Long-Term Redistribution of Peripheral Lymphocyte Subpopulations after Switching from Calcineurin to mTOR Inhibitors in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Population
2.2. Determination of HLA Antibodies
2.3. Immunophenotyping Analysis
2.4. Statistical Analysis
2.5. Data Availability
3. Results
3.1. Study Population and Clinical Follow-Up
3.2. Conversion from Tacrolimus to mTOR Inhibitor was not Associated with a Significant Development of de novo Donor Specific Antibodies
3.3. Peripheral Blood T Cell Numbers were not Affected by the mTOR Inhibitor Conversion
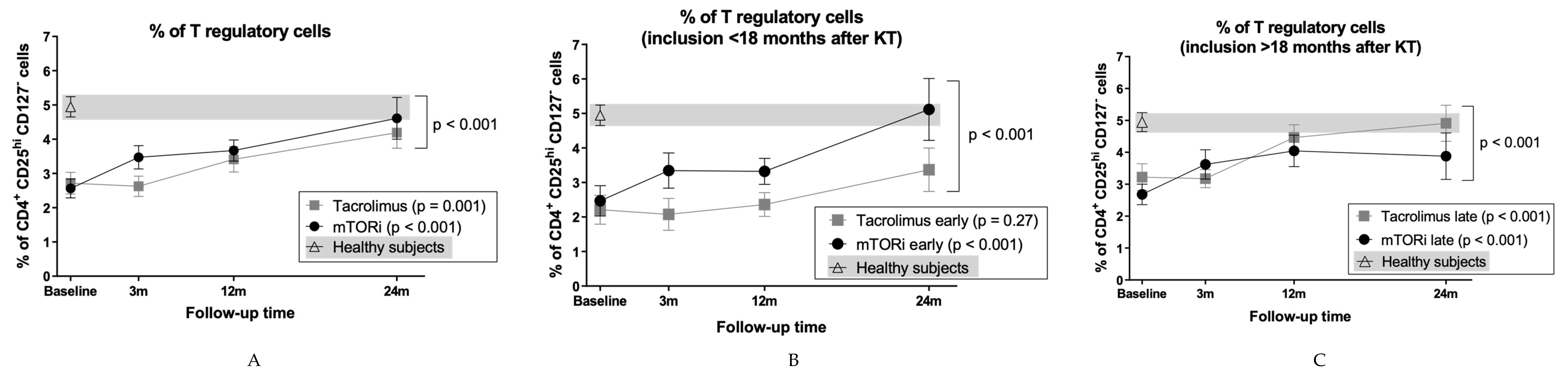
3.4. mTOR Inhibitors and Time Posttransplantation Promote Expansion of T Regulatory Cells
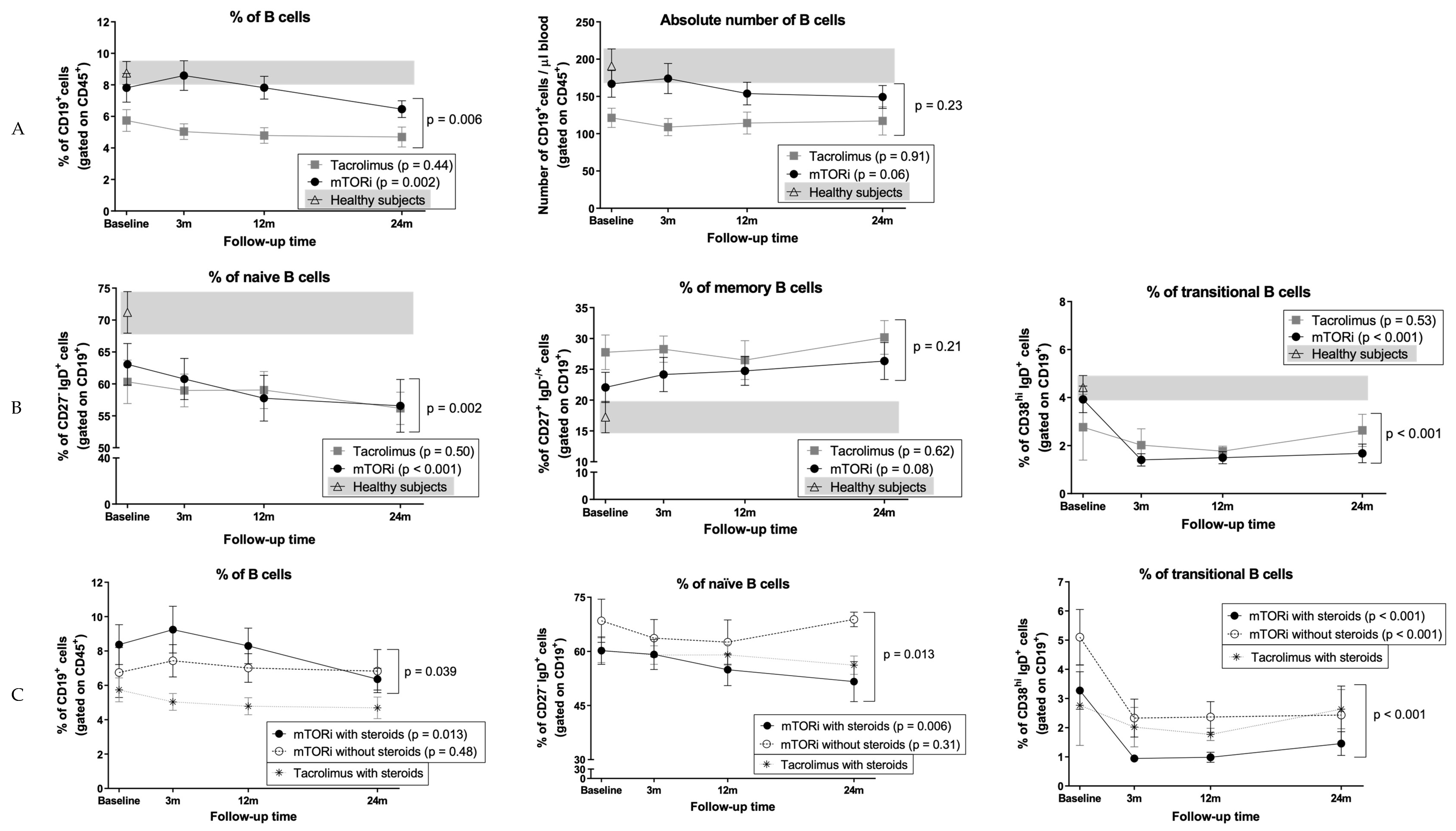
3.5. B Cells and Naïve B Cells Decrease After Conversion to mTOR Inhibitors
3.6. Conversion to mTOR Inhibitors Promotes a Decrease in Circulating Transitional B Cells
3.7. Impact of mTOR Inhibitors on B Cells in Patients who did not Receive Steroids
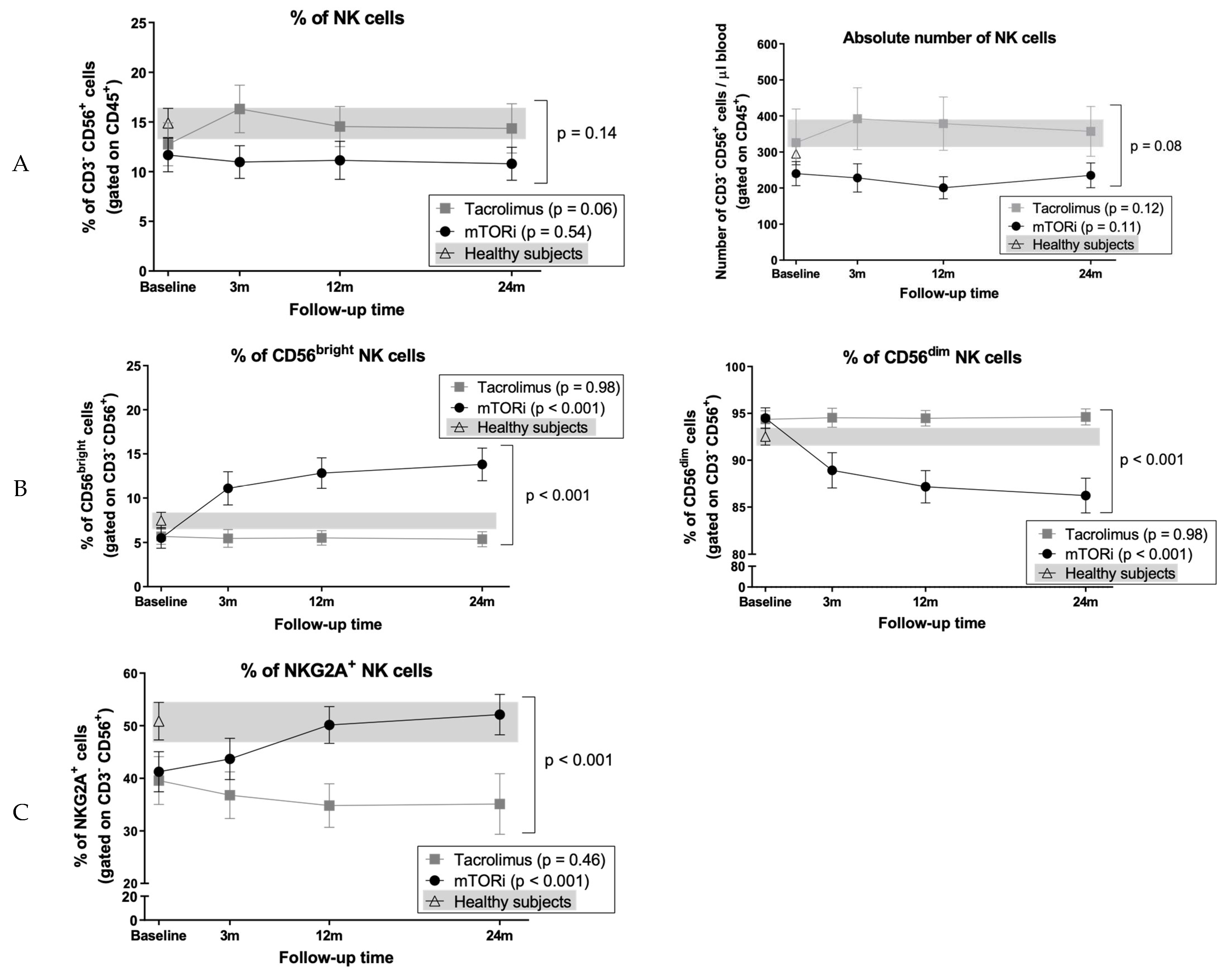
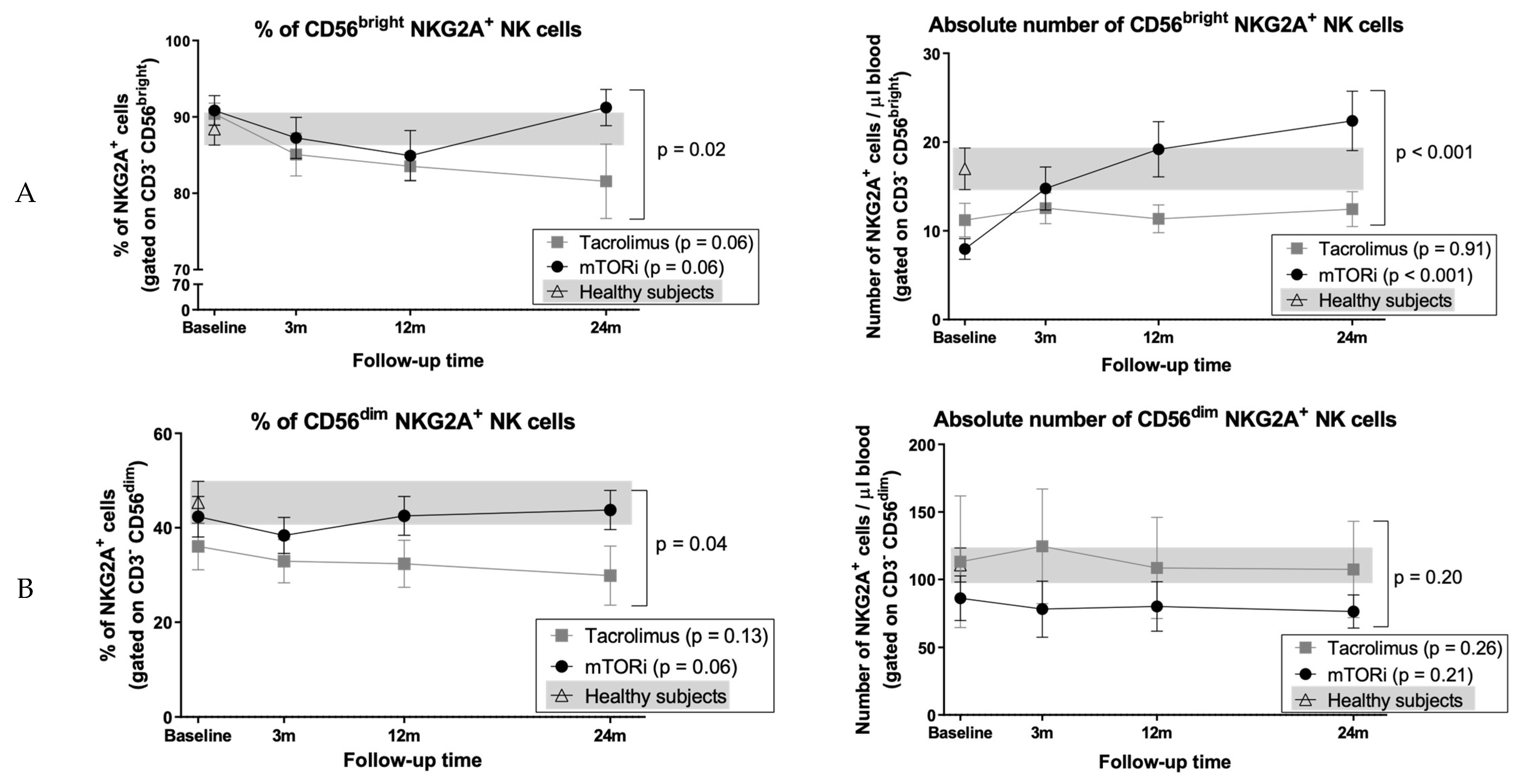
3.8. Conversion to mTOR Inhibitor Induces an Increase in the Proportion of CD56bright Cells and NKG2A+ NK Cell Subsets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Akinlolu, O.O.; Ettenger, R.E. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloveras, J.; Arcos, E.; Comas, J.; Crespo, M.; Pascual, J. A Paired Survival Analysis Comparing Hemodialysis and Kidney Transplantation from Deceased Elderly Donors Older than 65 Years. Transplantation 2015, 99, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Hanson, J.A.; Meier-Kriesche, H.; Okechukwu, C.N.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Kaplan, B.; Port, F.K. Survival in Recipients of Marginal Cadaveric Donor Kidneys Compared with Other Recipients and Wait-Listed Transplant Candidates. J. Am. Soc. Nephrol. 2001, 12, 589–597. [Google Scholar] [PubMed]
- Nieto, T.; Inston, N.; Cockwell, P. Renal Transplantation in Adults. Br. Med. J. 2016, 355, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.E.; Lodhi, S.; Meier-Kriesche, H.-U. Long-Term Renal Allograft Survival in the United States: A Critical Reappraisal. Am. J. Transplant. 2011, 11, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Gondos, A.; Dohler, B.; Brenner, H.; Opelz, G. Kidney Graft Survival in Europe and the United States: Strikingly Different Long-Term Outcomes. Transplantation 2013, 95, 267–274. [Google Scholar] [CrossRef]
- Coemans, M.; Süsal, C.; Döhler, B.; Anglicheau, D.; Giral, M.; Bestard, O.; Legendre, C.; Emonds, M.P.; Kuypers, D.; Molenberghs, G.; et al. Analyses of the Short- and Long-Term Graft Survival after Kidney Transplantation in Europe between 1986 and 2015. Kidney Int. 2018, 94, 964–973. [Google Scholar] [CrossRef]
