Abstract
This study aimed to investigate the association of circulating biomarkers with echocardiographic parameters of atrial remodelling and their potential for predicting atrial fibrillation (AF). In patients with and without AF (n = 21 and n = 60) the following serum biomarkers were determined: soluble ST2 (sST2), Galectin−3 (Gal-3), N-terminal pro-brain natriuretic peptide (NT-proBNP), microRNA (miR)−21, −29a, −133a, −146b and −328. Comprehensive transthoracic echocardiography was performed in all participants. Biomarkers were significantly altered in patients with AF. The echocardiographic parameter septal PA-TDI, indicating left atrial (LA) remodelling, correlated with concentrations of sST2 (r = 0.249, p = 0.048), miR−21 (r = −0.277, p = 0.012), miR−29a (r = −0.269, p = 0.015), miR−146b (r = −0.319, p = 0.004) and miR−328 (r = −0.296, p = 0.008). In particular, NT-proBNP showed a strong correlation with echocardiographic markers of LA remodelling and dysfunction (septal PA-TDI: r = 0.444, p < 0.001, LAVI/a’: r = 0.457, p = 0.001, SRa: r = 0.581, p < 0.001). Multivariate Cox regressions analysis highlighted miR−21 and NT-proBNP as predictive markers for AF (miR−21: hazard ratio (HR) 0.16; 95% confidence interval (CI) 0.04–0.7, p = 0.009; NT-proBNP: HR 1.002 95%CI 1.001–1.004, p = 0.006). Combination of NT-proBNP and miR−21 had the best accuracy to discriminate patients with AF from those without AF (area under the curve (AUC)= 0.843). Our findings indicate that miR−21 and NT-proBNP correlate with echocardiographic parameters of atrial remodeling and predict AF, in particular if combined.
1. Introduction
Atrial fibrillation (AF) is one of the main causes of stroke, particular in the elderly [1]. Stroke represents one of the most common reasons for death, disability and incapacity for work in western countries. Therefore, there is very large need for early detection of AF to prevent cardio-embolic stroke and this has become a research focus recently [2]. Diagnosis of AF is challenging as AF often occurs without any clinical symptoms, leading to a delay of diagnosis and delayed therapeutic anticoagulation in patients at high risk for cardio-embolic events.
Therefore, studies discussed prolongation of electrocardiogram (ECG) monitoring in patients after stroke even beyond 72 h as recommended by the European Society of Cardiology guidelines [3,4,5]. However, extended ECG monitoring is resource consuming and potentially compromises patients [6,7,8,9]. Therefore, it would be of interest to identify predictors indicating patients at high risk for AF which can selectively be assigned to extended ECG-monitoring.
It is well known that left atrial (LA) remodeling, dysfunction and fibrosis contribute to the onset and maintenance of AF [10,11]. As echocardiography is an important imaging tool to evaluate parameters of LA function, remodeling and fibrosis in patients with AF, echocardiography might contribute to early diagnosis of AF [10,12,13,14,15]. Recently, we identified septal total atrial conduction time (PA-TDI interval) as a predictor of AF in patients in sinus rhythm [16]. Furthermore, the ratio of LA volume index to tissue Doppler a′ (LAVI/a′) and the second negative peak strain rate during LA contraction (SRa) assessed by speckle tracking echocardiography, correlate with LA remodeling and fibrosis and are predictors of AF [11,14,16].
N-terminal fragment of pro-B-type natriuretic peptide (NT-proBNP), Galectin−3 (Gal3) and soluble suppression of tumorigenicity 2 (sST2), are associated with myocardial fibrosis [17,18,19] and have a predictive value for an adverse outcome in heart failure patients [20,21]. Gal3 levels are associated with the incident of AF and correlate with atrial fibrosis [22,23], BNP and sST2 are associated with AF reccurence [24].
MicroRNAs (miRs) comprise endogenous small non-coding RNA molecules, which negatively regulate gene/protein expression post-transcriptionally by complementary binding of messenger RNA-targets, thereby stabilizing them or blocking their translation [25]. miRs are emerging as therapeutic targets in cardiovascular diseases [26,27,28]. In addition, since they are stable and readily detectable in the bloodstream, miRs represent promising biomarkers for early detection of cardiac diseases. In previous studies, evidence was provided for miRs to be associated with atrial remodeling, atrial fibrosis and atrial fibrillation [29,30,31].
Hence, the present study investigated whether different biomarkers and miRs associated with LA remodeling and fibrosis correlate with echocardiographic parameters of LA function and remodeling, and could serve as predictive biomarkers for the detection of subclinical AF.
2. Methods
2.1. Study Design and Participants
The present study is a prospective, semi-blinded, single center controlled study, which was approved by the local ethics committee of Hannover Medical School (application number: 3316–2016). The study complies with the Declaration of Helsinki and all participants gave written informed consent. This study is a sub-study of a recent published manuscript describing prediction of AF by echocardiographic parameters in patients after embolic stroke of undetermined source and control cohorts, which gives further detailed information about in-/exclusion criteria and clinical work-flow [16]. Subsequently, included participants were categorized into cohorts and groups: (1) Control cohort: young participants without AF, old participants without AF, and patients with acute ischemic stroke without AF. (2) AF-cohort: patients with paroxysmal AF, patients with stroke and new-onset of AF. All participants underwent 12-lead ECG, and detailed transthoracic echocardiography.
