Deproteinization as a Rapid Method of Saliva Purification for the Determination of Carbamazepine and Carbamazepine-10,11 Epoxide
Abstract
:1. Introduction
2. Experimental Section
2.1. Instruments
2.2. Chemicals and Solvents
2.3. Saliva Sampling
2.4. Saliva Treatment
2.5. Method Validation
2.5.1. Linearity
2.5.2. Selectivity
2.5.3. Precision and Accuracy
2.5.4. Limits of Quantification
2.5.5. Absolute Recovery and Extraction Recovery
2.5.6. Stability
2.6. Clinical Application
3. Results
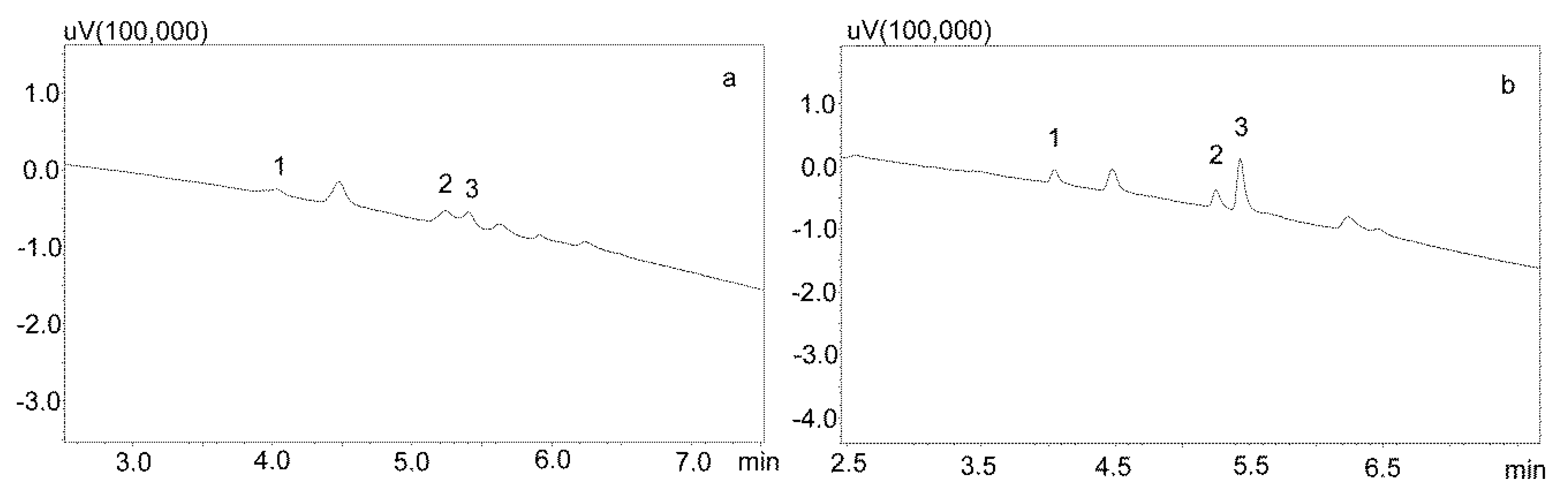
3.1. Chromatographic Analysis
3.2. Method Development
3.2.1. Extraction with Phree Columns
3.2.2. Modification of the Deproteinization Process
3.3. Method validation
3.4. Extraction and Absolute Recovery
3.5. Stability
3.6. Clinical Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caporossi, L.; Santoro, A.; Papaleo, B. Saliva as an analytical matrix: State of the art and application for biomonitoring. Biomarkers 2010, 15, 475–487. [Google Scholar] [CrossRef]
- Rylance, G.W.; Moreland, T.A. Saliva carbamazepine and phenytoin level monitoring. Arch. Dis. Child. 1981, 56, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Eeg-Olofsson, O.; Nilsson, H.L.; Tonnby, B.; Arvidsson, J.; Grahn, P.A.; Gylje, H.; Larsson, C.; Norén, L. Diurnal variation of carbamazepine and carbamazepine-10,11-epoxide in plasma and saliva in children with epilepsy: A comparison between conventional and slow-release formulations. J. Child. Neurol. 1990, 5, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.; Oldigs, H.-D.; Günther, E. Use of saliva in monitoring carbamazepine medication in epileptic children. Eur. J. Pediatr. 1977, 126, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rylance, G.W.; Butcher, G.M.; Moreland, T. Saliva carbamazepine levels in children. Br. Med. J. 1977, 2, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKichan, J.J.; Duffner, P.K.; Cohen, M.E. Salivary concentrations and plasma protein binding of carbamazepine and carbamazepine 10,11-epoxide in epileptic patients. Br. J. Clin. Pharmacol. 1981, 12, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Arranz Peña, M.I.; Saenz Lope, E. Can a single measurement of carbamazepine suffice for therapeutic monitoring? Clin. Chem. 1987, 33, 812–813. [Google Scholar] [CrossRef]
- Petit, P.; Lonjon, R.; Cociglio, M.; Sluzewska, A.; Blayac, J.P.; Hue, B.; Alric, R.; Pouget, R. Carbamazepine and its 10,11-epoxide metabolite in acute mania: Clinical and pharmacokinetic correlates. Eur. J. Clin. Pharmacol. 1991, 41, 541–546. [Google Scholar] [CrossRef]
- Callaghan, N.; Goggin, T. A comparison of plasma and saliva levels of carbamazepine and phenytoin as monotherapy. Ir. J. Med. Sci. 1984, 153, 170–173. [Google Scholar] [CrossRef]
- Datar, P.A. Quantitative bioanalytical and analytical method development of dibenzazepine derivative, carbamazepine: A review. J. Pharm. Anal. 2015, 5, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Patsalos, P.N.; Berry, D.J. Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther. Drug Monit. 2013, 35, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H. Carbamazepine. In Casebook in Clinical Pharmacokinetics and Drug Dosing; Cohen, H., Ed.; McGraw-Hill: New York, NY, USA, 2015; Available online: http://accesspharmacy.mhmedical.com/content.aspx?bookid=1514§ionid=88803447 (accessed on 8 January 2020).