- Sellarés, J.; De Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef]
- Pascual, J.; Pérez-Sáez, M.J.; Mir, M.; Crespo, M. Chronic Renal Allograft Injury: Early Detection, Accurate Diagnosis, and Management. Transplant. Rev. 2012, 26, 280–290. [Google Scholar] [CrossRef]
- Arias-Cabrales, C.; Redondo-Pachón, D.; Pérez-Sáez, M.J.; Gimeno, J.; Sánchez-Güerri, I.; Bermejo, S.; Sierra, A.; Burballa, C.; Mir, M.; Crespo, M.; et al. Renal Graft Survival According to Banff 2013 Classification in Indication Biopsies. Nefrología 2016, 36, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Nankivell, B.J.; Kuypers, D.R.J. Diagnosis and Prevention of Chronic Kidney Allograft Loss. Lancet 2011, 378, 1428–1437. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C. Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Gillingham, K.J.; Humar, A.; Kandaswamy, R.; Sutherland, D.E.R.; Payne, W.D.; Dunn, T.B.; Najarian, J.S. 2202 Kidney Transplant Recipients with 10 Years of Graft Function: What Happens Next? Am. J. Transplant. 2008, 8, 2410–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Husseini, A.; Aghil, A.; Ramirez, J.; Sawaya, B.; Rajagopalan, N.; Baz, M.; Mei, X.; Davenport, D.L.; Gedaly, R. Outcome of Kidney Transplant in Primary, Repeat, and Kidney-after-Nonrenal Solid-Organ Transplantation: 15-Year Analysis of Recent UNOS Database. Clin. Transplant. 2017, 31, e13108. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R. Chronic Calcineurin Inhibitor Nephrotoxicity-lest We Forget. Am. J. Transplant. 2011, 11, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Morelon, E.; Rostaing, L.; Goffin, E.; Brocard, A.; Tromme, I.; Broeders, N.; Del Marmol, V.; Chatelet, V.; Dompmartin, A.; et al. Sirolimus and Secondary Skin-Cancer Prevention in Kidney Transplantation. N. Engl. J. Med. 2012, 367, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.H.; Eris, J.; Kanellis, J.; Pussell, B.; Wiid, Z.; Witcombe, D.; Russ, G.R. A Systematic Review of Conversion from Calcineurin Inhibitor to Mammalian Target of Rapamycin Inhibitors for Maintenance Immunosuppression in Kidney Transplant Recipients. Am. J. Transplant. 2014, 14, 2106–2119. [Google Scholar] [CrossRef]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory Functions of MTOR Inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Fantus, D.; Rogers, N.M.; Grahammer, F.; Huber, T.B.; Thomson, A.W. Roles of MTOR Complexes in the Kidney: Implications for Renal Disease and Transplantation. Nat. Rev. Nephrol. 2016, 12, 587–609. [Google Scholar] [CrossRef]