2.2. Echocardiography
Data of transthoracic echocardiography were collected prospectively according to the American Society of Echocardiography guidelines [32]. Echocardiographic parameters of LA function and remodeling were determined as recently described [16]. In brief, dedicated acquisitions from the apical approach were performed for correct assessment of LA volumes according to recent recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) [33]. Left atrial volume was determined by biplane area length method in apical 4– and 2– chamber views at the ventricular end-systole. Subsequently, left atrial volume was indexed to surface area. In tissue Doppler imaging septal and lateral late diastolic peak tissue Doppler velocity (a′) was determined. The assessment of LAVI/a′ was performed with the average of septal and lateral a′. The interval between the onset of p-wave in lead II of the ECG on echocardiographic images and the peak a′-wave of the septal or lateral mitral valve (MV) annulus in tissue Doppler imaging was defined as PA-TDI septal or lateral and is illustrated for PA-TDI septal in Figure 1. Of note, only patients in sinus rhythm during echocardiographic examination were included in this study as parameters for LA remodeling cannot be determined in the presence of AF. Echocardiography was performed by one expert investigator, who was unaware of patients’ diagnosis and results of biomarker and microRNA-testing.
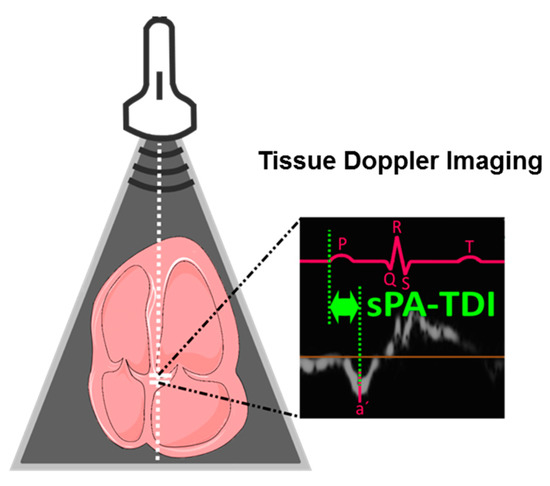
Figure 1.
Determination of septal PA-TDI (sPA-TDI). Septal PA-TDI was determined in tissue Doppler imaging and defined as interval between the onset of P-wave in lead II of the electrocardiogram (ECG) and the peak a′-wave of the septal mitral valve annulus.
2.3. Holter Electrocardiogram (ECG) Monitoring
Management of Holter ECG-Monitoring was previously described [16]. In brief, all participants had a 12-channel surface ECG. Participants without stroke were included in the control group, if there was no history of AF and/or no documented AF in a 24 h-long-term-ECG. In patients after a stroke, a Holter-ECG-monitoring was scheduled for 72 h. All ECG recordings were analyzed by two independent and blinded professionals applying current guidelines on atrial fibrillation [34].
2.4. Biomarker Assays
Plasma and serum samples were collected after echocardiographic examination. RNase/DNase clean aliquots of ethylenediaminetetraacetate (EDTA) plasma and serum were stored at −80 °C. Levels of NT-proBNP were determined in EDTA plasma at the Department of Clinical Chemistry at Hannover Medical School. The measurement of plasma Gal3 (Human Galectin−3 Platinum Elisa, eBiosciences, San Diego, CA, USA) and sST2 (Presage® ST2 Assay, Critical Diagnostics, San Diego, CA, USA) were conducted according to the manufacturer’s instructions.
2.5. RNA Isolation from EDTA Plasma
Purification and isolation of total RNA from 150 µL plasma was performed using miRNeasyMiniKit (QIAGEN, Venlo, Netherlands) according to the manufacturer’s instructions. Caenorhabditis elegans (cel miR−39, 3.5 µL 0.267 pmol/µL) as spiked-in RNA was added before starting the isolation procedure. 10 μL of RNA-solution was obtained for further analysis.
2.6. Real-Time Polymerase Chain Reaction (PCR)-Based Amplification of MicroRNAs
A TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) was used according to the manufacturer’s protocol to transcribe isolated RNA to complementary DNA (cDNA). HSA-miR−21–5p, hsa-miR−29a−3p, hsa-miR−133a−3p, hsa-miR−146b−5p, hsa-miR−328−3p, hsa-miR−486−5p (endogenous control) and cel-miR−39−3p primers (spiked-in control) were used (Primer sequences of miRs are shown in Table 1). Subsequently, the defined miRs were amplified with quantitative real-time polymerase chain reaction (qRT-PCR) utilizing specific TaqMan MicroRNA assays (Applied Biosystems). Plasma levels of the described miRs were normalized using the following miRs: 1. Synthetic Caenorhabditis elegans miR−39 (cel MiR−39) as spiked-in RNA as external control and qPCR normalizer [35,36], 2. Endogenous, stably expressed and abundant in plasma miR−486 [37,38,39] as previously described.

Table 1.
MicroRNA primer sequences.
2.7. Statistical Analysis
Statistical analysis and graphical presentation were performed using SPSS Statistics 25 (IBM SPSS Statistics 25) and GraphPad Prism 7.04 (Graph Pad Software, San Diego, CA, USA). Categorical variables are given as n (%). Continuous variables are presented as mean ± standard deviation (SD) for quantification of normally distributed variables, or median and interquartile ranges (IQR) for non-normally distributed variables. Normality and variance homogeneity were checked by Shapiro–Wilk and D’Agostino Pearson tests. Comparison between the groups was performed using the Student’s t-test for Gaussian distributed data and the Mann–Whitney test in non-normally distributed data. Analysis of variance (ANOVA) was performed followed by a Bonferroni test or Dunn’s test for multiple comparisons, respectively. Categorical variables were evaluated by the Chi-square test. To compare correlation with biomarkers, the Spearman’s rank order correlations was used.