- Ruiz, M.E.; Conforti, P.; Fagiolino, P.; Volonté, M.G. The use of saliva as a biological fluid in relative bioavailability studies: Comparison and correlation with plasma results. Biopharm. Drug Dispos. 2010, 31, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Singh, M.; Kaleekal, T.; Gupta, Y.K.; Tripathi, M. Concentration of antiepileptic drugs in persons with epilepsy: A comparative study in serum and saliva. Int. J. Neurosci. 2016, 126, 972–978. [Google Scholar] [CrossRef]
- Rylance, G.W.; Moreland, T.A.; Butcher, G.M. Carbamazepine dose-frequency requirement in children. Arch. Dis. Child. 1979, 54, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Moreland, T.A.; Priestman, D.A.; Rylance, G.W. Saliva carbamazepine levels in children before and during multiple dosing. Br. J. Clin. Pharmacol. 1982, 13, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Hockings, N.; Pall, A.; Moody, J.; Davidson, A.V.; Davidson, D.L. The effects of age on carbamazepine pharmacokinetics and adverse effects. Br. J. Clin. Pharmacol. 1986, 22, 725–728. [Google Scholar] [CrossRef] [Green Version]
- Miles, M.V.; Tennison, M.B.; Greenwood, R.S. Intraindividual variability of carbamazepine, phenobarbital, and phenytoin concentrations in saliva. Ther. Drug Monit. 1991, 13, 166–171. [Google Scholar] [CrossRef]
- Carvalho, J.; Rosado, T.; Barroso, M.; Gallardo, E. Determination of antiepileptic drugs using dried saliva spots. J. Anal. Toxicol. 2019, 43, 61–71. [Google Scholar] [CrossRef]
- Chee, K.Y.; Lee, D.; Byron, D.; Naidoo, D.; Bye, A. A simple collection method for saliva in children: Potential for home monitoring of carbamazepine therapy. Br. J. Clin. Pharmacol. 1993, 35, 311–313. [Google Scholar] [CrossRef] [Green Version]
- Dziurkowska, E.; Wesolowski, M. Solid phase extraction purification of saliva samples for antipsychotic drug quantitation. Molecules 2018, 23, 2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziurkowska, E.; Wesolowski, M. Simultaneous quantification of antipsychotic and antiepileptic drugs and their metabolites in human saliva using UHPLC-DAD. Molecules 2019, 24, 2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexishvili, M.M.; Rukhadze, M.D.; Okujava, V.M. Simultaneous determination of carbamazepine and carbamazepine 10,11-epoxide by using microcolumn HPLC: Study of pharmacokinetics of carbamazepine in a volunteer. Biomed. Chromatogr. 1997, 11, 36–41. [Google Scholar] [CrossRef]
- Dordević, S.; Kilibarda, V.; Stojanović, T. Determination of carbamazepine in serum and saliva samples by high performance liquid chromatography with ultraviolet detection. Vojnosanit. Pregl. 2009, 66, 347–352. [Google Scholar]
- Rukhadze, M.D.; Tsagareli, S.K.; Sidamonidze, N.S.; Meyer, V.R. Cloud-point extraction for the determination of the free fraction of antiepileptic drugs in blood plasma and saliva. Anal. Biochem. 2000, 287, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, R.F.; Ouvrier, R.A. Salivary anticonvulsant levels in children: A comparison of methods. Ther. Drug Monit. 1981, 3, 151–157. [Google Scholar] [CrossRef] [PubMed]





| Time (min) | Gradient (Percentage of Solvent B by Volume) |
|---|---|
| 0.01 | 10 |
| 0.5 | 10 |
| 10.0 | 55 |
| 12.0 | 90 |
| 12.2 | 10 |
| 15.0 | 10 |
| Calibration curve y = ax + b (n = 4) | Carbamazepine | Carbamazepine-10,11 epoxide |