- Kim, K.W.; Chung, B.H.; Kim, B.-M.; Cho, M.-L.; Yang, C.W. The Effect of Mammalian Target of Rapamycin Inhibition on T Helper Type 17 and Regulatory T Cell Differentiation in Vitro and in Vivo in Kidney Transplant Recipients. Immunology 2014, 144, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Levitsky, J.; Miller, J.; Huang, X.; Gallon, L.; Leventhal, J.R.; Mathew, J.M. Immunoregulatory Effects of Everolimus on in Vitro Alloimmune Responses. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- San Segundo, D.; Fernandez-Fresnedo, G.; Gago, M.; Beares, I.; Ruiz-Criado, J.; Gonzalez, M.; Ruiz, J.C.; Gómez-Alamillo, C.; Arias, M.; López-Hoyos, M. Number of Peripheral Blood Regulatory T Cells and Lymphocyte Activation at 3 Months after Conversion to Mtor Inhibitor Therapy. Transplant. Proc. 2010, 42, 2871–2873. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Chen, Z.; Wang, J.; Fang, A.; Li, Y.; Xiang, Y.; Ming, C.; Zhang, W. Immunoregulatory Effects of Sirolimus vs. Tacrolimus Treatment in Kidney Allograft Recipients. Cell. Immunol. 2015, 297, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Casiraghi, F.; Todeschini, M.; Cravedi, P.; Cugini, D.; Monteferrante, G.; Aiello, S.; Cassis, L.; Gotti, E.; Gaspari, F.; et al. Regulatory T Cells and T Cell Depletion: Role of Immunosuppressive Drugs. J. Am. Soc. Nephrol. 2007, 18, 1007–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggenenti, P.; Perico, N.; Gotti, E.; Cravedi, P.; D’Agati, V.; Gagliardini, E.; Abbate, M.; Gaspari, F.; Cattaneo, D.; Noris, M.; et al. Sirolimus Versus Cyclosporine Therapy Increases Circulating Regulatory T Cells, But Does Not Protect Renal Transplant Patients Given Alemtuzumab Induction From Chronic Allograft Injury. Transplantation 2007, 84, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Latorre, I.; Esteve-Sole, A.; Redondo, D.; Giest, S.; Argilaguet, J.; Alvarez, S.; Peligero, C.; Forstmann, I.; Crespo, M.; Pascual, J.; et al. Calcineurin and MTOR Inhibitors Have Opposing Effects on Regulatory T Cells While Reducing Regulatory B Cell Populations in Kidney Transplant Recipients. Transpl. Immunol. 2016, 35, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Libetta, C.; Esposito, P.; Gregorini, M.; Margiotta, E.; Martinelli, C.; Borettaz, I.; Canevari, M.; Rampino, T.; Ticozzelli, E.; Abelli, M.; et al. Sirolimus vs Cyclosporine after Induction with Basiliximab Does Not Promote Regulatory T Cell Expansion in de Novo Kidney Transplantation: Results from a Single-Center Randomized Trial. Transpl. Immunol. 2015, 33, 117–124. [Google Scholar] [CrossRef]
- Stallone, G.; Pontrelli, P.; Infante, B.; Gigante, M.; Netti, G.S.; Ranieri, E.; Grandaliano, G.; Gesualdo, L. Rapamycin Induces ILT3high ILT4high Dendritic Cells Promoting a New Immunoregulatory Pathway. Kidney Int. 2014, 85, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Sabbatini, M.; Ruggiero, G.; Palatucci, A.T.; Rubino, V.; Federico, S.; Giovazzino, A.; Apicella, L.; Santopaolo, M.; Matarese, G.; Galgani, M.; et al. Oscillatory MTOR Inhibition and Treg Increase in Kidney Transplantation. Clin. Exp. Immunol. 2015, 182, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Heidt, S.; Roelen, D.L.; Eijsink, C.; Van Kooten, C.; Claas, F.H.J.; Mulder, A. Effects of Immunosuppressive Drugs on Purified Human B Cells: Evidence Supporting the Use of MMF and Rapamycin. Transplantation 2008, 86, 1292–1300. [Google Scholar] [CrossRef]
