Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lipid Sample Preparation for 1H-NMR and GC-MS Analyses
2.3. 1H-NMR Spectroscopic Analysis
NMR Data Processing
2.4. GC-MS Analysis
2.5. RNA Isolation and Real-Time Analysis of mRNA Levels
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, G.; Li, L.; Zhu, B.; Li, Y. Lipidome in colorectal cancer. Oncotarget 2016, 7, 33429–33439. [Google Scholar] [CrossRef] [PubMed]
- García-Barros, M.; Coant, N.; Truman, J.-P.; Snider, A.J.; Hannun, Y.A. Sphingolipids in colon cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portolés, C.; Fernández, L.P.; De Molina, A.R. Precision nutrition for targeting lipid metabolism in colorectal cancer. Nutrients 2017, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mal, M.; Koh, P.K.; Cheah, P.Y.; Chan, E.C.Y. Metabotyping of human colorectal cancer using two-dimensional gas chromatography mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 483–493. [Google Scholar] [CrossRef]
- Mika, A.; Sledzinski, T.; Stepnowski, P. Current Progress of Lipid Analysis in Metabolic Diseases by Mass Spectrometry Methods. Curr. Med. Chem. 2019, 26, 60–103. [Google Scholar] [CrossRef]
- Lemonnier, L.A.; Dillehay, D.L.; Vespremi, M.J.; Abrams, J.; Brody, E.; Schmelz, E.M. Sphingomyelin in the suppression of colon tumors: Prevention versus intervention. Arch. Biochem. Biophys. 2003, 419, 129–138. [Google Scholar] [CrossRef]
- Miccadei, S.; Masella, R.; Mileo, A.M.; Gessani, S. ω3 polyunsaturated fatty acids as immunomodulators in colorectal cancer: New potential role in adjuvant therapies. Front. Immunol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tsugane, S. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer 2011, 129, 1718–1729. [Google Scholar] [CrossRef]
- Volpato, M.; Hull, M.A. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Rev. 2018, 37, 545–555. [Google Scholar] [CrossRef]
- Morland, S.L.; Martins, K.J.B.; Mazurak, V.C. N-3 Polyunsaturated Fatty Acid Supplementation During Cancer Chemotherapy. J. Nutr. Intermed. Metab. 2016, 5, 107–116. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, K.M.; Kim, S.H.; Kwon, Y.J.; Chun, Y.J.; Choi, H.K. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget 2016, 7, 67111–67128. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ren, X.-L.; Fu, Y.-Q.; Gao, J.-L.; Li, D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: A meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1302–1309. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Li, L.; Wei, J.; Xiong, S.; Zhao, Z. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta 2016, 150, 88–96. [Google Scholar] [CrossRef]
