always lead to dementia? A review. J. Neurol. Sci. 2016, 369, 57–62. [Google Scholar] [CrossRef]
- Pandya, S.Y.; Lacritz, L.H.; Weiner, M.F.; Deschner, M.; Woon, F.L. Predictors of Reversion from Mild Cognitive Impairment to Normal Cognition. Dement. Geriatr. Cogn. Disord. 2017, 43, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Doi, T.; Lee, S.; Makizako, H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimers Res. Ther. 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Lee, S.; Doi, T.; Lee, S.; Tsutsumimoto, K.; Harada, K.; Hotta, R.; Bae, S.; Nakakubo, S.; et al. Impact of Cognitive Frailty on Daily Activities in Older Persons. J. Nutr. Health Aging 2016, 20, 729–735. [Google Scholar] [CrossRef]
- Ngandu, T.; von Strauss, E.; Helkala, E.L.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Education and dementia: What lies behind the association? Neurology 2007, 69, 1442–1450. [Google Scholar] [CrossRef]
- Schafer, J.; Strimmer, K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat. Appl. Genet. Mol. Biol. 2005, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Park, H.; Doi, T.; Lee, S. Validity of the National Center for Geriatrics and Gerontology-Functional Assessment Tool and Mini-Mental State Examination for detecting the incidence of dementia in older Japanese adults. Geriatr. Gerontol. Int. 2017, 17, 2383–2388. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour, and Welfare of Japan. Annual Health, Labour, and Welfare Report 2011–2012. Available online: https://www.mhlw.go.jp/english/wp/wp-hw6/dl/02e.pdf (accessed on 26 March 2020).
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Montesi, F.; Lucicesare, A.; Pisacane, N.; Rietti, E.; Dalmonte, E.; Bianchin, M.; Mecocci, P. Mild cognitive impairment: Epidemiology and dementia risk in an elderly Italian population. J. Am. Geriatr. Soc. 2008, 56, 51–58. [Google Scholar] [CrossRef]
- Dotson, V.M.; Beydoun, M.A.; Zonderman, A.B. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Kuiper, J.S.; Zuidersma, M.; Oude Voshaar, R.C.; Zuidema, S.U.; van den Heuvel, E.R.; Stolk, R.P.; Smidt, N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2015, 22, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.C.; Yiu, K.K.L.; Kwok, T.C.Y.; Wong, S.Y.S.; Tsoi, K.K.F. Depression and Antidepressants as Potential Risk Factors in Dementia: A Systematic Review and Meta-analysis of 18 Longitudinal Studies. J. Am. Med. Dir. Assoc. 2019, 20, 279–286.e1. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.E.M.; Martyr, A.; Collins, R.; Brayne, C.; Clare, L. Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis. J. Alzheimers Dis 2019, 70, S119–S144. [Google Scholar] [CrossRef] [Green Version]
- Min, D.; Cho, E. Associations among health behaviors, body mass index, hypertension, and diabetes mellitus: A path analysis. Medicine 2018, 97, e10981. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Mendes De Leon, C.F.; Barnes, L.L.; Schneider, J.A.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002, 287, 742–748. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Gustafson, D.R. Adiposity and Alzheimer’s disease. Curr. Opin. Clin. Nutr. Metab Care 2009, 12, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Swan, G.E.; Lessov-Schlaggar, C.N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Sachdev, P. Brain reserve and dementia: A systematic review. Psychol. Med. 2006, 36, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health Labour, and Welfare of Japan. National Health and Nutrition Survey. Available online: https://www.mhlw.go.jp/english/ (accessed on 25 March 2020).