- Grimbert, P.; Thaunat, O. MTOR Inhibitors and Risk of Chronic Antibody-Mediated Rejection after Kidney Transplantation: Where Are We Now? Transpl. Int. 2017, 30, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelon, E.; Lefrançois, N.; Besson, C.; Prévautel, J.; Brunet, M.; Touraine, J.L.; Badet, L.; Touraine-Moulin, F.; Thaunat, O.; Malcus, C. Preferential Increase in Memory and Regulatory Subsets during T-Lymphocyte Immune Reconstitution after Thymoglobulin Induction Therapy with Maintenance Sirolimus vs Cyclosporine. Transpl. Immunol. 2010, 23, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Neudoerfl, C.; Mueller, B.J.; Blume, C.; Daemen, K.; Stevanovic-Meyer, M.; Keil, J.; Lehner, F.; Haller, H.; Falk, C.S. The Peripheral NK Cell Repertoire after Kidney Transplantation Is Modulated by Different Immunosuppressive Drugs. Front. Immunol. 2013, 4, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, U.; Neudörfl, C.; Daemen, K.; Keil, J.; Stevanovic- Meyer, M.; Lehner, F.; Haller, H.; Blume, C.; Falk, C.S. NK Cells of Kidney Transplant Recipients Display an Activated Phenotype That Is Influenced by Immunosuppression and Pathological Staging. PLoS ONE 2015, 10, e0132484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redondo-Pachón, D.; Pérez-Sáez, M.J.; Mir, M.; Gimeno, J.; Llinás, L.; García, C.; Hernández, J.J.; Jose, Y.; Pascual, J.; Crespo, M. Impact of Persistent and Cleared Preformed HLA DSA on Kidney Transplant Outcomes. Hum. Immunol. 2018, 79, 424–431. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, J.G.; Samaniego, M.; Barrio, M.C.; Potena, L.; Zeevi, A.; Djamali, A.; Cozzi, E. The Influence of Immunosuppressive Agents on the Risk of de Novo Donor-Specific HLA Antibody Production in Solid Organ Transplant Recipients. Transplantation 2016, 100, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Croze, L.E.; Tetaz, R.; Roustit, M.; Malvezzi, P.; Janbon, B.; Jouve, T.; Pinel, N.; Masson, D.; Quesada, J.L.; Bayle, F.; et al. Conversion to Mammalian Target of Rapamycin Inhibitors Increases Risk of de Novo Donor-Specific Antibodies. Transpl. Int. 2014, 27, 775–783. [Google Scholar] [CrossRef]
- Liefeldt, L.; Brakemeier, S.; Glander, P.; Waiser, J.; Lachmann, N.; Schönemann, C.; Zukunft, B.; Illigens, P.; Schmidt, D.; Wu, K.; et al. Donor-Specific HLA Antibodies in a Cohort Comparing Everolimus with Cyclosporine after Kidney Transplantation. Am. J. Transplant. 2012, 12, 1192–1198. [Google Scholar] [CrossRef]