- Xia, S.-H.; Wang, J.; Kang, J.X. Decreased n -6/ n -3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes. Carcinogenesis 2005, 26, 779–784. [Google Scholar] [CrossRef]
- Swierczynski, J.; Hebanowska, A.; Sledzinski, T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J. Gastroenterol. 2014, 20, 2279–2303. [Google Scholar] [CrossRef]
- Notarnicola, M.; Lorusso, D.; Tutino, V.; De Nunzio, V.; De Leonardis, G.; Marangelli, G.; Guerra, V.; Veronese, N.; Caruso, M.G.; Giannelli, G. Differential tissue fatty acids profiling between colorectal cancer patients with and without synchronous metastasis. Int. J. Mol. Sci. 2018, 19, 962. [Google Scholar] [CrossRef]
- Wang, S.; Xie, J.; Li, H.; Yang, K. Differences of polyunsaturated fatty acid in patients with colorectal cancer and healthy people. J. Cancer Res. Ther. 2015, 11, 459. [Google Scholar]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Veronese, N.; Lorusso, D.; Di Masi, M.; Benedetto, M.L.; Notarnicola, M. Elevated aa/epa ratio represents an inflammatory biomarker in tumor tissue of metastatic colorectal cancer patients. Int. J. Mol. Sci. 2019, 20, 2050. [Google Scholar] [CrossRef]
- Coviello, G.; Tutino, V.; Notarnicola, M.; Caruso, M.G. Erythrocyte membrane fatty acids profile in colorectal cancer patients: A preliminary study. Anticancer Res. 2014, 34, 4775–4779. [Google Scholar] [PubMed]
- Hamilton, S.; Rubio, C.; Volgenstein, B.; Sobin, L.; Kudo, S.; Fogt, F.; Winawer, S.J.; Goldgar, D.; Jass, J. Carcinoma of the colon and rectum. In World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Lung; Hamilton, S.R., Aaltonen, L.A., Eds.; IARC Press: Lyon, Franch, 2000; pp. 105–120. [Google Scholar]
- Mirnezami, R.; Jiménez, B.; Li, J.V.; Kinross, J.M.; Veselkov, K.; Goldin, R.D.; Holmes, E.; Nicholson, J.K.; Darzi, A. Rapid diagnosis and staging of colorectal cancer via high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy of intact tissue biopsies. Ann. Surg. 2014, 259, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, T.; Huang, J.; Zhang, L.; Xu, S.; Xiong, B.; Wang, Y.; Tang, H. Tissue metabonomic phenotyping for diagnosis and prognosis of human colorectal cancer. