Background Frequency Patterns in Standard Electroencephalography as an Early Prognostic Tool in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Management and Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. The Prognostic Value of EEG Findings: Compared with Previous Studies
4.2. The Prognostic Value of Background EEG Frequency
4.3. Other Clinical Prognostic Factors Combined with EEG Findings
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coppler, P.J.; Elmer, J.; Calderon, L.; Sabedra, A.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C.; Dezfulian, C. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation 2015, 89, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Rittenberger, J.C.; Tisherman, S.A.; Holm, M.B.; Guyette, F.X.; Callaway, C.W. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 2011, 82, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M. Part 8: Post–cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J. Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Taccone, F.S.; Baar, I.; De Deyne, C.; Druwe, P.; Legros, B.; Meyfroidt, G.; Ossemann, M.; Gaspard, N. Neuroprognostication after adult cardiac arrest treated with targeted temperature management: Task force for Belgian recommendations. Acta Neurol. Belg. 2017, 117, 3–15. [Google Scholar] [CrossRef]
- Lemiale, V.; Dumas, F.; Mongardon, N.; Giovanetti, O.; Charpentier, J.; Chiche, J.-D.; Carli, P.; Mira, J.-P.; Nolan, J.; Cariou, A. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013, 39, 1972–1980. [Google Scholar] [CrossRef]
- Elmer, J.; Torres, C.; Aufderheide, T.P.; Austin, M.A.; Callaway, C.W.; Golan, E.; Herren, H.; Jasti, J.; Kudenchuk, P.J.; Scales, D.C. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016, 102, 127–135. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Weissman, A.; Baldwin, M.; Flickinger, K.; Repine, M.J.; Guyette, F.X.; Doshi, A.A.; Dezfulian, C.; Callaway, C.W.; Elmer, J. Preliminary experience with point-of-care EEG in post-cardiac arrest patients. Resuscitation 2019, 135, 98–102. [Google Scholar] [CrossRef]
- Hirsch, L.; LaRoche, S.; Gaspard, N.; Gerard, E.; Svoronos, A.; Herman, S.; Mani, R.; Arif, H.; Jette, N.; Minazad, Y. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J. Clin. Neurophysiol. 2013, 30, 1–27. [Google Scholar] [CrossRef]