- de Fijter, J.W.; Holdaas, H.; Øyen, O.; Sanders, J.S.; Sundar, S.; Bemelman, F.J.; Sommerer, C.; Pascual, J.; Avihingsanon, Y.; Pongskul, C.; et al. Early Conversion from Calcineurin Inhibitor- to Everolimus-Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. Am. J. Transplant. 2017, 17, 1853–1867. [Google Scholar] [CrossRef] [Green Version]
- Lebranchu, Y.; Thierry, A.; Thervet, E.; Büchler, M.; Etienne, I.; Westeel, P.F.; De Ligny, B.H.; Moulin, B.; Rérolle, J.P.; Frouget, T.; et al. Efficacy and Safety of Early Cyclosporine Conversion to Sirolimus with Continued MMF-Four-Year Results of the Postconcept Study. Am. J. Transplant. 2011, 11, 1665–1675. [Google Scholar] [CrossRef]
- Kamar, N.; Del Bello, A.; Congy-Jolivet, N.; Guilbeau-Frugier, C.; Cardeau-Desangles, I.; Fort, M.; Esposito, L.; Guitard, J.; Gamé, X.; Rostaing, L. Incidence of Donor-Specific Antibodies in Kidney Transplant Patients Following Conversion to an Everolimus-Based Calcineurin Inhibitor-Free Regimen. Clin. Transplant. 2013, 27, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-Human Leukocyte Antigen and Donor-Specific Antibodies Detected by Luminex Posttransplant Serve as Biomarkers for Chronic Rejection of Renal Allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Llinàs-Mallol, L.; Redondo-Pachón, D.; Pérez-Sáez, M.J.; Raïch-Regué, D.; Mir, M.; Yélamos, J.; López-Botet, M.; Pascual, J.; Crespo, M. Peripheral Blood Lymphocyte Subsets Change after Steroid Withdrawal in Renal Allograft Recipients: A Prospective Study. Sci. Rep. 2019, 9, 7453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, I.; Wei, C.; Lee, F.E.H.; Anolik, J. Phenotypic and Functional Heterogeneity of Human Memory B Cells. Semin. Immunol. 2008, 20, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.; Yelamos, J.; Redondo-Pachón, D.; Muntasell, A.; Perez-Saéz, M.J.; López-Montañés, M.; García, C.; Torio, A.; Mir, M.; Hernández, J.J.; et al. Circulating NK-Cell Subsets in Renal Allograft Recipients with Anti-HLA Donor-Specific Antibodies. Am. J. Transplant. 2015, 15, 806–814. [Google Scholar] [CrossRef]
- Budde, K.; Becker, T.; Arns, W.; Sommerer, C.; Reinke, P.; Eisenberger, U.; Kramer, S.; Fischer, W.; Gschaidmeier, H.; Pietruck, F. Everolimus-Based, Calcineurin-Inhibitor-Free Regimen in Recipients of de-Novo Kidney Transplants: An Open-Label, Randomised, Controlled Trial. Lancet 2011, 377, 837–847. [Google Scholar] [CrossRef]
- Lebranchu, Y.; Thierry, A.; Toupance, O.; Westeel, P.F.; Etienne, I.; Thervet, E.; Moulin, B.; Frouget, T.; Le Meur, Y.; Glotz, D.; et al. Efficacy on Renal Function of Early Conversion from Cyclosporine to Sirolimus 3 Months after Renal Transplantation: Concept Study. Am. J. Transplant. 2009, 9, 1115–1123. [Google Scholar] [CrossRef]
