Metabolomic Analysis of Patients with Chronic Myeloid Leukemia and Cardiovascular Adverse Events after Treatment with Tyrosine Kinase Inhibitors
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Sample Preparation and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. GC-MS Analysis
2.4. Statistical analysis
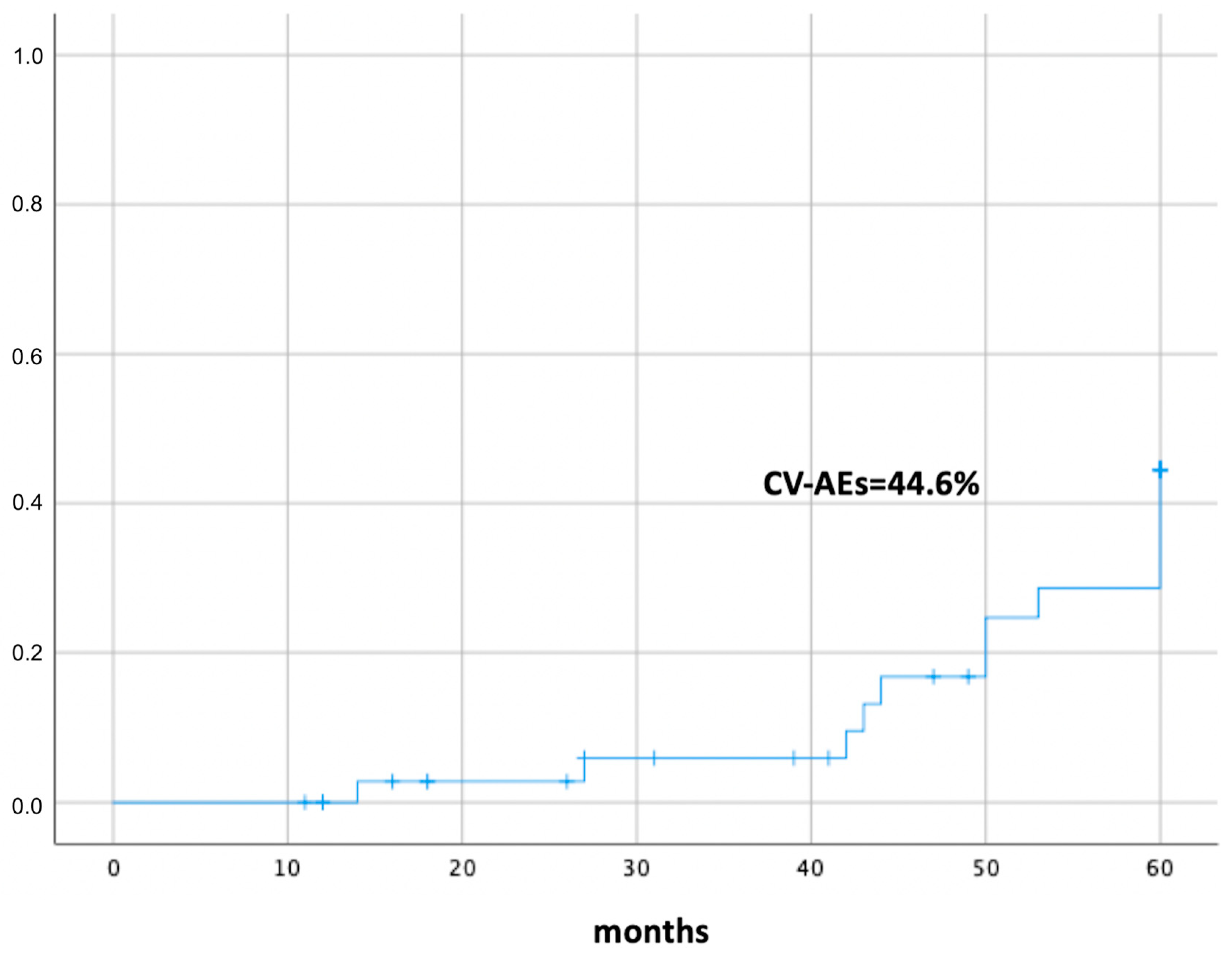
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldman, J.M.; Melo, J.V. Chronic Myeloid Leukemia—Advances in Biology and New Approaches to Treatment. N. Engl. J. Med. 2003, 349, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Björkholm, M.; Dickman, P.; Höglund, M.; Lambert, P.; Andersson, T.M.-L. Life Expectancy of Patients with Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Atzeni, S.; Orrù, N.; Azzena, L.; Martorana, L.; Littera, R.; Ledda, A.; La Nasa, G. Gynecomastia in a male after dasatinib treatment for chronic myeloid leukemia. Leukemia 2008, 22, 2127–2128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aghel, N.; Delgado, D.H.; Lipton, J.H. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: Preventive strategies and cardiovascular surveillance. Vasc. Health Risk Manag. 2017, 13, 293–303. [Google Scholar] [CrossRef]
- Caocci, G.; Mulas, O.; Annunziata, M.; Luciano, L.; Bonifacio, M.; Orlandi, E.M.; Pregno, P.; Galimberti, S.; Rossi, A.R.; Abruzzese, E.; et al. Cardiovascular toxicity in patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors in the real-life practice: Identification of risk factors and the role of prophylaxis. Am. J. Hematol. 2018, 93, E159–E161. [Google Scholar] [CrossRef]
- Caocci, G.; Mulas, O.; Abruzzese, E.; Luciano, L.; Iurlo, A.; Attolico, I.; Castagnetti, F.; Galimberti, S.; Sgherza, N.; Bonifacio, M.; et al. Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematol. Oncol. 2019, 37, 296–302. [Google Scholar] [CrossRef]
- Caocci, G.; Mulas, O.; Abruzzese, E.; Iurlo, A.; Annunziata, M.; Orlandi, E.M.; Galimberti, S.; Binotto, G.; Sgherza, N.; Luciano, L.; et al. Incidence and evaluation of predisposition to cardiovascular toxicity in chronic myeloid leukemia patients treated with bosutinib in the real-life practice. Ann. Hematol. 2019, 98, 1885–1890. [Google Scholar] [CrossRef]
- Ren, R. Mechanisms of BCR–ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer 2005, 5, 172–183. [Google Scholar] [CrossRef]
- Deidda, M.; Noto, A.; Bassareo, P.P.; Dessalvi, C.C.; Mercuro, G. Metabolomic Approach to Redox and Nitrosative Reactions in Cardiovascular Diseases. Front. Physiol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Deidda, M.; Piras, C.; Bassareo, P.P.; Dessalvi, C.C.; Mercuro, G. Metabolomics, a promising approach to translational research in cardiology. IJC Metab. Endocr. 2015, 9, 31–38. [Google Scholar] [CrossRef]