- Rossetti, A.O.; Tovar Quiroga, D.F.; Juan, E.; Novy, J.; White, R.D.; Ben-Hamouda, N.; Britton, J.W.; Oddo, M.; Rabinstein, A.A. Electroencephalography predicts poor and good outcomes after cardiac arrest: A two-center study. Crit. Care Med. 2017, 45, e674–e682. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.L.; Taccone, F.S.; Depondt, C.; Lamanna, I.; Gaspard, N.; Ligot, N.; Mavroudakis, N.; Naeije, G.; Vincent, J.-L.; Legros, B. The prognostic value of 48-h continuous EEG during therapeutic hypothermia after cardiac arrest. Neurocrit. Care 2016, 24, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, B.J.; Hofmeijer, J.; Tjepkema-Cloostermans, M.C.; van Putten, M. The prognostic value of discontinuous EEG patterns in postanoxic coma. Clin. Neurophysiol. 2018, 129, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Kim, Y.-J.; Lee, Y.-J.; Ryoo, S.M.; Sohn, C.H.; Seo, D.-W.; Lee, Y.-S.; Lee, J.H.; Lim, K.S.; Kim, W.Y. Serial evaluation of SOFA and APACHE II scores to predict neurologic outcomes of out-of-hospital cardiac arrest survivors with targeted temperature management. PLoS ONE 2018, 13, e0195628. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Kim, Y.J.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Kim, W.Y. Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. J. Clin. Med. 2020, 9, E159. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Callaway, C.W.; Neumar, R.W.; Geocadin, R.G.; Zimmerman, J.L.; Donnino, M.; Gabrielli, A.; Silvers, S.M.; Zaritsky, A.L.; Merchant, R. Part 9: Post–cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010, 122, S768–S786. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, J.M.; Kim, M.S.; Ryoo, S.M.; Park, Y.S.; Kim, S.S.; Kim, Y.O.; Kim, W.Y. Independent Risk Factors for the Shivering Occurrence During Induction Period in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management. Ther. Hypothermia Temp. Manag. 2019, 9, 70–75. [Google Scholar] [CrossRef]
- Westhall, E.; Rossetti, A.O.; van Rootselaar, A.-F.; Kjaer, T.W.; Horn, J.; Ullén, S.; Friberg, H.; Nielsen, N.; Rosén, I.; Åneman, A. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 2016, 86, 1482–1490. [Google Scholar] [CrossRef]
- Backman, S.; Cronberg, T.; Friberg, H.; Ullen, S.; Horn, J.; Kjaergaard, J.; Hassager, C.; Wanscher, M.; Nielsen, N.; Westhall, E. Highly malignant routine EEG predicts poor prognosis after cardiac arrest in the Target Temperature Management trial. Resuscitation 2018, 131, 24–28. [Google Scholar] [CrossRef]