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Loke, M.F.; Chua, E.G.; Gan, H.M.; Thulasi, K.; Wanyiri, J.W.; Thevambiga, I.; Goh, K.L.; Wong, W.F.; Vadivelu, J. Metabolomics and 16S rRNA sequencing of human colorectal cancers and adjacent mucosa. PLoS ONE 2018, 13, e0208584. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, 1–128. [Google Scholar] [CrossRef]
- Mika, A.; Kobiela, J.; Czumaj, A.; Chmielewski, M.; Stepnowski, P.; Sledzinski, T. Hyper-Elongation in Colorectal Cancer Tissue—Cerotic Acid is a Potential Novel Serum Metabolic Marker of Colorectal Malignancies. Cell. Physiol. Biochem. 2017, 41, 722–730. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Ye, X.; Chen, L.; Zhang, L.; Gao, Y.; Kang, J.X.; Cai, C. Characteristics of fatty acid distribution is associated with colorectal cancer prognosis. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 355–360. [Google Scholar] [CrossRef]
- Yang, K.; Li, H.; Dong, J.; Dong, Y.; Wang, C.-Z. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J. Gastroenterol. 2015, 21, 2405. [Google Scholar] [CrossRef]
- Mika, A.; Kobiela, J.; Pakiet, A.; Czumaj, A.; Sokołowska, E.; Makarewicz, W.; Chmielewski, M.; Stepnowski, P.; Marino-Gamazza, A.; Sledzinski, T. Preferential uptake of polyunsaturated fatty acids by colorectal cancer cells. Sci. Rep. 2020, 10, 1954. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, X.; Cheng, C.; Li, N.; Liu, Y.; Cao, Y. The implications of signaling lipids in cancer metastasis. Exp. Mol. Med. 2018, 50, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Zhang, H.; Deng, P.; Chen, J.; Zhou, B.; Hu, J.; Zou, J.; Lu, W.; Xiang, P.; et al. 1H NMR-based metabolic profiling of human rectal cancer tissue. Mol. Cancer 2013, 12, 1. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Li, D.; Deng, P.; Shao, X.; Hu, J.; Liu, C.; Jie, H.; Lin, Y.; Li, Z.; et al. 1 H-NMR-based metabolic profiling of a colorectal cancer CT-26 lung metastasis model in mice. Oncol. Rep. 2017, 38, 3044–3054. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef]