- Deidda, M.; Mercurio, V.; Cuomo, A.; Noto, A.; Mercuro, G.; Dessalvi, C.C. Metabolomic Perspectives in Antiblastic Cardiotoxicity and Cardioprotection. Int. J. Mol. Sci. 2019, 20, 4928. [Google Scholar] [CrossRef] [PubMed]
- Barberini, L.; Noto, A.; Fattuoni, C.; Satta, G.; Zucca, M.; Cabras, M.G.; Mura, E.; Cocco, P. The Metabolomic Profile of Lymphoma Subtypes: A Pilot Study. Molecules 2019, 24, 2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, G.; Wang, H.; Chen, P.; Hao, J.; Wang, Y. Identification of novel serum biomarker for the detection of acute myeloid leukemia based on liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2019, 166, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Karlíková, R.; Široká, J.; Friedecký, D.; Faber, E.; Hrdá, M.; Mičová, K.; Fikarová, I.; Gardlo, A.; Janečková, H.; Vrobel, I.; et al. Metabolite Profiling of the Plasma and Leukocytes of Chronic Myeloid Leukemia Patients. J. Proteome Res. 2016, 15, 3158–3166. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Xie, Y.; Xu, L.; Wu, X.; Wu, D. Monitoring tyrosine kinase inhibitor therapeutic responses with a panel of metabolic biomarkers in chronic myeloid leukemia patients. Cancer Sci. 2018, 109, 777–784. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, W.M.M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012). Int. J. Behav. Med. 2012, 19, 403–488. [Google Scholar] [CrossRef]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef]
- Cross, N.C.P.; White, H.E.; Müller, M.C.; Saglio, G.; Hochhaus, A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012, 26, 2172–2175. [Google Scholar] [CrossRef]
- Dunn, W.; The Human Serum Metabolome (HUSERMET) Consortium; Broadhurst, D.; Begley, P.; Zelená, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.; Halsall, A.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Oberkersch, R.E.; Santoro, M.M. Role of amino acid metabolism in angiogenesis. Vasc. Pharmacol. 2019, 112, 17–23. [Google Scholar] [CrossRef]
- Molnár, G.A.; Kun, S.; Sélley, E.; Kertész, M.; Szélig, L.; Csontos, C.; Böddi, K.; Bogar, L.; Miseta, A.; Wittmann, I. Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress—A Review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Szijárto, I.; Molnár, G.; Mikolás, E.; Fisi, V.; Cseh, J.; Laczy, B.; Kovacs, T.; Böddi, K.; Takátsy, A.; Gollasch, M.; et al. Elevated Vascular Level of ortho-Tyrosine Contributes to the Impairment of Insulin-Induced Arterial Relaxation. Horm. Metab. Res. 2014, 46, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Ercal, N.; Mare, S.; Pennathur, S.; Orhan, H.; Heinecke, J.W. Misincorporation of free m-tyrosine into cellular proteins: A potential cytotoxic mechanism for oxidized amino acids. Biochem. J. 2006, 395, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.P. Regulation of Endothelial Adherens Junctions by Tyrosine Phosphorylation. Mediat. Inflamm. 