- Tjepkema-Cloostermans, M.C.; Hofmeijer, J.; Trof, R.J.; Blans, M.J.; Beishuizen, A.; van Putten, M.J. Electroencephalogram predicts outcome in patients with postanoxic coma during mild therapeutic hypothermia. Crit. Care Med. 2015, 43, 159–167. [Google Scholar] [CrossRef]
- Sivaraju, A.; Gilmore, E.J.; Wira, C.R.; Stevens, A.; Rampal, N.; Moeller, J.J.; Greer, D.M.; Hirsch, L.J.; Gaspard, N. Prognostication of post-cardiac arrest coma: Early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015, 41, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, I.; Solari, D.; Novy, J.; Oddo, M.; Rossetti, A.O. Standardized EEG interpretation in patients after cardiac arrest: Correlation with other prognostic predictors. Resuscitation 2018, 126, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Torre, J.L.; Lopez-Delgado, A.; Hernandez-Hernandez, M.A.; Paramio-Paz, A.; Pia-Martinez, C.; Orizaola, P.; Martin-Garcia, M. Postanoxic alpha, theta or alpha-theta coma: Clinical setting and neurological outcome. Resuscitation 2018, 124, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Park, K.N.; Shon, Y.-M.; Kim, Y.-M.; Kim, H.J.; Youn, C.S.; Kim, S.H.; Choi, S.P.; Kim, S.C. Continuous amplitude-integrated electroencephalographic monitoring is a useful prognostic tool for hypothermia-treated cardiac arrest patients. Circulation 2015, 132, 1094–1103. [Google Scholar] [CrossRef]
- Duez, C.H.V.; Johnsen, B.; Ebbesen, M.Q.; Kvaloy, M.B.; Grejs, A.M.; Jeppesen, A.N.; Soreide, E.; Nielsen, J.F.; Kirkegaard, H. Post resuscitation prognostication by EEG in 24 vs 48h of targeted temperature management. Resuscitation 2019, 135, 145–152. [Google Scholar] [CrossRef]
- Muhlhofer, W.; Szaflarski, J.P. Prognostic value of EEG in patients after cardiac arrest—An updated review. Curr. Neurol. Neurosci. Rep. 2018, 18, 16. [Google Scholar] [CrossRef]
- Ebell, M.H.; Jang, W.; Shen, Y.; Geocadin, R.G. Development and validation of the Good Outcome Following Attempted Resuscitation (GO-FAR) score to predict neurologically intact survival after in-hospital cardiopulmonary resuscitation. JAMA Intern. Med. 2013, 173, 1872–1878. [Google Scholar] [CrossRef]
- Ruijter, B.J.; van Putten, M.J.A.M.; van den Bergh, W.M.; Tromp, S.C.; Hofmeijer, J. Propofol does not affect the reliability of early EEG for outcome prediction of comatose patients after cardiac arrest. Clin. Neurophysiol. 2019, 130, 1263–1270. [Google Scholar] [CrossRef]