All patients (n = 81) were stratified to two categories in a random manner: training set n = 35 and test set n = 46. The training set was analyzed to build a miR prediction model that was later confirmed in test and entire sets. In the training set, we screened out miRs with a significant p-value of less than 0.1 indicating potential association with AF (s-ST2, Gal3, miR−21, miR−29a, miR−133a, miR−146b, miR−328, NT-proBNP) by using univariate logistic regression (provided in Table S1). Thereafter, this signature was validated in the test set and the entire set. receiver operating characteristic (ROC) analysis was also carried out to validate accuracy and feasibility of the miR model (presented in Table S2). Subsequently predictors of AF were determined using a stepwise multivariate logistic regression analysis with variables, which significantly linked to AF in univariate analysis (p < 0.05; Gal3, miR−21, miR−29a, miR−146b, miR−328, NT-proBNP). Results from the regression analyses are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
The discriminative ability of the risk prediction model was assessed by the area under the ROC curve. Youden’s index was determined to ascertain cut-off values of the variables independently associated with AF. Subsequently, under the assumption of a dichotomous distribution using cut-off values, biomarkers and miRs independently predicting AF were compared by a McNemar test (presented in Table S3). A two-sided p-value of <0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
In a recently published pilot-study with the aim to identify echocardiographic parameters predicting AF in patients with embolic stroke of undetermined source and controls between 1 August 2016 and 30 April 2017 a total of 175 patients were included [16]. In the present sub-study, we investigated 81 patients who approved additional biomarker analysis with the intention to predict AF and correlate results of the biomarker analysis with echocardiographic parameters associated with LA function and remodeling.
Participants were classified according to previously diagnosed AF and/or stroke into the following groups: young participants without stroke and without AF (n = 13), old participants without AF and without stroke (n = 10), patients with acute ischemic stroke without AF (n = 37), patients with paroxysmal AF without stroke (n = 11), and patients with stroke and new-onset of AF (n = 10). Baseline characteristics of study participants and echocardiographic parameters are presented in Table 2. Medication documented at study inclusion is provided in Table S4. Participants in the young control group without AF and stroke were significantly younger. Patients did not receive unfractionated heparin prior to blood sampling. Low molecular heparin was administered for a minimum of 12 h prior to blood sampling. No patient received Vitamin K antagonists and mineralocorticoid receptor antagonists at inclusion to the study. Medication with non-vitamin K antagonist oral anticoagulants, angiotensin-converting enzyme inhibitors, and ß-blockers were significantly frequent in patients with AF. However, in patients with AF hypertension was more frequent in comparison to patients without AF, explaining the wider use of angiotensin-converting enzyme inhibitors and ß-blockers in these patients. No significant difference was observed between patients with and without AF regarding left ventricular ejection fraction. In patients with AF echocardiographic parameters of left atrial function (LAVI/a′, SRa) and septal PA-TDI were significantly altered, compared to participants without AF as provided in Figure 2. Of note, there was no statistical significant difference between patients with and without stroke regarding these parameters.

Table 2.
Patient characteristics and echocardiographic parameters.
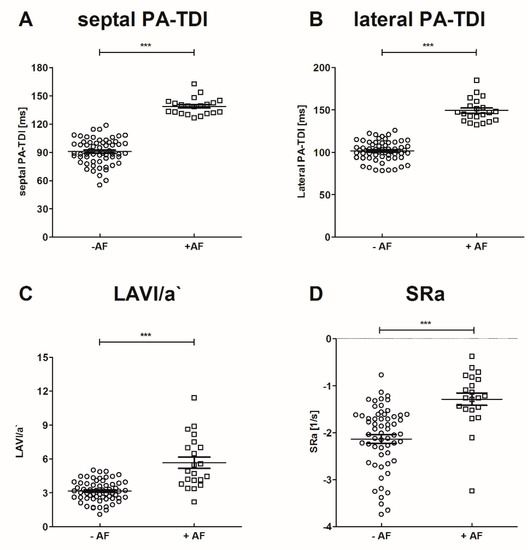
Figure 2.
Echocardiographic parameters of left atrial (LA) function in patients with and without AF. *** p < 0.001.
3.2. Soluble ST−2, Gal3 and NT-proBNP
Results of sST−2, Gal3 and NT-proBNP biomarker analysis are presented in Table 3 and Figure 3: Soluble ST−2, Gal3 and NT-proBNP were significantly increased in patients with AF in comparison to patients without AF. Furthermore, NT-proBNP was significantly increased in patients with AF independent of stroke presence compared to controls. Soluble ST−2 and Gal3 were significantly increased in patients with stroke and AF compared to patients with stroke without AF. Levels of s-ST2 and Gal3 were significantly higher with the presence of stroke (s-ST2 27.70 ± 9.91 vs. 22.33 ± 7.86, p = 0.01; Gal3 5.29 ± 2.14 vs. 3.99 ± 1.32, p = 0.006). The tested biomarkers did not correlate with the levels of C-reactive protein (s-ST2 r = 0.188, p = 0.148; Gal3 r = 0.009, p = 0.945; NT-proBNP r = 0.013, p = 0.917).

Table 3.
Biomarkers.
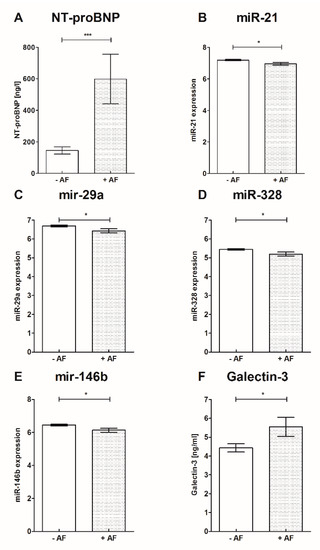
Figure 3.
Biomarkers and miRs were altered in patients with and without AF. A: NT-proBNP, B: miR−21, C: miR−29a, D: miR−328, E: miR−146b, F: Galectin−3 mean ± SD; * p < 0.05, *** p < 0.001; miR- Micro ribonucleic acid, NT-proBNP- N-terminal pro-brain natriuretic peptide.
3.3. MicroRNA-Panel Results
In our analysis the microRNA panel included miRs which are associated with atrial remodeling (miR−29a, miR−146b), electrical remodeling (miR−328), cardiac fibrosis (miR−21, miR−133a, miR−146b) and atrial fibrillation (miR−21, miR−29a).