2015, 2015, 272858. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.Y.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. α-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Dias, S.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Martino, B.; Greco, M.; Abruzzese, E.; Trawinska, M.M.; Lai, S.; Ragatzu, P.; Galimberti, S.; Baratè, C.; Mulas, O.; et al. Killer immunoglobulin-like receptors can predict TKI treatment-free remission in chronic myeloid leukemia patients. Exp. Hematol. 2015, 43, 1015–1018.e1. [Google Scholar] [CrossRef]
- Bocchia, M.; Sicuranza, A.; Abruzzese, E.; Iurlo, A.; Sirianni, S.; Gozzini, A.; Galimberti, S.; Aprile, L.; Martino, B.; Pregno, P.; et al. Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission. Front. Oncol. 2018, 8, 194. [Google Scholar] [CrossRef]

| Sex, n (%) | Response to treatment, n (%) | ||||
|---|---|---|---|---|---|
| Male | 29 | (74.4) | MR4 | 20 | (51.3) |
| Female | 10 | (25.6) | No MR4 | 19 | (48.7) |
| Age at diagnosis, mean years (range) | 49 | (24–70) | CVD risk factors, n (%) | ||
| Median follow-up, mean years (range) | 3.7 | (0.9–5) | Hypertension | 7 | (17.9) |
| Leukocyte 103/L, mean value (range) | 124 | (6.6–300) | Dyslipidemia | 10 | (25.6) |
| Hemoglobin g/dL, mean value (range) | 11.6 | (6.4–14.7) | Obesity (BMI > 24.5 kg/m2) | 9 | (23.1) |
| Platelet 103/L, mean value (range) | 502 | (76–2059) | Severe renal insufficiency | 0 | (0) |
| Splenomegaly, n (%) | 14 | (36) | Diabetes | 7 | (17.9) |
| Sokal score, n (%) | High CV risk score | 11 | (28.2) | ||
| Low | 27 | (69) | Low CV risk score | 28 | (71.8) |
| Intermediate | 8 | (21) | CVD conditions before TKIs, n (%) | ||
| High | 4 | (10) | Hypertension | 2 | (5.1) |
| Type of TKI, n (%) | Myocardial infarction/angina | 1 | (2.6) | ||
| Imatinib | 16 | (41) | Arrhythmia | 2 | (5.1) |
| Dasatinb | 8 | (20.5) | CVD events after TKIs, n (%) | ||
| Nilotinib | 14 | (35.9) | Pleural or pericardial effusions | 5 | (12.8) |
| Ponatinib | 1 | (2.6) | Hypertension | 1 | (2.6) |
| Line of treatment, n (%) | Atrial fibrillation | 1 | (2.6) | ||
| First | 22 | (56.4) | ST-elevation myocardial infarction | 1 | (2.6) |
| Second | 13 | (33.4) | Reduction of cardiac ejection fraction | 1 | (2.6) |
| Third | 2 | (5.1) | Dissecting aneurysm of the aorta | 1 | (2.6) |
| Fourth | 2 | (5.1) | Metabolic effects after TKIs, n (%) | ||
| Reason for switching, n (%) | Hypercholesterolemia | 7 | (17.9) | ||
| Inefficacy | 6 | (15.3) | |||
| Intolerance | 11 | (28.2) |