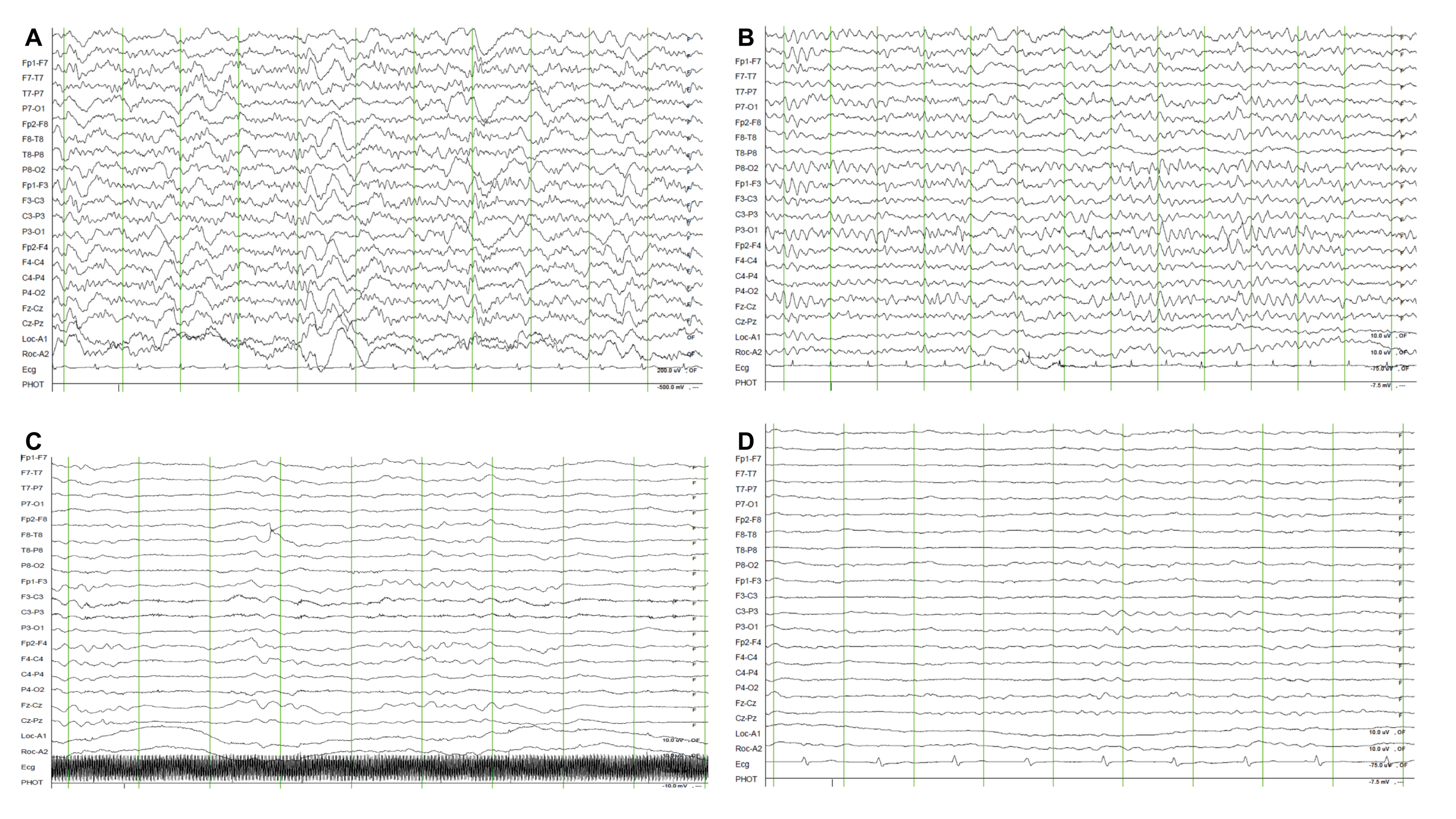
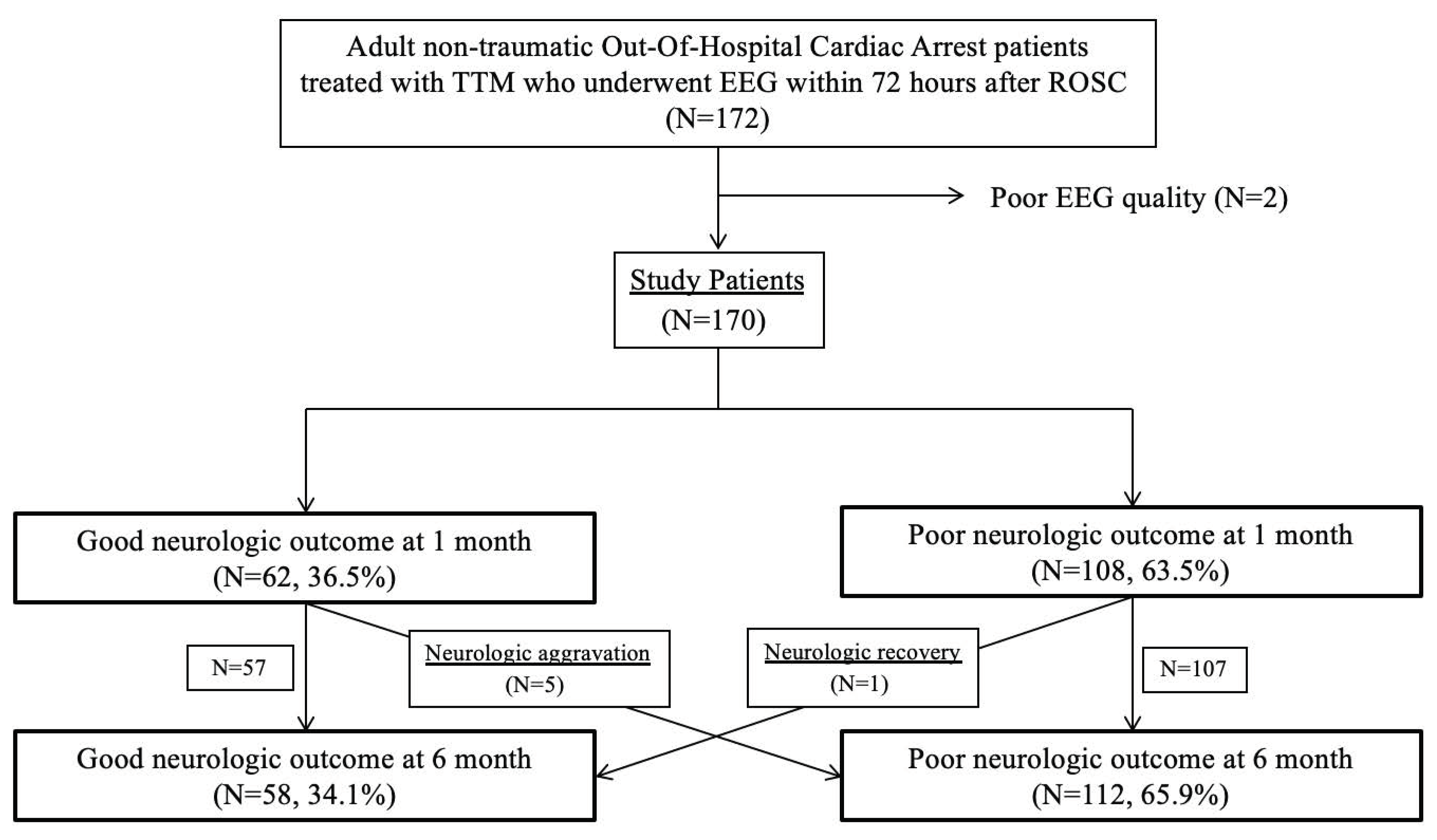
| Characteristics | Total (n = 170) | Favourable Neurologic Outcome (n = 58) | Unfavourable Neurologic Outcome (n = 112) | p-Value |
|---|---|---|---|---|
| Age, years | 60.0 (45.8–71.0) | 54.5 (39.0–64.3) | 62.0 (49.0–73.0) | <0.001 |
| Age < 65 years | 107 (62.9%) | 48 (82.8%) | 59 (52.7%) | <0.001 |
| Male | 113 (66.5%) | 42 (72.4%) | 71 (63.4%) | 0.238 |
| Previous medical history | ||||
| Hypertension | 58 (34.1%) | 14 (24.1%) | 44 (39.3%) | 0.048 |
| Diabetes mellitus | 43 (25.3%) | 8 (13.8%) | 34 (31.5%) | 0.013 |
| Acute myocardial infarction | 8 (4.7%) | 1 (1.7%) | 7 (6.3%) | 0.267 |
| Congestive heart failure | 12 (7.1%) | 4 (6.9%) | 8 (7.1%) | >0.999 |
| Chronic kidney disease | 20 (11.8%) | 3 (5.2%) | 17 (15.2%) | 0.055 |
| Malignancy | 13 (7.6%) | 3 (5.2%) | 10 (8.9%) | 0.382 |
| Arrest characteristics | ||||
| Witnessed | 127 (74.7%) | 49 (84.5%) | 78 (69.6%) | 0.035 |
| Bystander CPR | 120 (70.6%) | 39 (67.2%) | 81 (72.3%) | 0.491 |
| Initial shockable rhythm | 66 (38.8%) | 39 (67.2%) | 27 (24.1%) | <0.001 |
| No flow time, min | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 (0.0–3.0) | 0.778 |
| Resuscitation duration, min | 22.0 (10.0–35.3) | 11.5 (6.0–20.8) | 26.5 (16.0–39.5) | <0.001 |
| Time from ROSC to target temperature, hours | 5.52 (3.93–7.43) | 5.75 (4.88–7.97) | 5.00 (3.76–7.47) | 0.069 |
| Target temperature | 0.679 | |||
| 33 °C | 150 (88.2%) | 52 (89.7%) | 98 (87.5%) | |
| 36 °C | 20 (11.8%) | 6 (10.3%) | 14 (12.5%) | |
| Phases of TTM at EEG examination | 0.730 | |||
| Induction | 15 (8.8%) | 6 (10.3%) | 9 (8.0%) | |
| Maintenance | 92 (54.1%) | 32 (55.2%) | 60 (53.6%) | |
| Rewarming | 20 (11.8%) | 8 (13.8%) | 12 (10.7%) | |
| Normothermia | 43 (25.3%) | 12 (20.7%) | 31 (27.7%) | |
| Time from ROSC to EEG examination, hours | 22.0 (12.8–40.3) | 21.0 (11.0–36.8) | 22.0 (13.3–42.5) | 0.581 |
| Treated sedatives | ||||
| Propofol | 139 (81.8%) | 56 (96.6%) | 83 (74.1%) | <0.001 |
| Max. propofol rate, mg/kg/h | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (1.5–4.0) | 0.166 |
| Remifentanil | 87 (51.2%) | 34 (58.6%) | 53 (47.3%) | 0.162 |
| Max remifentanil rate, µg/kg/h | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.258 |
| Morphine | 54 (31.8%) | 21 (36.2%) | 33 (29.5%) | 0.371 |