Comparing patients with AF to patients without AF, miR−21, miR−29a, miR−146b and miR−328 were significantly downregulated in patients with AF, while miR−133a was not significantly altered. None of the miRs correlated with the presence of stroke (miR−21 r = 0.014, p = 0.90; miR−29a r = 0.052, p = 0.65; miR−133a r = 0.084, p = 0.49; miR−146b r = 0.005, p = 0.97; miR−328 r = 0.038, p = 0.74) and the acute-phase protein C-reactive protein (miR−21 r = 0.171, p = 0.17; miR−29a r = 0.072, p = 0.57; miR−133a r = 0.078, p = 0.54; miR−146 r = 0.09, p = 0.47; miR−328 r = 0.08, p = 0.54). MicroRNA-array results are provided in Table 3 and Figure 3.
3.4. Correlation of Biomarkers and Echocardiographic Parameters of Left Atrial (LA) Function and Remodeling
In this study of patients with preserved ejection fraction, miR−21, miR−29a, miR−133a, miR−146b, miR−328, s-ST2, Gal3 and NT-proBNP did not correlate with left ventricular ejection fraction and parameters of diastolic dysfunction assessed by echocardiography. The echocardiographic parameter sPA-TDI, reflecting the septal total atrial conduction time, significantly correlated with serum concentrations of sST2 (r = 0.249, p = 0.048), miR−21 (r = −0.277, p = 0.012), miR−29a (r = −0.296, p = 0.015), miR−146b (r = −0.319, p = 0.004), miR−328 (r = −0.296, p = 0.008) and NT-proBNP (r = 0.426, p =< 0.001).
Furthermore, NT-proBNP correlated highly significantly with additional echocardiographic parameters associated with left atrial remodelling and dysfunction (LAVI/a′ r = 0.457, p = 0.001; SRa (second negative peak strain rate during LA contraction) r = 0.462, p = 0.001). However, miR−133a was the only miR not correlating with echocardiographic parameters of LA function and remodeling (septal PA-TDI r = −0.153, p = 0.172; LAVI/a′ r = 0.07, p = 0.52; SRa r = 0.06, p = 0.61). Correlation between septal PA-TDI and Biomarkers/miR are provided in Figure 4.
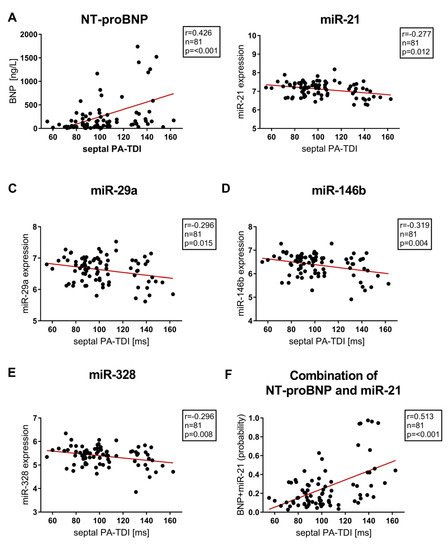
Figure 4.
Correlation between septal PA-TDI, NT-proBNP, single micro RNAs (miR−21, miR−29a, miR−146b, miR−328) and combined miR−21 & NT-proBNP to distinguish between patients with and without AF. A: NT-proBNP, B: miR−21, C: miR−29a, D: miR−146b, E: miR−328, F: Combination of NT-proBNP and miR−21 (probability) miR- Micro ribonucleic acid, NT-proBNP- N-terminal pro-brain natriuretic peptide, septal PA-TDI- septal total atrial conduction time.
3.5. Biomarkers and Prediction of Atrial Fibrillation (AF)
To exclude the influence of statistical outliers and to analyze a prediction model the entire cohort was divided in a random manner into a training and a test set. The prediction model was built in the training model and subsequent confirmed in the test and entire set.
The predictive validity of NT-proBNP and miR−21 was consistent in regression analysis and the ROC analysis of the training and test set. Regression analysis and ROC-analysis of the training and test set are provided in Tables S1 and S2.
Univariate regression analysis of the complete cohort showed Gal3, miR−21, miR−29a, miR−146b, miR−328 and NT-proBNP as predictors of AF. However, multivariable regression analysis highlighted miR−21 (HR 0.16 (95%CI 0.04–0.7), p = 0.009) and NT-proBNP (HR 1.002 (95%CI 1.001–1.004), p = 0.006) as independent predictors with the presence of AF in the complete cohort (as provided in Table 4).

Table 4.
Biomarker predicting AF in complete cohort (∑n = 81, AF n = 21).
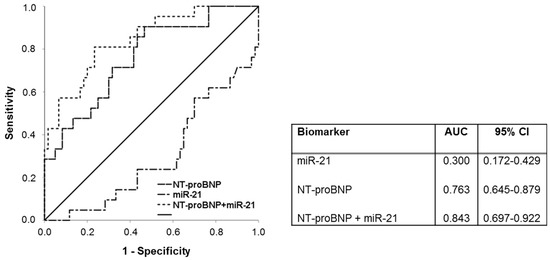
Figure 5.
ROC-curve analysis of NT-proBNP, miR−21 and combined NT-proBNP and miR−21. miR- Micro ribonucleic acid, NT-proBNP- N-terminal pro-brain natriuretic peptide.
The discriminative ability of miR−21, NT-proBNP and the combination of miR−21 and NT-proBNP were verified by the McNemar test. Upon acceptance of a dichotomous distribution, McNemar test revealed a better specificity for combination of NT-proBNP and miR−21 compared to NT-proBNP and miR−21. Furthermore, the combination of NT-proBNP and miR−21 had a comparable sensitivity to NT-proBNP and a better sensitivity in comparison to miR−21. Results from the McNemar test are provided in Table S3. The combination of NT-proBNP and miR−21 correlated strongly with septal PA-TDI (Figure 4F).
4. Discussion
In our current prospective study we analyzed different circulating miRs and biomarkers involved in atrial remodeling and fibrosis to identify specific blood-based biomarkers predicting AF.