- Cataldo, J.K.; Prochaska, J.J.; Glantz, S.A. Cigarette smoking is a risk factor for Alzheimer’s Disease: An analysis controlling for tobacco industry affiliation. J. Alzheimers Dis. 2010, 19, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Coley, N.; Ngandu, T.; Lehtisalo, J.; Soininen, H.; Vellas, B.; Richard, E.; Kivipelto, M.; Andrieu, S.; Hatice, F.; MAPT/DSA groups. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimers Dement. 2019, 15, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Zhang, L.; Wang, X.; Hao, G.; Zhang, Z.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C.; et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015. Circulation 2018, 137, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, L.A.; Sullivan, J.C. Sex Differences in Hypertension: Where We Have Been and Where We Are Going. Am. J. Hypertens 2018, 31, 1247–1254. [Google Scholar] [CrossRef]
- Song, J.J.; Ma, Z.; Wang, J.; Chen, L.X.; Zhong, J.C. Gender Differences in Hypertension. J. Cardiovasc. Transl. Res. 2020, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
| Reverters (n = 244) | Nonreverters (n = 545) | p Value | |
|---|---|---|---|
| Age (years) * | 69.5 (3.4) | 73.1 (5.7) | <0.01 a |
| Sex (% male) | 41.8 | 48.8 | >0.05 b |
| Education (years) * | 11.8 (2.4) | 11.0 (2.4) | <0.01 a |
| Heart disease (% yes) | 16.4 | 17.4 | >0.05 b |
| Pulmonary disease (% yes) | 7.4 | 10.1 | >0.05 b |
| Hypertension (% yes) | 8.2 | 10.4 | >0.05 b |
| Walking speed (m/s) * | 1.3 (0.2) | 1.2 (0.2) | >0.05 a |
| Mini-mental state examination (points) * | 26.9(1.9) | 26.4(1.8) | >0.05 a |
| Geriatric depression scale (points) * | 2.6 (2.3) | 3.3 (2.8) | <0.01 a |
| Category of MCI (%) | <0.01 b | ||
| amnestic MCI single domain | 16.4 | 13.2 | |
| non-amnestic MCI single domain | 72.1 c | 57.4 d | |
| amnestic MCI multiple domain | 4.5 d | 8.8 c | |
| non-amnestic MCI multiple domain | 7.0 d | 20.6 c | |
| Modifiable risk factors (% yes) | |||
| Less education (10 years ≥) | 24.2 | 40.2 | <0.01 b |
| Hearing loss | 3.0 | 4.3 | >0.05 b |
| Hypertension | 41.4 | 50.3 | <0.05 b |
| Obesity (BMI 25≤) | 25.5 | 29.0 | >0.05 b |
| Smoking | 34.4 | 42.6 | <0.05 b |
| Depression (GDS 5≤) | 16.8 | 26.3 | <0.01 b |
| Physical inactivity | 22.5 | 34.3 | <0.01 b |
| Social isolation | 27.5 | 40.7 | <0.01 b |
| Diabetes | 12.3 | 16.0 | >0.05 b |
| Classes (N) | Parameters (N) | Likelihood | BIC | AIC | AIC3 |
|---|---|---|---|---|---|
| 3 | 29 | −3865.8 | 7925.0 | 7789.5 | 7818.5 |
| 4 | 39 | −3852.3 | 7964.8 | 7782.6 | 7821.6 |
| 5 | 49 | −3839.6 | 8006.0 | 7777.2 | 7826.2 |
| 6 | 59 | −3832.5 | 8058.6 | 7783.0 | 7842.0 |
| Class 1 (Psychosocial Factors) | Class 2 (Health Behavior Factors) | Class 3 (Low Risk Factors) | Class 4 (Educational Factor) | Class 5 (Smoking Factor) | |
|---|---|---|---|---|---|
| Class prevalence (%) | 25.9 | 23.9 | 20.3 | 18.5 | 11.4 |
| Less education (10 years ≥) | 0.4547 | 0.1676 | 0.0331 | 0.8196 | 0.3203 |
| Hearing loss | 0.0501 | 0.0485 | 0.0255 | 0.0322 | 0.0255 |
| Hypertension | 0.4638 | 0.8573 | 0.2521 | 0.5248 | 0.0155 |
| Obesity (BMI 25≤) | 0.2782 | 0.5323 | 0.0768 | 0.2764 | 0.1026 |
| Smoking | 0.4258 | 0.5957 | 0.1713 | 0.0135 | 0.9713 |
| Depression (GDS 5≤) | 0.7428 | 0.0741 | 0.0823 | 0.0298 | 0.0097 |
| Physical inactivity | 0.4749 | 0.2936 | 0.0695 | 0.2915 | 0.4015 |
| Social isolation | 0.7365 | 0.2378 | 0.167 | 0.3016 | 0.2667 |
| Diabetes | 0.1635 | 0.2361 | 0.0759 | 0.0983 | 0.1434 |
| Psychosocial Factors n = 186 (23.6%) | Health Behavior Factors n = 179 (22.7%) | Low Risk Factors n = 159 (20.2%) | Educational Factors n = 146 (18.5%) | Smoking Factorsn n = 119 (15.1%) | p Value | Post Hoc | |
|---|---|---|---|---|---|---|---|
| Age (years) * | 73.3 (5.9) | 71.1 (4.4) | 70.35 (4.2) | 73.7 (5.9) | 71.4 (5.5) | <0.05 a | 1,4>3 1,4>2 1>5 4>5 |