- Murakami, N.; Riella, L.V.; Funakoshi, T. Risk of Metabolic Complications in Kidney Transplantation after Conversion to MTOR Inhibitor: A Systematic Review and Meta-Analysis. Am. J. Transplant. 2014, 14, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- De Sandes-Freitas, T.V.; Felipe, C.R.; Campos, É.F.; De Lima, M.G.; Soares, M.F.; De Franco, M.F.; Aguiar, W.F.; Tedesco-Silva, H.; Medina-Pestana, J.O. Subclinical Lesions and Donor-Specific Antibodies in Kidney Transplant Recipients Receiving Tacrolimus-Based Immunosuppressive Regimen Followed by Early Conversion to Sirolimus. Transplantation 2015, 99, 2372–2381. [Google Scholar] [CrossRef]
- Gatault, P.; Bertrand, D.; Büchler, M.; Colosio, C.; Hurault De Ligny, B.; Weestel, P.F.; Rerolle, J.P.; Thierry, A.; Sayegh, J.; Moulin, B.; et al. Eight-Year Results of the Spiesser Study, a Randomized Trial Comparing de Novo Sirolimus and Cyclosporine in Renal Transplantation. Transpl. Int. 2016, 29, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mjörnstedt, L.; Schwartz Sørensen, S.; Von Zur Mühlen, B.; Jespersen, B.; Hansen, J.M.; Bistrup, C.; Andersson, H.; Gustafsson, B.; Solbu, D.; Holdaas, H. Renal Function Three Years after Early Conversion from a Calcineurin Inhibitor to Everolimus: Results from a Randomized Trial in Kidney Transplantation. Transpl. Int. 2015, 28, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Hertig, A.; Albano, L.; Anglicheau, D.; Durrbach, A.; Vuiblet, V.; Moulin, B.; Merville, P.; Hazzan, M.; Lang, P.; et al. Fibrosis Progression According to Epithelial-Mesenchymal Transition Profile: A Randomized Trial of Everolimus versus CsA. Am. J. Transplant. 2015, 15, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Thaunat, O.; Koenig, A.; Leibler, C.; Grimbert, P. Effect of Immunosuppressive Drugs on Humoral Allosensitization after Kidney Transplant. J. Am. Soc. Nephrol. 2016, 27, 1890–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nova-Lamperti, E.; Fanelli, G.; Becker, P.D.; Chana, P.; Elgueta, R.; Dodd, P.C.; Lord, G.M.; Lombardi, G.; Hernandez-Fuentes, M.P. IL-10-Produced by Human Transitional B-Cells down-Regulates CD86 Expression on B-Cells Leading to Inhibition of CD4(+)T-Cell Responses. Sci. Rep. 2016, 6, 20044. [Google Scholar] [CrossRef] [Green Version]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity, 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherukuri, A.; Salama, A.D.; Carter, C.R.; Landsittel, D.; Arumugakani, G.; Clark, B.; Rothstein, D.M.; Baker, R.J. Reduced Human Transitional B Cell T1/T2 Ratio Is Associated with Subsequent Deterioration in Renal Allograft Function. Kidney Int. 2017, 91, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Igarashi, H.; Medina, K.L.; Yokota, T.; Rossi, M.I.D.; Sakaguchi, N.; Comp, P.C.; Kincade, P.W. Early Lymphoid Progenitors in Mouse and Man Are Highly Sensitive to Glucocorticoids. Int. Immunol. 2005, 17, 501–511. [Google Scholar] [CrossRef]