- Jiménez, B.; Mirnezami, R.; Kinross, J.; Cloarec, O.; Keun, H.C.; Holmes, E.; Goldin, R.D.; Ziprin, P.; Darzi, A.; Nicholson, J.K. 1H HR-MAS NMR Spectroscopy of Tumor-Induced Local Metabolic “Field-Effects” Enables Colorectal Cancer Staging and Prognostication. J. Proteome Res. 2013, 12, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Kaczynski, Z.; Stepnowski, P.; Kaczor, M.; Proczko-Stepaniak, M.; Kaska, L.; Sledzinski, T. Potential Application of 1H NMR for Routine Serum Lipidome Analysis -Evaluation of Effects of Bariatric Surgery. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Hinz, S.; Uckermann, O.; Hönscheid, P.; von Schönfels, W.; Burmeister, G.; Hendricks, A.; Ackerman, J.M.; Baretton, G.B.; Hampe, J.; et al. Shotgun lipidomics-based characterization of the landscape of lipid metabolism in colorectal cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.W.; Liu, D.B.; Han, C.Z.; Du, L.L.; Jing, J.X.; Wang, Y. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J. Gastroenterol. 2014, 20, 8646–8652. [Google Scholar] [CrossRef]
- Czumaj, A.; Zabielska, J.; Pakiet, A.; Mika, A.; Rostkowska, O.; Makarewicz, W.; Kobiela, J.; Sledzinski, T.; Stelmanska, E. In vivo effectiveness of orlistat in the suppression of human colorectal cancer cell proliferation. Anticancer Res. 2019, 39, 3815–3822. [Google Scholar] [CrossRef]
- Ran, H.; Zhu, Y.; Deng, R.; Zhang, Q.; Liu, X.; Feng, M.; Zhong, J.; Lin, S.; Tong, X.; Su, Q. Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J. Exp. Clin. Cancer Res. 2018, 37, 54. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Guerrouahen, B.S.; Pasquier, J.; Diboun, I.; Karoly, E.D.; Suhre, K.; Rafii, A. Metabolic signatures differentiate ovarian from colon cancer cell lines. J. Transl. Med. 2015, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Yan Lim, J.; Yee Kwan, H. Roles of Lipids in Cancer. In Lipid Metabolism; IntechOpen: London, UK, 2018. [Google Scholar]
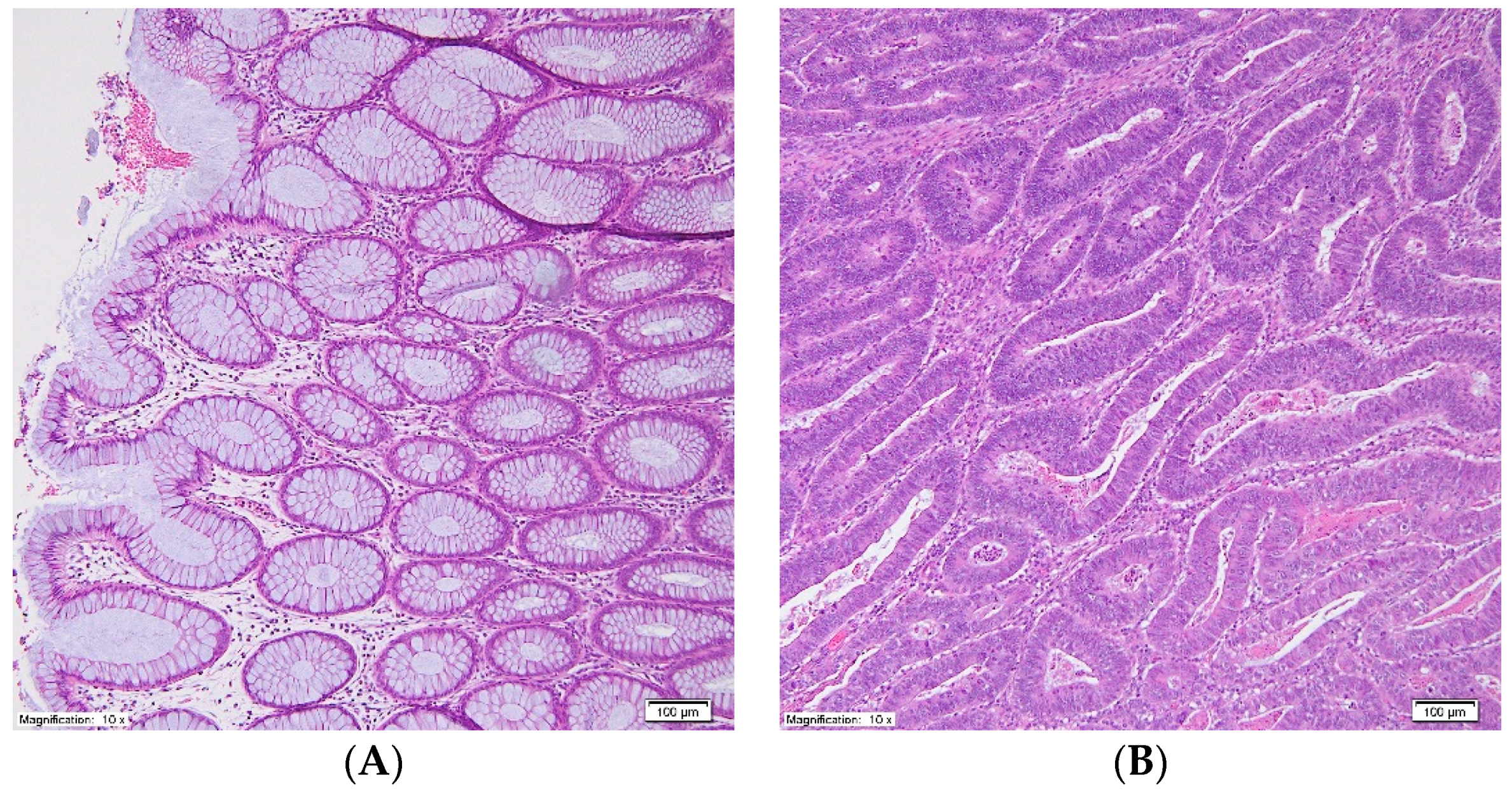
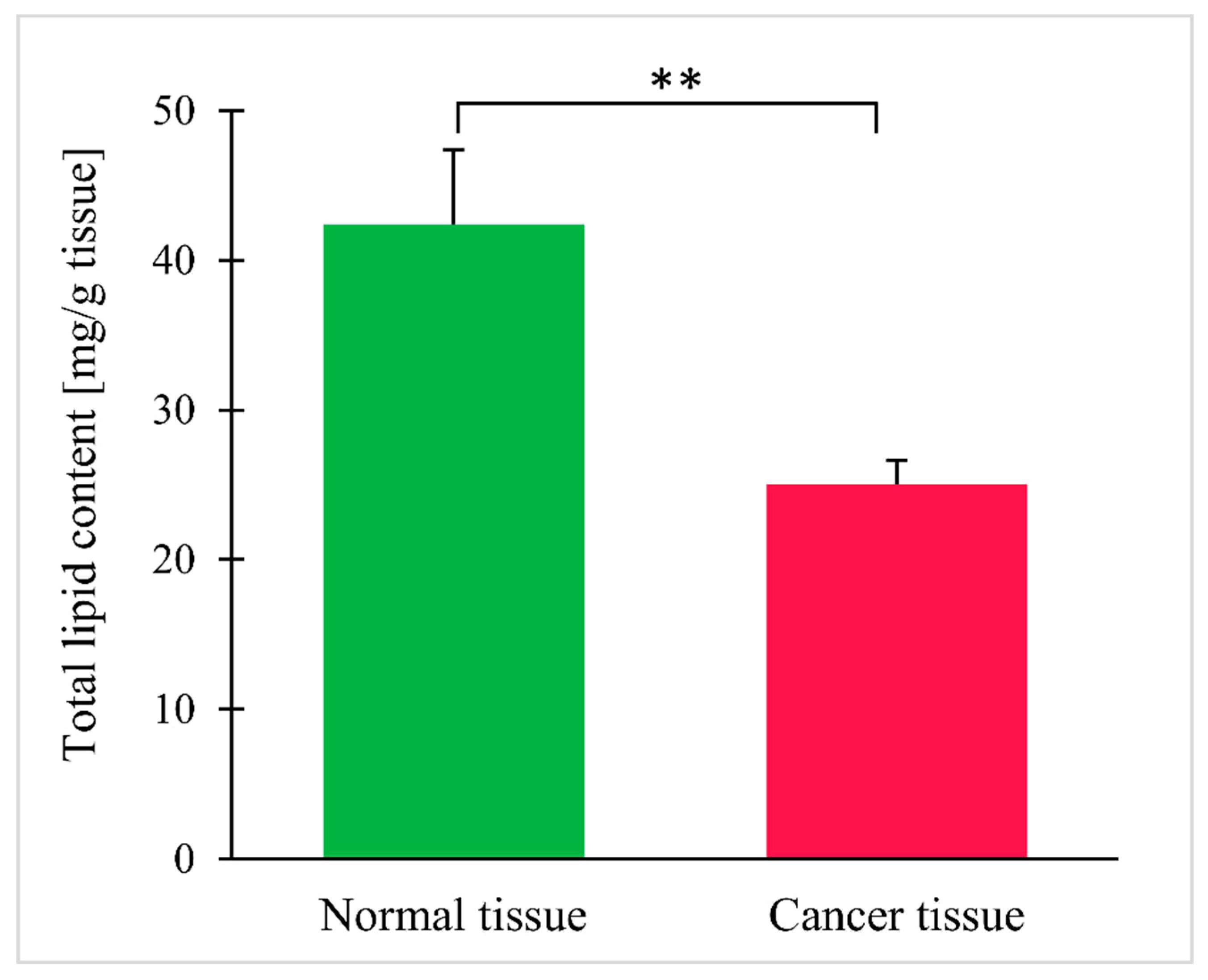



| Parameter | Mean ± SEM |
|---|---|
| Age (years) | 69.8 ± 2.66 |
| BMI (kg/m2) | 28.4 ± 1.09 |
| CRP-hs (mg/L) | 3.53 ± 0.737 |
| Total serum cholesterol (mg/dL) | 155 ± 12.7 |
| Triacylglycerols (mg/dL) | 123 ± 11.2 |
| HDL (mg/dL) | 36.7 ± 2.57 |
| LDL (mg/dL) | 99.2 ± 8.44 |
| Glucose (mg/dL) | 105 ± 8.53 |
| Albumin (g/dL) | 3.11 ± 0.186 |
| Total protein (g/dL) | 6.10 ± 0.331 |
| Stroma content in tumor (%) | 22.9 ± 2.42 |
| Connective tissue cells content in tumor (%) | 15.1 ± 2.22 |
| Inflammatory cells content in tumor (%) | 7.79 ± 1.72 |
| Epithelial cells content in tumor (%) | 59.5 ± 4.51 |
| Tumor-stroma ratio | 5.36 ± 0.577 |
| Parameter | N |
| Sex | |
| Male | 16 |
