Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years
Abstract
:1. Introduction
2. Methods
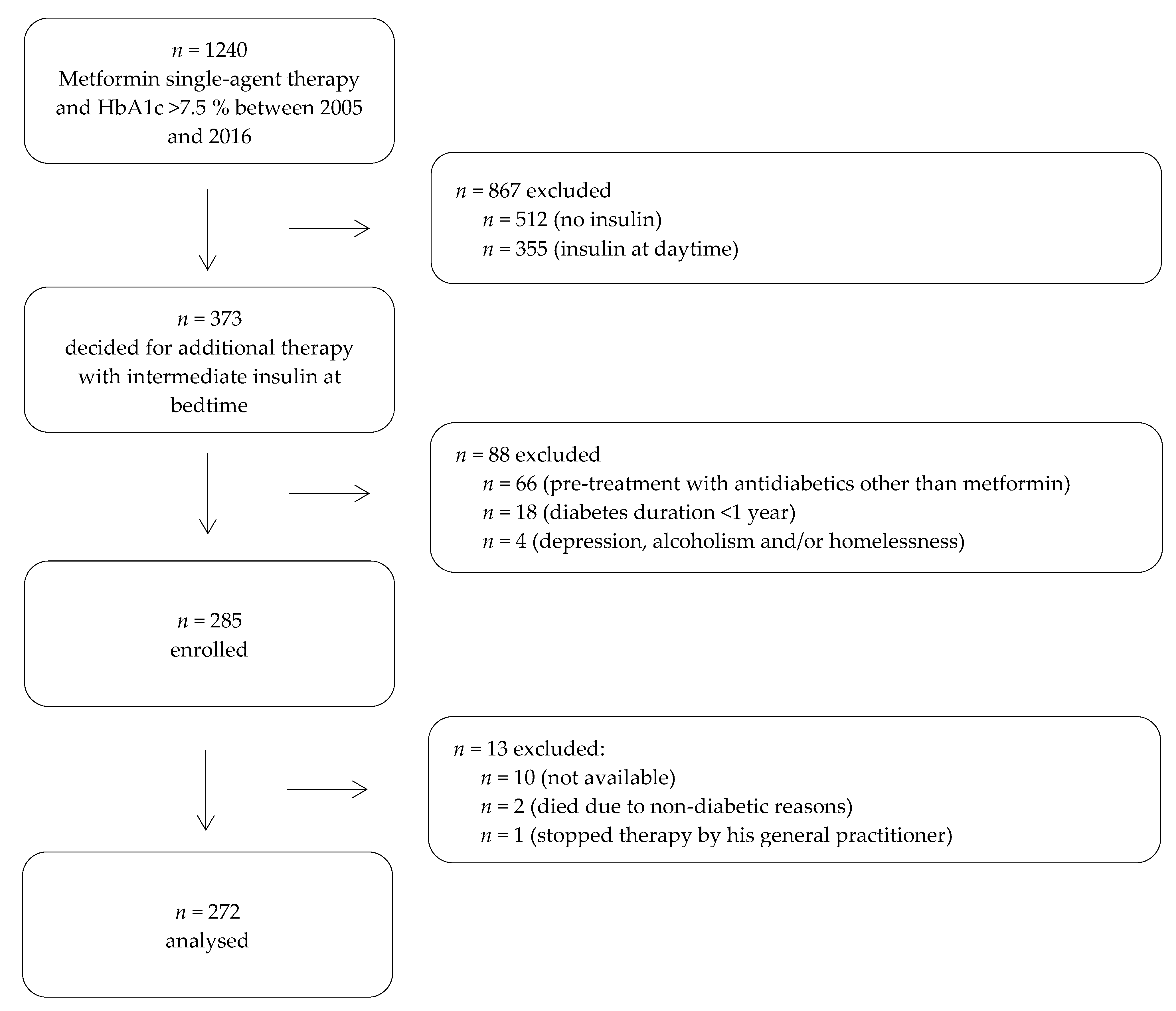
2.1. Participants and Study Design
2.2. Treatment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| HbA1c | Glycated haemoglobin |
| IU | international units |
| NPH | Neural Protamine Hagedorn |
| STTP | structured treatment and teaching programme |
References
- Bundesärztekammer (BÄK); Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Therapie des Typ-2-Diabetes—Langfassung, 1. Auflage. Version 4. 2013, Zuletzt Geändert. November 2014. Available online: www.dm-therapie.versorgungsleitlinien.de (accessed on 29 August 2019).