- Traitanon, O.; Mathew, J.M.; La Monica, G.; Xu, L.; Mas, V.; Gallon, L. Differential Effects of Tacrolimus versus Sirolimus on the Proliferation, Activation and Differentiation of Human B Cells. PLoS ONE 2015, 10, e0129658. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.P.; Valenzuela, N.M.; Ziegler, M.E.; Rozengurt, E.; Reed, E.F. Everolimus Inhibits Anti-HLA I Antibody-Mediated Endothelial Cell Signaling, Migration and Proliferation More Potently than Sirolimus. Am. J. Transplant. 2014, 14, 806–819. [Google Scholar] [CrossRef]
- Wang, F.; Meng, M.; Mo, B.; Yang, Y.; Ji, Y.; Huang, P.; Lai, W.; Pan, X.; You, T.; Luo, H.; et al. Crosstalks between MTORC1 and MTORC2 Variagate Cytokine Signaling to Control NK Maturation and Effector Function. Nat. Commun. 2018, 9, 4874. [Google Scholar] [CrossRef] [PubMed]
- Juelke, K.; Killig, M.; Luetke-Eversloh, M.; Parente, E.; Gruen, J.; Morandi, B.; Ferlazzo, G.; Thiel, A.; Schmitt-Knosalla, I.; Romagnani, C. CD62L Expression Identifies a Unique Subset of Polyfunctional CD56dim NK Cells. Blood 2010, 116, 1299–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E Is a Major Ligand for the Natural Killer Inhibitory Receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braud, V.M.; Allan, D.S.J.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]

| Tacrolimus Group (n = 16) | mTORi Group (n = 29) | p-Value | |
|---|---|---|---|
| Recipient age (years) (mean (SD)) | 52.4 (13.9) | 52.5 (15.9) | 0.990 |
| Recipient sex (female) (n, %) | 3 (19%) | 9 (31%) | 0.491 |
| Race (caucasian) (n, %) | 14 (88%) | 25 (86%) | 1.000 |
| Type of donor (deceased) (n, %) | 15 (94%) | 25 (86%) | 0.641 |
| Donor age (years) (mean (SD)) | 46.9 (15.7) | 43.2 (12.4) | 0.385 |
| HLA mismatch class I (A/B)/class II (DR) (mean (SD)) | 3 (1)/1 (1) | 3 (1)/1 (1) | 0.794/0.922 |
| Sensitizing events before KT (yes) (n, %) | 3 (19%) | 9 (31%) | 0.491 |
| Induction immunosuppression (antilymphocyte antibodies) (n, %) | 0 (0%) | 2 (7%) | 0.531 |
| Delayed graft function (n, %) | 4 (25%) | 5 (17%) | 0.700 |
| Acute rejection pre inclusion (n, %) | 1 (6%) | 0 | 0.356 |
| Anti-HLA DSA/no DSA antibodies prior to the study (n, %) | 0 (0%)/0 (0%) | 2 (6.9%)/1 (3%) | 0.531/1.000 |
| Time after KT (months) (median (p25-p75)) | 17.0 (3.0–48.8) | 15.6 (3.3–50.1) | 0.827 |
| Immunosuppression treatment at inclusion | |||
| CNI (n, %) MPA (n, %) Steroids (n, %) | 16 (100%) 16 (100%) 16 (100%) | 29 (100%) 23 (79%) 19 (66%) | |
| Immunosuppression treatment at the end of study * | |||