Clinically silent AF often is underdiagnosed and screening of patients at risk seems to be a meaningful strategy [34]. Prolonged rhythm monitoring extending even beyond 72 h ECG-monitoring as recommended by current AF ESC guidelines in patients after stroke has been suggested [3,34,40]. However, prolonged cardiac rhythm monitoring for detection of subclinical AF requires significant diagnostic resources and potentially affects patients’ integrity as well as compliance. Therefore, predictors of subclinical AF in patients at risk and patients after stroke would be important as potential screening markers to detect patients with high chances for the presence of subclinical AF, which can be further evaluated with risk-adapted rhythm monitoring.
In the present study, we demonstrated in a cohort with sinus rhythm, preserved ejection fraction and without myocardial infarction that miR−21, miR−29a, miR−146b and miR−328 were significantly decreased in patients with clinical silent AF in comparison to patients without AF. In contrast, s-ST2, Gal−3 and NT-proBNP were significantly increased in patients with diagnosed AF. Multivariable regression analysis in a test and in a training set showed the predictive value of miR−21 and NT-proBNP. Furthermore, in the entire set multivariable regression analysis highlighted miR−21 and NT-proBNP as independent predictors of AF.
In patients with heart failure based on impaired systolic or diastolic ventricular function NT-proBNP, Gal3 and s-ST2 are associated with poor clinical outcome [20,21,41]. In contrast, in patients with AF and without structural heart disease circulating NT-proBNP is increased even in the presence of sinus rhythm [42,43]. However, NT-proBNP is associated with the presence and burden of AF [44]. After ablation of AF, NT-proBNP is a predictor of AF relapse [45]. Gal3 conciliates fibrotic pathways and is associated with detrimental ventricular and atrial remodeling [23,46,47]. In addition, the presence of AF is associated with elevated Gal3 plasma levels [48]. This is in line with our findings showing elevation of sST2, Gal3 and NT-proBNP in patients with AF.
Previous studies depicted miR−21 as a regulator of cardiac fibrosis. Inhibition of miR−21 suppresses fibrosis in mice after transverse aortic constriction and reduces AF duration in rats after initiation of myocardial infarction [26,49]. In patients with AF a decrease of circulating miR−21 was shown [50,51]. Thus, our results validate these findings. In the present study miR−29, miR−146b and miR 328 were significantly downregulated in patients with AF. miR−29 is potentially associated with collagen regulation of the atrium. In the study of Dawson et al., circulating miR−29 levels were also decreased in patients with AF [30]. Adverse electrical remodeling in an experimental model with AF in dogs was associated with levels of miR−328 [52]. Additionally, mal-structural remodeling by fibrosis is probably mediated via TIMP−4 by miR−146b [53]. miR−133 has pro-fibrotic properties mediated by inhibition of connective tissue growth factor [54]. However, in the present study the expression of circulating miR−133 was not significantly altered in patients with AF. Besides, in patients with paroxysmal, symptomatic AF unresponsive to medical treatment effective AF ablation is associated with a favorable reverse atrial remodeling and increased expression of miR-21- a regulator of atrial fibrosis-, miR-150-associated with fibrosis and remodeling-, and miR-409-associated with the progression of AF burden-, representing potential targets to prevent relapse after AF ablation approach [55].
Subsequently, we correlate the tested biomarkers to echocardiographic parameters. LA-remodeling and fibrosis are well established arrhythmic substrates, which correlate to echocardiographic parameters of LA function and remodeling [13]. A ratio of indexed LA volume and mitral annulus velocity during atrial contraction (LAVI/a′) express atrial ejection function and is a predictor of AF [11,14,16].
Additionally, left atrial strain and left atrial strain rate assessed by 2D speckle-tracking echocardiography facilitate evaluation of regional LA-dysfunction and fibrosis [13,56]. The strain rate parameter SRa assessed during the contractile phase of the LA is associated with AF [16,57]. However, besides adding additional diagnostic value, speckle trecking analysis of the LA involves some methodological complexities: Implementation of LA strain is challenging, as the left atrial wall is thin compared with the wall of the LV, making adequate tracking more difficult. Furthermore, LA strain is directly influenced by the LV filling pressures and, therefore, by the LV function, leading to a high variability of the measurements [58]. Another important limitation of LA strain is its lack of standardization, leading to a broad range of the normal reference [59]. This lack of a clear consensus on the normal reference ranges for left atrial strain makes it difficult to interpret in clinical routine. However, in the present monocentric study, determination of LA strain in our hands was performed in a standardized manner. Therefore, we think LA strain data are of significant value for interpretation in the context of the overall data set in this selected patient population. Furthermore, total atrial conduction time, determined at the lateral mitral valve ring, has been shown to correlate with the extent of left atrial fibrosis [15]. Moreover, a recent published echocardiographic parameter, septal PA-TDI, seems to be a predictor of subclinical AF and a promising parameter for risk-stratified reduction of ECG monitoring duration after stroke [16].
Gal3 and miR−133a were not associated with echocardiographic parameters. Actually, in the present study echocardiographic parameters reflecting LA dysfunction and remodeling correlate with levels of sST2, miR−21, miR−29a, miR−146b, miR−328 and NT-proBNP. In particular, septal PA-TDI correlates with sST2, miR−21, miR−29a, miR−146b, miR−328 and NT-proBNP, which are connected to fibrotic changes and associated with atrial remodeling. Hence, these finding might account for potential association of septal PA-TDI with atrial fibrosis. However, the intra- and extracellular effects of miRs, their origin as well as their metabolism are not entirely investigated. Therefore, our results do not provide a conclusion of tissue-specific release of miRs and pathophysiological background of septal PA-TDI.
Furthermore, in our study the combination of NT-proBNP and miR−21 was associated with a preferable accuracy in predicting AF. Multivariate analysis depicted the combination of NT-proBNP and miR−21 as the most powerful predictor of AF in our study. The discriminative ability was enhanced by the combination of both biomarkers as revealed in ROC curve analysis (AUC = 0.84) compared to NT-proBNP (AUC = 0.76) and miR−21 (AUC = 0.30) alone.