| Female | 9 |
| Location of primary tumor | |
| Cecum | 9 |
| Ascending colon | 3 |
| Transverse colon | 2 |
| Descending/proximal sigmoid colon | 4 |
| Rectosigmoid | 3 |
| Rectum | 4 |
| T stage | |
| T1 | 2 |
| T2 | 9 |
| T3 | 12 |
| T4 | 2 |
| UICC stage | |
| I | 10 |
| II | 3 |
| III | 9 |
| IV | 3 |
| Lymph node status | |
| N0 | 13 |
| N1/N2 | 12 |
| L.p. | 1H NMR signal | Chemical Shift * (ppm) | Normal Colon Tissue (Signal Intensity) Mean ± SEM | Colon Cancer Tissue (Signal Intensity) Mean ± SEM | p |
|---|---|---|---|---|---|
| 1 | -C18H3 in total cholesterol | 0.70 | 79.6 ± 6.93 | 141 ± 10.9 | <0.001 |
| 2 | -C26H3/-C27H3 in total cholesterol | 0.86 | 199 ± 13.8 | 256 ± 17.8 | 0.004 |
| 3 | -C21H3 in free cholesterol | 0.93 | 167 ± 17.2 | 221 ± 16.1 | 0.011 |
| 4 | -C19H3 in free cholesterol | 1.02 | 96.5 ± 9.25 | 150 ± 14.1 | <0.001 |
| 5 | -C19H3 in esterified cholesterol | 1.04 | 48.0 ± 11.9 | 58.4 ± 5.82 | 0.424 |
| 6 | -CH3 in fatty acyl chain | 0.89 | 1813 ± 71.8 | 1444 ± 101 | <0.001 |
| 7 | -(CH2)n in fatty acyl chain | 1.27 | 7444 ± 321 | 6315 ± 441 | 0.013 |
| 8 | CHCH2CH2(CH2)- in fatty acyl chain | 1.32 | 4810 ± 221 | 3078 ± 236 | <0.001 |
| 9 | -CO-CH2- in fatty acyl chain | 2.32 | 1139 ± 49.3 | 769 ± 57.2 | <0.001 |
| 10 | -CO-CH2CH2- in fatty acyl chain | 1.61 | 1272 ± 62.2 | 892 ± 70.0 | <0.001 |
| 11 | -HC=CH- in fatty acyl chain | 5.36 | 725 ± 42.1 | 551 ± 71.4 | 0.028 |
| 12 | -CH2HC=C in fatty acyl chain: 18:1 | 2.02 | 1013 ± 51.4 | 618 ± 52.9 | <0.001 |
| 13 | -CH2HC= in fatty acyl chain: 18:2n-6/20:4n-6 | 2.08 | 205 ± 17.0 | 163 ± 24.4 | 0.146 |
| 14 | CHCH2CH= in fatty acyl chain: 18:2n-6 | 2.78 | 58.7 ± 7.21 | 40.7 ± 7.90 | 0.106 |
| 15 | -CO-CH2- in fatty acyl chain: 22:6n-3 | 2.42 | 17.3 ± 1.44 | 21.8 ± 2.44 | 0.049 |
| 16 | CHCH2CH= in fatty acyl chain: 20:4n-6/22:6n-3 | 2.84 | 66.8 ± 14.2 | 144 ± 30.7 | 0.022 |
| 17 | -CH2-CH2-NH2 of PE | 3.11 | 17.7 ± 2.41 | 46.0 ± 5.08 | <0.001 |
| 18 | C2H in glycerol backbone of PE | 3.26 | 15.3 ± 1.85 | 27.3 ± 3.94 | 0.007 |
| 19 | -N+(CH3)3 in SM head group | 3.21 | 84.6 ± 7.53 | 141 ± 10.3 | <0.001 |
| 20 | -CH2N+(CH3)3 in SM head group | 3.62 | 92.8 ± 6.76 | 161 ± 13.3 | <0.001 |
| 21 | -CH2CH2N+(CH3)3 in SM head group | 4.25 | 97.0 ± 8.87 | 139 ± 13.7 | 0.005 |
| 22 | -CH2N+(CH3)3 in PC head group | 3.22 | 177 ± 71.8 | 1139 ± 338 | <0.001 |
| 23 | -N+(CH3)3 in PC head group | 3.68 | 255 ± 23.7 | 495 ± 40.6 | <0.001 |