| Max morphine rate, mg/h | 2.5 (1.9–3.0) | 3.0 (2.0–3.5) | 2.0 (1.0–3.0) | 0.348 |
| Midazolam | 37 (21.8%) | 14 (24.1%) | 23 (20.5%) | 0.589 |
| Max midazolam rate, mg/kg/h | 0.10 (0.08–0.16) | 0.08 (0.04–0.11) | 0.10 (0.08–0.20) | 0.219 |
| Fentanyl | 2 (1.2%) | 0 (0%) | 2 (1.8%) | 0.548 |
| Treated neuromuscular blocking agent | 35 (20.6%) | 15 (25.9%) | 20 (17.9%) | 0.221 |
| Characteristics | Total (n = 170) | Favourable Neurologic Outcome (n = 58) | Unfavourable Neurologic Outcome (n = 112) | p-Value |
|---|---|---|---|---|
| EEG background frequency | <0.001 | |||
| Dominant alpha waves | 61 (35.9%) | 36 (62.1%) | 25 (22.3%) | |
| Dominant theta waves | 17 (10.0%) | 14 (24.1%) | 3 (2.7%) | |
| Dominant delta waves | 15 (8.8%) | 2 (3.4%) | 13 (11.6%) | |
| Undetermined | 77 (45.3%) | 6 (10.3%) | 71 (63.4%) | |
| EEG background voltage | <0.001 | |||
| Attenuation or Suppressed (<10 μV) | 64 (37.6%) | 4 (6.9%) | 60 (53.6%) | |
| Low voltage (10–20 μV) | 13 (7.6%) | 4 (6.9%) | 9 (8.0%) | |
| Normal (>20 μV) | 93 (54.7%) | 50 (86.2%) | 43 (38.4%) | |
| Other background EEG findings | ||||
| Burst suppression or Burst attenuation | 22 (12.9%) | 2 (3.4%) | 20 (17.9%) | 0.008 |
| Reactivity to pain stimuli | 28 (16.5%) | 16 (27.6%) | 12 (10.7%) | 0.005 |
| Stage II Sleep transients | 9 (5.3%) | 6 (10.3%) | 3 (2.7%) | 0.064 |
| Epileptic form discharge | ||||
| Spike and wave or Sharp and wave | 14 (8.2%) | 4 (6.9%) | 10 (8.9%) | 0.774 |
| Rhythmic delta activity | 42 (24.7%) | 27 (46.6%) | 15 (13.4%) | <0.001 |
| Characteristics | Crude OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 0.950 | 0.921–0.980 | 0.001 | |||
| Female | 0.660 | 0.330–1.318 | 0.239 | |||
| Initial shockable rhythm | 6.462 | 3.213–12.996 | <0.001 | 7.158 | 2.779–20.334 | <0.001 |
| Witnessed | 2.373 | 1.048–5.372 | 0.038 | |||
| Bystander CPR | 0.786 | 0.395–1.562 | 0.491 | |||
| No flow time, min | 1.011 | 0.953–1.072 | 0.725 | |||
| Resuscitation duration, min | 0.959 | 0.938–0.982 | <0.001 | 0.966 | 0.939–0.993 | 0.015 |
| Predominant background EEG frequency | ||||||
| Undetermined | Reference | Reference | ||||
| Dominant alpha waves | 17.040 | 6.414–45.271 | <0.001 | 9.576 | 3.087–29.703 | <0.001 |
| Dominant theta waves | 55.222 | 12.325–247.425 | <0.001 | 31.843 | 5.861–173.017 | <0.001 |
| Dominant delta waves | 1.821 | 0.331–10.026 | 0.491 | 2.333 | 0.347–15.691 | 0.384 |
| Voltage of background EEG frequency | ||||||