To our knowledge, no other study compared the predictive value of NT-proBNP, s-ST2, Gal3 and miRs for AF in patients with and without stroke. Furthermore, we described for the first time the potential association of biomarkers involved in atrial fibrosis and the echocardiographic parameter septal PA-TDI, which seemed to be a strong predictor of AF. Especially, after AF-ablation NT-proBNP, miR-21 and septal PA-TDI may be promising to investigate the therapy-response and to allocate patients to new monitoring systems to avoid AF recurrence [60]. In everyday clinical practice the identified biomarkers – in particular NT-pro-BNP - could be used to screen patients at risk for AF (e.g., after embolic stroke of undetermined source). Subsequent patients with elevated biomarker levels should be allocated to a comprehensive echocardiographic evaluation including septal PA-TDI for further risk stratification. The clinical value of this workflow should be tested in a prospective study.
In this study limitations should be considered: (1) This study included a small sample size within different groups. However, a detailed patient characterization with a rigorous diagnostic process with a complete echocardiographic protocol, ECG-monitoring and blood sample acquisition, the prospective design, and the inclusion of patients with and without stroke strengthen this study. Therefore, the predictive performance of NT-proBNP and miR-21 can be applied in both cohorts. (2) The present study is only descriptive and cannot contribute to an understanding of the pathomechanisms behind the regulated miR and biomarkers in AF. (3) A potential selection bias owing to biomarker and miR selection has to be considered. However, it should be recognized that other miR and biomarkers may also display predictive properties in AF.
5. Conclusions
This study confirmed the potential role of biomarkers for the prediction of AF. We demonstrated that biomarkers and especially miRs are associated with AF and correlate with echocardiographic parameters reflecting LA function and remodeling. In particular, NT-proBNP and miR−21 are independent predictors of AF in this study. The combination of both enhances the ability to discriminate between patients with and without AF. Thus, miR−21 and NT-proBNP are candidate AF-biomarkers for additive risk-stratified decision-making. Further studies in greater scale are warranted to verify this hypothesis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/4/1118/s1, Table S1: Regression analysis of training and test set, Table S2: ROC-analysis of training and test set, Table S3: Mc Nemar test for dependent samples, Table S4: Medication documented at study inclusion.
Author Contributions
J.-T.S. and T.J.P. contributed equally as first authors to the manuscript. U.B., T.T., and C.B. contributed equally as senior authors to the manuscript. J.-T.S. and U.B. perceived the original idea. T.J.P. and D.H.-K. performed sST−2 and Gal3 measurements and analysis. C.B., S.C. and T.T. performed miR-measurements and analysis. R.L. performed measurements and analysis of NT-proBNP. J.-T.S., T.J.P., S.B. and U.B. analyzed and interpreted data. J.-T.S., D.B. and U.B. enrolled patients at the cardiology ward. K.W. and G.M.G. enrolled patients at the stroke unit. J.-T.S., J.H. and S.B. collected data. J.-T.S., T.J.P., C.B., A.A.D., J.B., T.T. and U.B. wrote the manuscript. The manuscript was approved by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
Saskia Biber and biomarker-analysis was supported by the “Else-Kröner-Fresenius-Stiftung” as part of the KlinStrucMed-Program at Hannover Medical School. TT received funding from the Federal Ministry of Education and Research (BMBF, Germany, research grant ERA-CVD JTC2016 EXPERT, 01KL1711) and Deutsche Forschungsgemeinschaft (DFG, Projects TH903/18−1 and TH903/20−2).
Acknowledgments
We thank Tabea Wolter, Martina Kasten, Julia Afanasjew, Stella Schlothauer, Anika Hübner and Isabelle Riedel for their expert technical assistance.
Conflicts of Interest
T.T. has filed and licensed patents about the use of miR−21 and other miRNAs as cardiovascular diagnostics and therapeutics. TT is founder and shareholder of Cardior Pharmaceuticals GmbH. J.T.S., T.J.P., S.B., S.C., K.W., G.M.G., J.H., A.A.D., D.B., R.L., J.B., C.B., and U.B. declare no competing interests.