| Sex (% male) | 45.2 | 68.2 c | 21.4 d | 13.0 d | 91.6 c | <0.01 b | |
| Education (years) * | 10.6 (2.2) | 12.5 (2.2) | 12.7 (1.9) | 8.6 (0.9) | 11.7 (2.3) | <0.05 a | 3>1,4,5 2>1,4,5 1>4 5>1 5>4 |
| Heart disease (% yes) | 19.9 | 21.2 | 13.8 | 16.4 | 11.8 | >0.05 b | |
| Pulmonary disease (% yes) | 8.1 | 10.6 | 7.5 | 8.9 | 11.8 | >0.05 b | |
| Hypertension (% yes) | 48.9 | 92.2 c | 26.4 d | 52.7 | 0.0 d | <0.01 b | |
| Walking speed (m/s) * | 1.2 (0.2) | 1.2 (0.2) | 1.4 (0.2) | 1.2 (0.2) | 1.3 (0.2) | <0.05 a | 3>1,2,4,5 2>1 5>1 5>4 |
| Mini-mental state examination (points) * | 26.4 (1.8) | 26.7 (1.8) | 27.1 (1.9) | 26.1 (1.7) | 26.4 (1.8) | <0.05 a | 3>1,4,5 2>4 |
| Geriatric depression scale (points) * | 6.7 (2.4) | 2.0 (1.5) | 1.8 (1.6) | 2.3 (1.3) | 1.8 (1.5) | <0.05 a | 1>2,3,4,5 |
| Category of MCI | >0.05 b | ||||||
| amnestic MCI single domain | 11.3 | 19.6 | 13.8 | 8.2 | 18.5 | ||
| non-amnestic MCI single domain | 59.7 | 57.5 | 68.6 | 64.4 | 60.5 | ||
| amnestic MCI multiple domain | 10.8 | 5.6 | 4.4 | 8.9 | 7.6 | ||
| non-amnestic MCI multiple domain | 18.3 | 17.3 | 13.2 | 18.5 | 13.4 | ||
| Modifiable risk factors (% yes) | |||||||
| Less education (10 years ≥) | 44.6 c | 8.9 d | 0.0 d | 100.0 c | 27.7 | <0.01 b | |
| Hearing loss | 4.3 | 5.0 | 3.1 | 3.4 | 2.5 | >0.05 b | |
| Hypertension | 48.9 | 92.2 c | 26.4 d | 52.7 | 0.0 d | <0.01 b | |
| Obesity (BMI 25≤) | 26.9 | 58.1 c | 7.5 d | 30.1 | 7.6 d | <0.01 b | |
| Smoking | 43.0 | 65.4 c | 0.0 d | 0.0 d | 100.0 c | <0.01 b | |
| Depression (GDS 5≤) | 91.4 c | 3.4 d | 5.0 d | 0.0 d | 0.0 d | <0.01 b | |
| Physical inactivity | 48.4 c | 5.0 d | 30.1 | 37.8 | <0.01 b | ||
| Social isolation | 73.1 c | 18.9 d | 33.6 | 27.7 d | <0.01 b | ||
| Diabetes | 15.6 | 5.7 d | 11.6 | 13.4 | <0.01 b |
| Unadjusted Models | Adjusted Models | |||||
|---|---|---|---|---|---|---|
| Nonreverters (n = 545) | Reverters (n = 244) | Nonreverters (n = 545) | Reverters (n = 244) | |||
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Age (years) | REF | 0.86 (0.83–0.90) | <0.01 | |||
| Sex (male) | REF | 0.93 (0.63–1.39) | >0.05 | |||
| Education history (years) | REF | 1.03 (0.95–1.13) | >0.05 | |||
| Heart disease (yes) | REF | 1.02 (0.65–1.60) | >0.05 | |||
| Pulmonary disease (yes) | REF | 0.84 (0.46–1.52) | >0.05 | |||
| MMSE (points) | REF | 1.08 (0.99–1.19) | >0.05 | |||
| GDS (points) | REF | 0.94 (0.85–1.04) | >0.05 | |||
| Class | ||||||
| Low risk factors | REF | 1 | REF | 1 | ||
| Psychosocial factors | REF | 0.25 (0.15–0.39) | <0.01 | REF | 0.46 (0.23–0.92) | <0.05 |
| Health behavior factors | REF | 0.39 (0.25–0.60) | <0.01 | REF | 0.42 (0.26–0.70) | <0.01 |
| Educational factors | REF | 0.29 (0.18–0.48) | <0.01 | REF | 0.51 (0.27–0.94) | <0.05 |
| Smoking factors | REF | 0.30 (0.18–0.51) | <0.01 | REF | 0.34 (0.18–0.63) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, O.; Lee, S.; Bae, S.; Makino, K.; Shinkai, Y.; Chiba, I.; Harada, K.; Shimada, H. Modifiable Risk Factor Possession Patterns of Dementia in Elderly with MCI: A 4-Year Repeated Measures Study. J. Clin. Med. 2020, 9, 1076. https://doi.org/10.3390/jcm9041076
Katayama O, Lee S, Bae S, Makino K, Shinkai Y, Chiba I, Harada K, Shimada H. Modifiable Risk Factor Possession Patterns of Dementia in Elderly with MCI: A 4-Year Repeated Measures Study. Journal of Clinical Medicine. 2020; 9(4):1076. https://doi.org/10.3390/jcm9041076
Chicago/Turabian StyleKatayama, Osamu, Sangyoon Lee, Seongryu Bae, Keitaro Makino, Yohei Shinkai, Ippei Chiba, Kenji Harada, and Hiroyuki Shimada. 2020. "Modifiable Risk Factor Possession Patterns of Dementia in Elderly with MCI: A 4-Year Repeated Measures Study" Journal of Clinical Medicine 9, no. 4: 1076. https://doi.org/10.3390/jcm9041076