| CNI (n, %) mTORi (n, %) MPA (n, %) Steroids (n, %) | 16 (100%) 0 (0%) 16 (100%) 16 (100%) | 6 (21%) 23 (82%) 21 (75%) 25 (89%) | |
| Renal function and proteinuria | |||
| Creatinine at the start of study (mg/dL) (mean (SD)) | 1.4 (0.5) | 1.3 (0.4) | 0.286 |
| eGFR at the start of study (mL/min/1.73 m2) (mean (SD)) | 57 (21) | 59 (14) | 0.763 |
| pCOR < 500 mg/g at the start of study (yes) (n, %) | 16 (100%) | 29 (100%) | NA |
| Creatinine at the end of study (mg/dL) (mean (SD)) | 1.6 (0.8) | 1.3 (0.5) ** | 0.246 |
| eGFR at the end of study (mL/min/1.73 m2) (mean (SD)) | 56 (22) | 61 (16) ** | 0.424 |
| pCOR < 500 mg/g at the end of study (yes) (n, %) | 16 (100%) | 17 (77%) ** | 0.067 |
| Adjusting Variable | β (95% CI) | p-value |
|---|---|---|
| Time Point of the Study | 1.46 (0.57; 2.34) | 0.001 |
| Group of the Study Tacrolimus Group mTORi Group | 0 1.32 (−19.63; 22.28) | 0.901 |
| Interaction Group and Time Point of the Study | 0.10 (−1.04; 1.23) | 0.868 |
| Time After KT | 2.97 (0.15; 5.80) | 0.039 |
| Recipient Sex Male Female | 0 20.01 (0.01; 40.01) | 0.050 |
| Recipient Age | −0.35 (−0.99; 0.29) | 0.281 |
| Delayed Graft Function No Yes | 0 1.62 (−24.13; 27.38) | 0.902 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llinàs-Mallol, L.; Redondo-Pachón, D.; Raïch-Regué, D.; Pérez-Sáez, M.J.; Yélamos, J.; Duran, X.; Faura, A.; López-Botet, M.; Pascual, J.; Crespo, M. Long-Term Redistribution of Peripheral Lymphocyte Subpopulations after Switching from Calcineurin to mTOR Inhibitors in Kidney Transplant Recipients. J. Clin. Med. 2020, 9, 1088. https://doi.org/10.3390/jcm9041088
Llinàs-Mallol L, Redondo-Pachón D, Raïch-Regué D, Pérez-Sáez MJ, Yélamos J, Duran X, Faura A, López-Botet M, Pascual J, Crespo M. Long-Term Redistribution of Peripheral Lymphocyte Subpopulations after Switching from Calcineurin to mTOR Inhibitors in Kidney Transplant Recipients. Journal of Clinical Medicine. 2020; 9(4):1088. https://doi.org/10.3390/jcm9041088
Chicago/Turabian StyleLlinàs-Mallol, Laura, Dolores Redondo-Pachón, Dàlia Raïch-Regué, María José Pérez-Sáez, José Yélamos, Xavier Duran, Anna Faura, Miguel López-Botet, Julio Pascual, and Marta Crespo. 2020. "Long-Term Redistribution of Peripheral Lymphocyte Subpopulations after Switching from Calcineurin to mTOR Inhibitors in Kidney Transplant Recipients" Journal of Clinical Medicine 9, no. 4: 1088. https://doi.org/10.3390/jcm9041088
APA StyleLlinàs-Mallol, L., Redondo-Pachón, D., Raïch-Regué, D., Pérez-Sáez, M. J., Yélamos, J., Duran, X., Faura, A., López-Botet, M., Pascual, J., & Crespo, M. (2020). Long-Term Redistribution of Peripheral Lymphocyte Subpopulations after Switching from Calcineurin to mTOR Inhibitors in Kidney Transplant Recipients. Journal of Clinical Medicine, 9(4), 1088. https://doi.org/10.3390/jcm9041088