Abbreviations
| AF | Atrial fibrillation |
| Cis | Confidence intervals |
| Gal3 | Galectin−3 |
| HR | Hazard ratio |
| LA | Left atrial |
| LAVI/á | Ration of LA volume index to tissue Doppler á |
| miR | MicroRNA |
| NT-proBNP | N-terminal fragment of pro-B-type natriuretic peptide |
| PA-TDI | Total atrial conduction time |
| ROC | Receiver operating characteristic |
| SRa | Second negative peak strain rate during LA contraction |
| sST2 | Soluble suppression of tumorigenicity 2 |
References
- Chinitz, J.S.; Castellano, J.M.; Kovacic, J.C.; Fuster, V. Atrial fibrillation, stroke, and quality of life. Ann. N. Y. Acad. Sci. 2012, 1254, 140–150. [Google Scholar] [CrossRef]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef]
- Haeusler, K.G.; Groschel, K.; Kohrmann, M.; Anker, S.D.; Brachmann, J.; Bohm, M.; Diener, H.C.; Doehner, W.; Endres, M.; Gerloff, C.; et al. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin. Res. Cardiol. 2018, 107, 871–880. [Google Scholar] [CrossRef]
- Flint, A.C.; Banki, N.M.; Ren, X.; Rao, V.A.; Go, A.S. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: The Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke 2012, 43, 2788–2790. [Google Scholar] [CrossRef] [PubMed]
- Tayal, A.H.; Callans, D.J. Occult atrial fibrillation in ischemic stroke: Seek and you shall find. Neurology 2010, 74, 1662–1663. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Cote, R.; et al. Coordinators Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.; Groschel, K.; Gelbrich, G.; Hamann, G.F.; Kermer, P.; Liman, J.; Seegers, J.; Wasser, K.; Schulte, A.; Jurries, F.; et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): An open-label randomised controlled trial. Lancet Neurol. 2017, 16, 282–290. [Google Scholar] [CrossRef]
- Israel, C.; Kitsiou, A.; Kalyani, M.; Deelawar, S.; Ejangue, L.E.; Rogalewski, A.; Hagemeister, C.; Minnerup, J.; Schabitz, W.R. Detection of atrial fibrillation in patients with embolic stroke of undetermined source by prolonged monitoring with implantable loop recorders. Thromb. Haemost. 2017, 117, 1962–1969. [Google Scholar] [CrossRef]
- Donal, E.; Lip, G.Y.; Galderisi, M.; Goette, A.; Shah, D.; Marwan, M.; Lederlin, M.; Mondillo, S.; Edvardsen, T.; Sitges, M.; et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 355–383. [Google Scholar] [CrossRef]
- Toh, N.; Kanzaki, H.; Nakatani, S.; Ohara, T.; Kim, J.; Kusano, K.F.; Hashimura, K.; Ohe, T.; Ito, H.; Kitakaze, M. Left atrial volume combined with atrial pump function identifies hypertensive patients with a history of paroxysmal atrial fibrillation. Hypertension 2010, 55, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joyce, E.; Debonnaire, P.; Katsanos, S.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Dysfunction in the Pathogenesis of Cryptogenic Stroke: Novel Insights from Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; Dibella, E.V.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Stahrenberg, R.; Edelmann, F.; Haase, B.; Lahno, R.; Seegers, J.; Weber-Kruger, M.; Mende, M.; Wohlfahrt, J.; Kermer, P.; Vollmann, D.; et al. Transthoracic echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or transient ischemic attack. Stroke 2011, 42, 3643–3645. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Hars, C.; Schiedat, F.; Bosche, L.I.; Gotzmann, M.; Strauch, J.; Dietrich, J.W.; Vogt, M.; Tannapfel, A.; Deneke, T.; et al. Correlation between total atrial conduction time estimated via tissue Doppler imaging (PA-TDI Interval), structural atrial remodeling and new-onset of atrial fibrillation after cardiac surgery. J. Cardiovasc. Electrophysiol. 2013, 24, 626–631. [Google Scholar] [CrossRef]
- Sieweke, J.T.; Biber, S.; Weissenborn, K.; Heuschmann, P.U.; Akin, M.; Zauner, F.; Gabriel, M.M.; Schuppner, R.; Berliner, D.; Bauersachs, J.; et al. Septal total atrial conduction time for prediction of atrial fibrillation in embolic stroke of unknown source: A pilot study. Clin. Res. Cardiol. 2019. [Google Scholar] [CrossRef]
- Liu, C.Y.; Heckbert, S.R.; Lai, S.; Ambale-Venkatesh, B.; Ostovaneh, M.R.; McClelland, R.L.; Lima, J.A.C.; Bluemke, D.A. Association of Elevated NT-proBNP With Myocardial Fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 2017, 70, 3102–3109. [Google Scholar] [CrossRef]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Cohen Solal, A.; Padeletti, L.; et al. Galectin−3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef]
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.V.; Shi, V.; Lefkowitz, M.; et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef]
- Felker, G.M.; Fiuzat, M.; Thompson, V.; Shaw, L.K.; Neely, M.L.; Adams, K.F.; Whellan, D.J.; Donahue, M.P.; Ahmad, T.; Kitzman, D.W.; et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ. Heart Fail. 2013, 6, 1172–1179. [Google Scholar] [CrossRef]
- Motiwala, S.R.; Szymonifka, J.; Belcher, A.; Weiner, R.B.; Baggish, A.L.; Sluss, P.; Gaggin, H.K.; Bhardwaj, A.; Januzzi, J.L. Serial measurement of galectin−3 in patients with chronic heart failure: Results from the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study. Eur. J. Heart Fail. 2013, 15, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Szadkowska, I.; Wlazel, R.N.; Migala, M.; Szadkowski, K.; Zielinska, M.; Paradowski, M.; Pawlicki, L. The association between galectin−3 and clinical parameters in patients with first acute myocardial infarction treated with primary percutaneous coronary angioplasty. Cardiol. J. 2013, 20, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Canpinar, H.; Canpolat, U.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Hazirolan, T.; et al. The Association of Serum Galectin−3 Levels with Atrial Electrical and Structural Remodeling. J. Cardiovasc. Electrophysiol. 2015, 26, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Marfella, R.; Santamaria, M.; Papini, S.; Parisi, Q.; Sacra, C.; Colaprete, D.; Paolisso, G.; Rizzo, M.R.; Barbieri, M. Stretch, Injury and Inflammation Markers Evaluation to Predict Clinical Outcomes After Implantable Cardioverter Defibrillator Therapy in Heart Failure Patients With Metabolic Syndrome. Front. Physiol. 2018, 9, 758. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA−21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Fiedler, J.; Jazbutyte, V.; Kirchmaier, B.C.; Gupta, S.K.; Lorenzen, J.; Hartmann, D.; Galuppo, P.; Kneitz, S.; Pena, J.T.; Sohn-Lee, C.; et al. MicroRNA−24 regulates vascularity after myocardial infarction. Circulation 2011, 124, 720–730. [Google Scholar] [CrossRef]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Adam, O.; Lohfelm, B.; Thum, T.; Gupta, S.K.; Puhl, S.L.; Schafers, H.J.; Bohm, M.; Laufs, U. Role of miR−21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol. 2012, 107, 278. [Google Scholar] [CrossRef]