| 24 | >C3H2 in glycerol backbone of PL | 4.01 | 171 ± 18.2 | 309 ± 27.2 | <0.001 |
| 25 | -C2H in glycerol backbone of PL | 5.24 | 38.3 ± 3.28 | 71.1 ± 7.43 | <0.001 |
| 26 | >C1H2/C3H2 in glycerol backbone of TG | 4.33 | 334 ± 20.9 | 137 ± 17.4 | <0.001 |
| 27 | -C2H in glycerol backbone of TG | 5.28 | 134 ± 10.0 | 41.6 ± 6.11 | <0.001 |
| 28 | >C1H2/C3H2 in glycerol backbone of TG and PL | 4.16 | 368 ± 21.0 | 210 ± 18.7 | <0.001 |
| Fatty Acids | Normal | Cancer Tissue | p |
|---|---|---|---|
| 16:0 | 22.0 ± 0.368 | 20.7 ± 0.318 | 0.017 |
| 18:0 | 7.14 ± 0.467 | 12.5 ± 0.567 | <0.001 |
| Total SFA | 32.6 ± 0.657 | 36.8 ± 0.519 a | <0.001 |
| 16:1 | 4.86 ± 0.353 | 3.34 ± 0.201 | <0.001 |
| 18:1 | 45.0 ± 0.953 | 35.6 ± 1.07 | <0.001 |
| Total MUFA | 51.1 ± 1.15 | 40.6 ± 1.21 b | <0.001 |
| 18:2 (LA) | 11.1 ± 0.398 | 11.2 ± 0.399 | 0.682 |
| 20:4 (ARA) | 2.99 ± 0.349 | 6.73 ± 0.500 | <0.001 |
| Total n-6 PUFA | 15.2 ± 0.722 | 20.5 ± 0.719 a | <0.001 |
| 18:3 (ALA) | 0.040 ± 0.004 | 0.050 ± 0.007 | 0.012 |
| 20:5 (EPA) | 0.210 ± 0.025 | 0.450 ± 0.041 | <0.001 |
| 22:6 (DHA) | 0.450 ± 0.044 | 1.02 ± 0.059 | <0.001 |
| Total n-3 PUFA | 1.02 ± 0.082 | 2.11 ± 0.107 a | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, A.; Pakiet, A.; Czumaj, A.; Kaczynski, Z.; Liakh, I.; Kobiela, J.; Perdyan, A.; Adrych, K.; Makarewicz, W.; Sledzinski, T. Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study. J. Clin. Med. 2020, 9, 1095. https://doi.org/10.3390/jcm9041095
Mika A, Pakiet A, Czumaj A, Kaczynski Z, Liakh I, Kobiela J, Perdyan A, Adrych K, Makarewicz W, Sledzinski T. Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study. Journal of Clinical Medicine. 2020; 9(4):1095. https://doi.org/10.3390/jcm9041095
Chicago/Turabian StyleMika, Adriana, Alicja Pakiet, Aleksandra Czumaj, Zbigniew Kaczynski, Ivan Liakh, Jarek Kobiela, Adrian Perdyan, Krystian Adrych, Wojciech Makarewicz, and Tomasz Sledzinski. 2020. "Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study" Journal of Clinical Medicine 9, no. 4: 1095. https://doi.org/10.3390/jcm9041095
APA StyleMika, A., Pakiet, A., Czumaj, A., Kaczynski, Z., Liakh, I., Kobiela, J., Perdyan, A., Adrych, K., Makarewicz, W., & Sledzinski, T. (2020). Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study. Journal of Clinical Medicine, 9(4), 1095. https://doi.org/10.3390/jcm9041095