| a. Metabolic Pathways of Patients without CV-AEs | Total | Statistics |
|---|---|---|
| Transfer of acetyl groups into mitochondria | 22 | 1.4285 × 10 −31 |
| Amino sugar metabolism | 33 | 1.1458 × 10 −31 |
| Glycine and serine metabolism | 59 | 1.5881 × 10 −31 |
| Glycerolipid metabolism | 25 | 6.7412 × 10 −32 |
| Purine metabolism | 74 | 1.3050 × 10 −31 |
| Fructose and mannose degradation | 32 | 3.8378 × 10 −32 |
| Warburg effect | 58 | 1.4761 × 10 −31 |
| Ammonia recycling | 32 | 1.8447 × 10 −31 |
| Cysteine metabolism | 26 | 1.9269 × 10 −31 |
| Alpha-linolenic acid and linoleic acid metabolism | 19 | 1.0902 × 10 −31 |
| b. Metabolic pathways of patients with CV-AEs | ||
| Transfer of acetyl groups into mitochondria | 22 | 9.8105 × 10 −32 |
| Amino sugar metabolism | 33 | 1.0445 × 10 −31 |
| Glycine and serine metabolism | 59 | 7.5674 × 10 −32 |
| Glycerolipid metabolism | 25 | 4.1298 × 10 −32 |
| Sphingolipid metabolism | 40 | 1.6407 × 10 −31 |
| Tryptophan metabolism | 60 | 6.8071 × 10 −31 |
| Citric acid cycle | 32 | 9.6546 × 10 −32 |
| Beta-alanine metabolism | 34 | 2.7986 × 10 −32 |
| Glutathione metabolism | 21 | 1.5996 × 10 −31 |
| Tyrosine and methionine metabolism | 43 | 1.2771 × 10 −32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caocci, G.; Deidda, M.; Noto, A.; Greco, M.; Simula, M.P.; Mulas, O.; Cocco, D.; Fattuoni, C.; Mercuro, G.; La Nasa, G.; et al. Metabolomic Analysis of Patients with Chronic Myeloid Leukemia and Cardiovascular Adverse Events after Treatment with Tyrosine Kinase Inhibitors. J. Clin. Med. 2020, 9, 1180. https://doi.org/10.3390/jcm9041180
Caocci G, Deidda M, Noto A, Greco M, Simula MP, Mulas O, Cocco D, Fattuoni C, Mercuro G, La Nasa G, et al. Metabolomic Analysis of Patients with Chronic Myeloid Leukemia and Cardiovascular Adverse Events after Treatment with Tyrosine Kinase Inhibitors. Journal of Clinical Medicine. 2020; 9(4):1180. https://doi.org/10.3390/jcm9041180
Chicago/Turabian StyleCaocci, Giovanni, Martino Deidda, Antonio Noto, Marianna Greco, Maria Pina Simula, Olga Mulas, Daniele Cocco, Claudia Fattuoni, Giuseppe Mercuro, Giorgio La Nasa, and et al. 2020. "Metabolomic Analysis of Patients with Chronic Myeloid Leukemia and Cardiovascular Adverse Events after Treatment with Tyrosine Kinase Inhibitors" Journal of Clinical Medicine 9, no. 4: 1180. https://doi.org/10.3390/jcm9041180
APA StyleCaocci, G., Deidda, M., Noto, A., Greco, M., Simula, M. P., Mulas, O., Cocco, D., Fattuoni, C., Mercuro, G., La Nasa, G., & Cadeddu Dessalvi, C. (2020). Metabolomic Analysis of Patients with Chronic Myeloid Leukemia and Cardiovascular Adverse Events after Treatment with Tyrosine Kinase Inhibitors. Journal of Clinical Medicine, 9(4), 1180. https://doi.org/10.3390/jcm9041180