- Dawson, K.; Wakili, R.; Ordog, B.; Clauss, S.; Chen, Y.; Iwasaki, Y.; Voigt, N.; Qi, X.Y.; Sinner, M.F.; Dobrev, D.; et al. MicroRNA29: A mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013, 127, 1466–1475. [Google Scholar] [CrossRef]
- Santulli, G.; Iaccarino, G.; De Luca, N.; Trimarco, B.; Condorelli, G. Atrial fibrillation and microRNAs. Front. Physiol. 2014, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheitlin, M.D.; Armstrong, W.F.; Aurigemma, G.P.; Beller, G.A.; Bierman, F.Z.; Davis, J.L.; Douglas, P.S.; Faxon, D.P.; Gillam, L.D.; Kimball, T.R.; et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003, 108, 1146–1162. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef] [PubMed]
- Derda, A.A.; Thum, S.; Lorenzen, J.M.; Bavendiek, U.; Heineke, J.; Keyser, B.; Stuhrmann, M.; Givens, R.C.; Kennel, P.J.; Schulze, P.C.; et al. Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int. J. Cardiol. 2015, 196, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.G.; Halliani, A.; Moerland, P.D.; Meijers, J.C.; Creemers, E.E.; Pinto-Sietsma, S.J. Normalization panels for the reliable quantification of circulating microRNAs by RT-qPCR. FASEB J. 2015, 29, 3853–3862. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Lanfear, D.E.; de Ronde, M.W.J.; Lupon, J.; Leenders, J.J.; Liu, Z.; Zuithoff, N.P.A.; Eijkemans, M.J.C.; Zamora, E.; De Antonio, M.; et al. Prognostic value of circulating microRNAs on heart failure-related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur. J. Heart Fail. 2018, 20, 67–75. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, J.H.; Choi, S.H.; Lee, N.; Park, W.J.; Oh, D.J.; Rhim, C.Y.; Lee, K.H. Determinants of brain natriuretic peptide levels in patients with lone atrial fibrillation. Circ. J. 2006, 70, 100–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellinor, P.T.; Low, A.F.; Patton, K.K.; Shea, M.A.; Macrae, C.A. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J. Am. Coll. Cardiol. 2005, 45, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.C.; Chen, L.Y.; Chong, J.P.; Austin, E.; Quay, C.N.; Gong, L.; Mark Richards, A.; Ling, L.H. Plasma mid-regional pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide improve discrimination of lone atrial fibrillation. Int. J. Cardiol. 2015, 188, 10–12. [Google Scholar] [CrossRef]
- Hussein, A.A.; Saliba, W.I.; Martin, D.O.; Shadman, M.; Kanj, M.; Bhargava, M.; Dresing, T.; Chung, M.; Callahan, T.; Baranowski, B.; et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation 2011, 123, 2077–2082. [Google Scholar] [CrossRef]
- De Boer, R.A.; Yu, L.; van Veldhuisen, D.J. Galectin−3 in cardiac remodeling and heart failure. Curr. Heart Fail. Rep. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Hernandez-Romero, D.; Vilchez, J.A.; Lahoz, A.; Romero-Aniorte, A.I.; Jover, E.; Garcia-Alberola, A.; Jara-Rubio, R.; Martinez, C.M.; Valdes, M.; Marin, F. Galectin−3 as a marker of interstitial atrial remodelling involved in atrial fibrillation. Sci. Rep. 2017, 7, 40378. [Google Scholar] [CrossRef]
- Fashanu, O.E.; Norby, F.L.; Aguilar, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Chen, L.Y.; Soliman, E.Z.; Alonso, A.; Folsom, A.R. Galectin−3 and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2017, 192, 19–25. [Google Scholar] [CrossRef]
- Cardin, S.; Guasch, E.; Luo, X.; Naud, P.; Le Quang, K.; Shi, Y.; Tardif, J.C.; Comtois, P.; Nattel, S. Role for MicroRNA−21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ. Arrhythmia Electrophysiol. 2012, 5, 1027–1035. [Google Scholar] [CrossRef]
- Galenko, O.; Jacobs, V.; Knight, S.; Taylor, M.; Cutler, M.J.; Muhlestein, J.B.; Carlquist, J.L.; Knowlton, K.U.; Jared Bunch, T. The role of microRNAs in the development, regulation, and treatment of atrial fibrillation. J. Interv. Card. Electrophysiol. 2019, 55, 297–305. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, Y.; Wang, S.; Ye, P.; Miao, X.; Xia, J. The expression levels of plasma micoRNAs in atrial fibrillation patients. PLoS ONE 2012, 7, e44906. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA−328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 2010, 122, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Han, J.; Li, Y.; Xie, C.; Xie, L.; Shi, J.; Zhang, J.; Yang, B.; Chen, D.; et al. Integrated analysis of microRNA and mRNA expression profiles in the left atrium of patients with nonvalvular paroxysmal atrial fibrillation: Role of miR−146b−5p in atrial fibrosis. Heart Rhythm 2015, 12, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR−133 and miR−30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Santamaria, M.; Paolisso, G.; Marfella, R. microRNA expression changes after atrial fibrillation catheter ablation. Pharmacogenomics J. 2015, 16, 1863–1877. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Schneider, C.; Malisius, R.; Krause, K.; Lampe, F.; Bahlmann, E.; Boczor, S.; Antz, M.; Ernst, S.; Kuck, K.H. Strain rate imaging for functional quantification of the left atrium: Atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur. Heart J. 2008, 29, 1397–1409. [Google Scholar] [CrossRef]
- Huynh, Q.L.; Kalam, K.; Iannaccone, A.; Negishi, K.; Thomas, L.; Marwick, T.H. Functional and Anatomic Responses of the Left Atrium to Change in Estimated Left Ventricular Filling Pressure. J. Am. Soc. Echocardiogr. 2015, 28, 1428–1433. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70. [Google Scholar] [CrossRef]
- Sardu, C.; Santamaria, M.; Rizzo, M.R.; Barbieri, M.; di Marino, M.; Paolisso, G.; Santulli, G.; Marfella, R. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): The TELECART Study. Int. J. Clin. Pract. 2016, 70, 569–576. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).