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abholz, H.-H.; Egidi, G.; Uebel, T.; Kochen, M.M. Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM): Kritik am, Nationalen Aktionsplan Diabetes. ZFA Z. Allg. 2008, 84, 239–242. [Google Scholar] [CrossRef]
- Schwabe, U.; Paffrath, D.; Ludwig, W.D.; Klauber, J. Arzneiverordnungs-Report 2018; Springer: Berlin, Germany, 2018. [Google Scholar]
- Holman, R.R.; Farmer, A.; Davies, M.J.; Levy, J.; Darbyshire, J.L.; Keenan, J.; Paul, S.K. Three-Year Efficacy of Complex Insulin Regimens in Type 2 Diabetes. N. Engl. J. Med. 2009, 361, 1736–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergenstal, R.M. Optimization of Insulin Therapy in Patients With Type 2 Diabetes. Endocr. Pr. 2000, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Bretzel, R.G.; Nuber, U.; Landgraf, W.; Owens, D.O.; Bradley, C.; Linn, T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): An open randomized controlled trial. Lancet 2008, 371, 1073–1084. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Charpentier, G.; Clauson, P.; Ravn, G.M.; Roberts, V.L.; Thorsteinsson, B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly Controlled Type 2 Diabetes. Clin. Ther. 2006, 28, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Yale, J.F.; Harris, S.B.; Issa, M.; Stewart, J.A.; Dempsey, E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes in either no oral glucose-lowering agents or submaximal doses of metformin and/or sulfonylureas. The Canadian INSIGHT (Implementing new Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet. Med. 2006, 23, 736–742. [Google Scholar] [PubMed]
- Yki-Järvinen, H.; Ryysy, L.; Nikkilä, K.; Tulokas, T.; Vanamo, R.; Heikkilä, M. Comparison of Bedtime Insulin Regimens in Patients with Type 2 Diabetes Mellitus. Ann. Intern. Med. 1999, 130, 389. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.C.; Van Avendonk, M.J.; Jansen, H.; Goudswaard, A.N.N.; Donk, M.V.D.; Gorter, K.; Kerssen, A.; Rutten, G.E. Insulin monotherapy compared with the addition of oral glucose-lowering agents to insulin for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskin, P.; Allen, E.; Hollander, P.; Lewin, A.; Gabbay, R.A.; Hu, P.; Bode, B.; Garber, A. For the INITIATE Study Group Initiating Insulin Therapy in Type 2 Diabetes: A comparison of biphasic and basal insulin analogs. Diabetes Care 2005, 28, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civera, M.; Merchante, A.; Salvador, M.; Sanz, J.; Martinez, I. Safety and efficacy of repaglinide in combination with metformin and bedtime NPH insulin as an insulin treatment regimen in type 2 diabetes. Diabetes Res. Clin. Pr. 2008, 79, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Grüßeer, M.; Jörgens, V. Strukturiertes Behandlungs-und Schulungsprogramm für Typ-2-Diabetiker mit Normalinsulin vor dem Essen Insulin, 7nd ed.; Deutscher Ärzte-Verlag: Köln, Germany, 2016. [Google Scholar]
- German Diabetes Association (DDG). Guidelines for Recognition of a Treatment Institution—Certified Diabetes Center DDG. 2015. Available online: https://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Zertifizierung/Basisanerkennung/Richtlinie_Zert_Diabeteszentrum_DDG_2015.pdf (accessed on 29 August 2019).
- Goudswaard, A.N.; Stolk, R.P.; Zuithoff, P.; De Valk, H.W.; Rutten, G.E. Starting insulin in type 2 diabetes: Continue oral hypoglycemic agents? A randomized trial in primary care. J. Fam. Pr. 2004, 53, 393–399. [Google Scholar]
- Lipska, K.J.; Hirsch, I.B.; Riddle, M.C. Human Insulin for Type 2 Diabetes: An Effective, Less-Expensive Option. JAMA 2017, 318, 23. [Google Scholar] [CrossRef] [PubMed]
- Kaiser Permanente. Type 2 Diabetes Screening and Treatment Guideline. Available online: https://wa.kaiserpermanente.org/static/pdf/public/guidelines/diabetes2.pdf (accessed on 29 August 2019).