|---|---|---|
| Range (ng/mL) | 10–5000 | 10–5000 |
| Determination Coefficient (R2) | 0.99958 ± 0.000171 | 0.99975 ± 0.000006 |
| Slope a ± SD | 0.00025 ± 0.0001 | 0.000175 ± 0.00015 |
| Intercept b ± SD | 0.004825 ± 0.002326 | 0.004875 ± 0.003333 |
| LOQ (ng/mL) | 10 | 10 |
| Analyte | Carbamazepine | Carbamazepine-10,11 epoxide | ||||
|---|---|---|---|---|---|---|
| Quality Concentration | Intra-day (% CV) | Inter-day (% CV) | Accuracy (%) | Intra-day (% CV) | Inter-day (% CV) | Accuracy (%) |
| Low (30 ng/mL) | 6.39 | 12.82 | 95.35 | 6.02 | 13.58 | 109.28 |
| Medium (2000 ng/mL) | 4.97 | 5.32 | 98.51 | 4.18 | 3.14 | 97.59 |
| High (3750 ng/mL) | 2.69 | 2.67 | 98.65 | 2.67 | 2.77 | 97.93 |
| Analyte | Carbamazepine | Carbamazepine-10,11 epoxide | ||
|---|---|---|---|---|
| Concentration (ng/mL) | 30 | 3750 | 30 | 3750 |
| Extraction Recovery (%) | 99.09 | 101.19 | 95.89 | 97.37 |
| Absolute Recovery (%) | 82.19 | 97.07 | 81.13 | 92.61 |
| Analyte | −21 °C | 8 °C | 15 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 2000 | 3750 | 30 | 2000 | 3750 | 30 | 2000 | 3750 | |
| Difference (%) | |||||||||
| Carbamazepine | −2.25 | −2.16 | −1.44 | −3.91 | −2.53 | −1.93 | −0.30 | −1.12 | −1.72 |
| Carbamazepine-10,11 epoxide | −2.45 | −1.92 | −0.48 | −6.23 | −3.56 | −2.75 | −3.64 | −2.3 | 0.38 |
| Patient | Gender | Age (Year) | Dose (mg/day) | Carbamazepine Concentration (ng/mL) | Carbamazepine-10,11 epoxide Concentration (ng/mL) |
|---|---|---|---|---|---|
| 1 | M | 31 | 800 | 1192.3 | 231.8 |
| 2 | M | 44 | 400 | 1817.8 | 428.0 |
| 3 | M | 50 | 400 | 4891.0 | 226.8 |
| 4 | M | 58 | 800 | 4839.8 | 348.5 |
| 5 | M | 49 | 700 | 1165.7 | 229.8 |
| 6 | M | 33 | 400 | 262.2 | 120.7 |
| 7 | M | 46 | 800 | 2334.2 | 664.1 |
| 8 | M | 44 | 800 | 344.7 | 122.0 |
| 9 | M | 55 | 400 | 690.7 | 168.9 |
| 10 | M | 51 | 800 | 1285.1 | 448.3 |
| 11 | F | 22 | 1200 | 464.0 | 161.1 |
| 12 | F | 49 | 400 | 282.2 | 127.5 |
| 13 | F | 26 | 600 | 472.9 | 172.9 |
| 14 | F | 37 | 1200 | 2456.8 | 559.1 |
| 15 | F | 31 | 1200 | 1668.1 | 569.1 |
| 16 | F | 29 | 1800 | 613.5 | 182.1 |
| 17 | F | 19 | 600 | 784.3 | 273.3 |
| 18 | F | 31 | 800 | 1119.9 | 152.7 |
| 19 | F | 69 | 900 | 607.6 | 147.7 |
| 20 | F | 30 | 400 | 1198.2 | 142.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurkowska, E.; Wesolowski, M. Deproteinization as a Rapid Method of Saliva Purification for the Determination of Carbamazepine and Carbamazepine-10,11 Epoxide. J. Clin. Med. 2020, 9, 915. https://doi.org/10.3390/jcm9040915
Dziurkowska E, Wesolowski M. Deproteinization as a Rapid Method of Saliva Purification for the Determination of Carbamazepine and Carbamazepine-10,11 Epoxide. Journal of Clinical Medicine. 2020; 9(4):915. https://doi.org/10.3390/jcm9040915
Chicago/Turabian StyleDziurkowska, Ewelina, and Marek Wesolowski. 2020. "Deproteinization as a Rapid Method of Saliva Purification for the Determination of Carbamazepine and Carbamazepine-10,11 Epoxide" Journal of Clinical Medicine 9, no. 4: 915. https://doi.org/10.3390/jcm9040915
APA StyleDziurkowska, E., & Wesolowski, M. (2020). Deproteinization as a Rapid Method of Saliva Purification for the Determination of Carbamazepine and Carbamazepine-10,11 Epoxide. Journal of Clinical Medicine, 9(4), 915. https://doi.org/10.3390/jcm9040915