| Normal | Reference | |||||
| Attenuation or Suppressed (<10 μV) | 0.057 | 0.019–0.171 | <0.001 | |||
| Low voltage (10–20 μV) | 0.382 | 0.110–1.329 | 0.130 | |||
| Other EEG findings | ||||||
| Burst suppression or Burst attenuation | 0.164 | 0.037–0.730 | 0.018 | |||
| Reactivity to pain stimuli | 3.175 | 1.383–7.286 | 0.006 | |||
| Stage II Sleep transients | 4.192 | 1.009–17.426 | 0.049 | |||
| Epileptic form discharge | ||||||
| Spike and wave or Sharp and wave | 0.756 | 0.226–2.522 | 0.649 | |||
| Rhythmic delta activity | 5.632 | 2.662–11.919 | <0.001 |
| Variables | Favourable Neurologic Outcome/Patient Numbers | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| EEG pattern | 50/78 | 86.2% | 75.0% | 64.1% | 91.3% | 78.8% |
| EEG pattern and/or rhythm | 55/100 | 94.8% | 59.8% | 55.0% | 95.7% | 71.8% |
| EEG pattern + age | 40/53 | 69.0% | 88.4% | 75.5% | 84.6% | 81.8% |
| EEG pattern + rhythm | 34/44 | 58.6% | 91.1% | 77.3% | 81.0% | 80.0% |
| EEG pattern + age + rhythm | 27/31 | 46.6% | 94.4% | 87.1% | 77.7% | 79.4% |
| EEG pattern + age + rhythm + resuscitation duration | 17/17 | 29.3% | 100.0% | 100.0% | 73.2% | 75.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Kim, M.-J.; Koo, Y.S.; Kim, W.Y. Background Frequency Patterns in Standard Electroencephalography as an Early Prognostic Tool in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management. J. Clin. Med. 2020, 9, 1113. https://doi.org/10.3390/jcm9041113
Kim Y-J, Kim M-J, Koo YS, Kim WY. Background Frequency Patterns in Standard Electroencephalography as an Early Prognostic Tool in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management. Journal of Clinical Medicine. 2020; 9(4):1113. https://doi.org/10.3390/jcm9041113
Chicago/Turabian StyleKim, Youn-Jung, Min-Jee Kim, Yong Seo Koo, and Won Young Kim. 2020. "Background Frequency Patterns in Standard Electroencephalography as an Early Prognostic Tool in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management" Journal of Clinical Medicine 9, no. 4: 1113. https://doi.org/10.3390/jcm9041113
APA StyleKim, Y.-J., Kim, M.-J., Koo, Y. S., & Kim, W. Y. (2020). Background Frequency Patterns in Standard Electroencephalography as an Early Prognostic Tool in Out-of-Hospital Cardiac Arrest Survivors Treated with Targeted Temperature Management. Journal of Clinical Medicine, 9(4), 1113. https://doi.org/10.3390/jcm9041113