- Kuniss, N.; Müller, U.A.; Kloos, C.; Müller, R.; Starrach, G.; Jörgens, V.; Kramer, G. Substantial improvement in HbA1c following a treatment and teaching programme for people with type 2 diabetes on conventional insulin therapy in an in- and outpatient setting. Acta Diabetol. 2017, 55, 131–137. [Google Scholar] [CrossRef] [PubMed]

| Parameter | All (n = 272) | Drop Out (n = 13) | Group A (n = 23) | Group B (n = 144) | Group C (n = 69) | Group D (n = 36) |
|---|---|---|---|---|---|---|
| Age (years) | 59.5 ± 11.0 | 56.8 ± 7.7 | 59.3 ± 10.2 | 58.9 ± 10.8 | 59.3 ± 11.6 | 62.6 ± 11.0 |
| Female n (%) | 95 (34.9) | 5 (38.5) | 10 (43.5) | 51 (35.4) | 21 (30.4) | 13 (36.1) |
| Diabetes duration (years) | 6.6 ± 4.7 | 5.4 ± 4.4 | 5.6 ± 4.7 | 6.6 ± 4.2 | 6.7 ± 5.1 | 7.4 ± 5.8 |
| Treatment duration with metformin (years) | 4.8 ± 3.8 | 3.8 ± 2.5 | 4.0 ± 4.4 | 4.8 ± 3.5 | 5.1 ± 3.9 | 4.8 ± 4.1 |
| Previous participation in a STTP n (%) | 220 (80.9) | 8 (61.5) | 20 (87.0) | 118 (81.9) | 53 (76.8) | 29 (80.6) |
| BMI (kg/m2) | 31.6 ± 5.8 | 31.3 ± 5.1 | 34.6 ± 6.9 † | 31.1 ± 5.2 † | 32.4 ± 6.8 † | 30.2 ± 4.3 † |
| Bodyweight (kg) | 92.6 ± 18.5 | 87.7 ± 17.2 | 98.7 ± 24.3 | 91.0 ± 17.4 | 95.0 ± 20.7 | 91.0 ± 13.4 |
| HbA1c (%) | 8.4 ± 0.9 | 8.8 ± 0.7 | 8.5 ± 1.0 | 8.5 ± 0.9 | 8.2 ± 0.8 | 8.5 ± 0.9 |
| HbA1c (mmol/mol) | 68.6 ± 9.6 | 72.3 ± 7.7 | 69.8 ± 11.0 | 69.3 ± 9.8 | 66.3 ± 8.5 | 69.3 ± 9.9 |
| eGFR (ml/min) | 120.6 ± 41.2 | 120.6 ± 33.9 | 135.7 ± 48.3 | 118.1 ± 39.3 | 122.0 ± 46.1 | 118.3 ± 32.6 |
| Concomitant diseases n (%) | ||||||
| Neuropathy | 96 (35.3) | 4 (30.8) | 9 (39.1) | 50 (34.7) | 23 (33.3) | 14 (38.9) |
| Retinopathy | 9 (3.3) | 0 (0) | 0 (0.0) | 3 (2.1) | 6 (8.7) | 0 (0.0) |
| Hypertension | 240 (88.2) | 11 (84.6) | 21 (91.3) | 127 (88.2) | 62 (89.9) | 30 (83.3) |
| Coronary heart disease | 67 (24.6) | 3 (23.1) | 6 (26.1) | 34 (23.6) | 17 (24.6) | 10 (27.8) |
| Peripheral arterial disease | 21 (7.7) | 0 (0) | 1 (4.3) | 12 (8.3) | 4 (5.8) | 4 (11.1) |
| Myocardial infarction | 21 (7.7) | 1 (7.7) | 1 (4.3) | 13 (9.0) | 4 (5.8) | 3 (8.3) |
| Apoplex | 7 (2.6) | 0 (0) | 1 (4.3) | 5 (3.5) | 1 (1.4) | 0 (0.0) |
| Participants | HbA1c (%) | Bodyweight (kg) | ||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| All (n = 272) | 8.4 ± 0.9 | 7.2 ± 0.8 † | 92.6 ± 18.5 | 90.9 ± 18.4 † |
| Group A (n = 23, 4.5 months) | 8.5 ± 1.0 | 8.5 ± 0.8 | 98.7 ± 24.3 | 97.8 ± 23.8 |
| Group B (n = 144, 32.7 months) | 8.5 ± 0.9 | 7.3 ± 0.4 † | 91.0 ± 17.4 | 90.4 ± 17.2 |
| Group C (n = 69, 25.0 months) | 8.2 ± 0.8 | 6.3 ± 0.5 † | 95.0 ± 20.7 | 91.0 ± 20.8 † |
| Group D (n = 36, 57.4 months) | 8.5 ± 0.9 | 7.2 ± 0.4 † | 91.0 ± 13.4 | 88.4 ± 13.1 † |
| Study | Intervention (years) | Duration | HbA1c (%) | Weight (kg) | Insulin Dose (IU) | Severe Hypoglycaemia | |
|---|---|---|---|---|---|---|---|
| Baseline | Change | ||||||
| Present study | bedtime intermediate acting insulin (human) plus metformin | 2 months –11 years | 8.4 | −1.2 | −1.7 | 13 | 0 |
| Holman et al. 2009 [6] | bedtime basal insulin (detemir) plus metformin/sulfonylurea | 3 years | 8.1 | −1.2 | +3.6 | 88 * | 1.7/patient/year ** |
| Bretzel et al. 2008 [8] | bedtime long-acting insulin (glargine) plus oral agents | 44 weeks | 8.7 | −1.7 | +3.0 | 42 | 0.03/patient/year ** |
| Philis-Tsimikas et al. 2006 [9] | evening intermediate acting insulin (human) plus oral drugs | 20 weeks | 9.2 | −1.7 | +1.6 | 33 | 0 ** |
| evening basal insulin (detemir) plus oral drugs | 8.9 | −1.5 | +0.7 | 37 | 2 cases in 169 patients ** | ||
| Gerstein et al. 2006 [10] | bedtime long-acting insulin (glargine) plus oral agents | 24 weeks | 8.6 | −1.6 | +1.9 | 38 | 1 case in 206 patients ** |
| Yki-Jarvinen et al. 1999 [11] | bedtime intermediate acting insulin (human) plus metformin and glyburide | 52 weeks | 9.9 | −2.1 | +1.2 | 20 | 0 ** |
| bedtime intermediate acting insulin (human) plus glyburide | 9.8 | −1.8 | +3.9 | 24 | 0 ** | ||
| bedtime intermediate acting insulin (human) plus metformin | 9.8 | −2.5 | +0.9 | 36 | 0 ** | ||
| Raskin et al. 2005 [13] | bedtime long-acting insulin (glargine) plus metformin/other drugs | 28 weeks | 9.8 | −2.4 | +3.5 | 51 | 1 case in 1 patient ** |
| Civera et al. 2008 [14] | bedtime intermediate acting insulin (human) plus metformin | 24 weeks | 9.6 | −0.7 | +1.7 | 21 | 0 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertes, B.; Gödde, S.; Piorkowski, M.; Kramer, G.; Müller, U.A.; Kuniss, N. Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years. J. Clin. Med. 2020, 9, 1153. https://doi.org/10.3390/jcm9041153
Mertes B, Gödde S, Piorkowski M, Kramer G, Müller UA, Kuniss N. Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years. Journal of Clinical Medicine. 2020; 9(4):1153. https://doi.org/10.3390/jcm9041153
Chicago/Turabian StyleMertes, Bernardo, Sybille Gödde, Michael Piorkowski, Guido Kramer, Ulrich Alfons Müller, and Nadine Kuniss. 2020. "Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years" Journal of Clinical Medicine 9, no. 4: 1153. https://doi.org/10.3390/jcm9041153
APA StyleMertes, B., Gödde, S., Piorkowski, M., Kramer, G., Müller, U. A., & Kuniss, N. (2020). Successful Treatment with Bedtime Basal Insulin Added to Metformin without Weight Gain or Hypoglycaemia over Three Years. Journal of Clinical Medicine, 9(4), 1153. https://doi.org/10.3390/jcm9041153





