Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug–Drug Interactions
Abstract
:1. Introduction
2. Approach
3. Discussion
3.1. Regulatory Environment of THC-Containing Products
3.2. Potential for Adverse Drug Events and Drug–Drug Interactions
3.2.1. Molecular Targets of THC
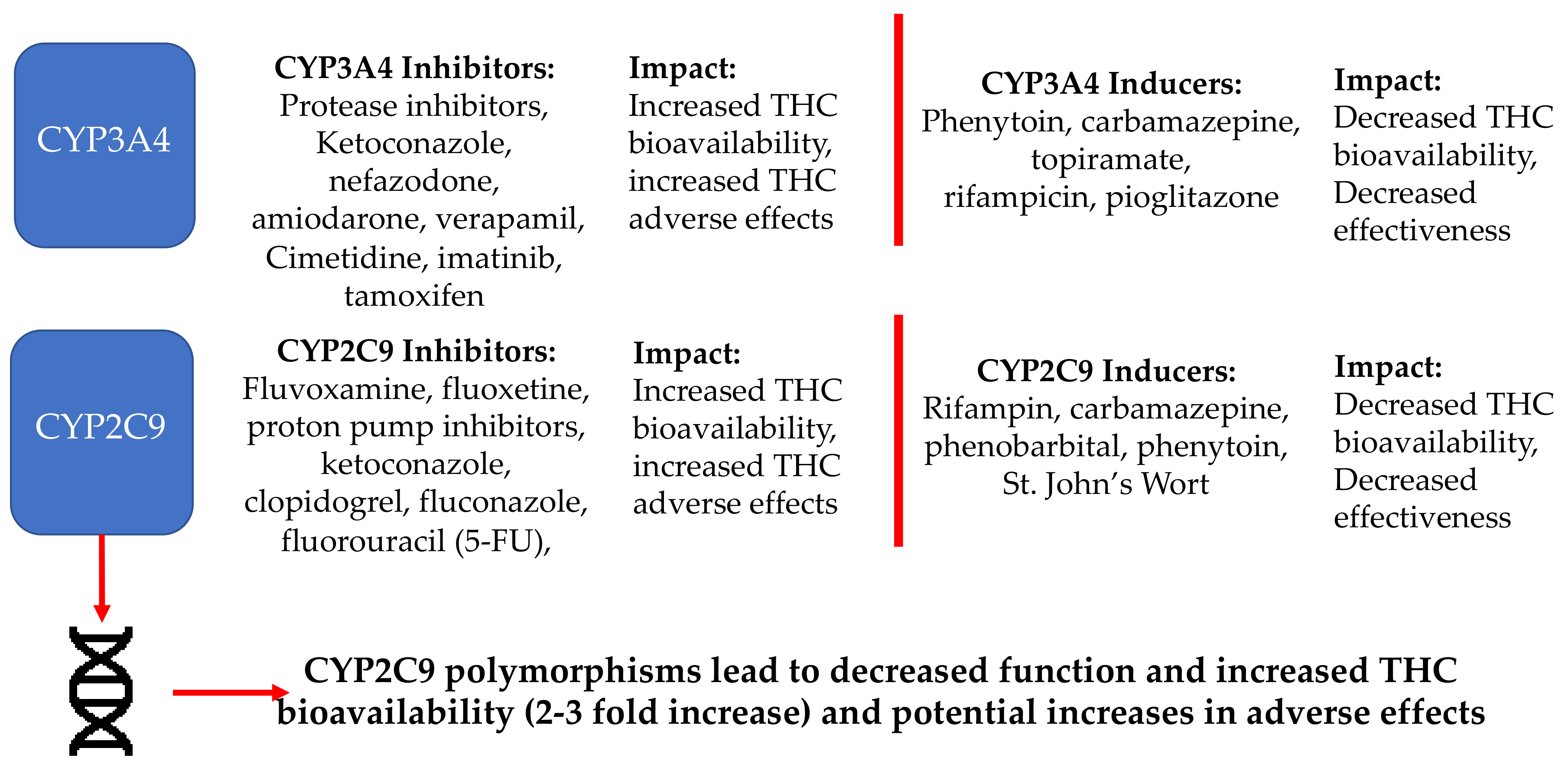
3.2.2. Metabolic Inhibition and Induction
3.2.3. Synergistic Pharmacodynamic Effects
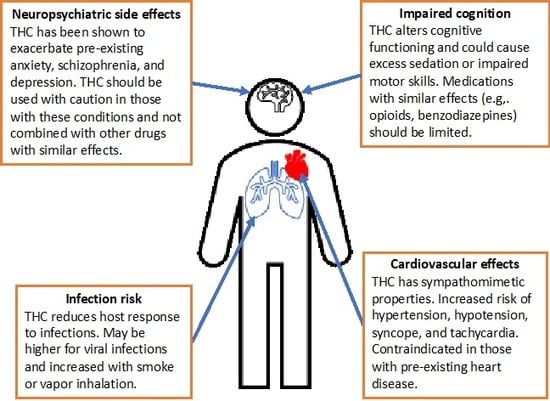
3.2.4. Neuropsychiatric Side Effects
3.2.5. Cognitive Adverse Reactions
3.2.6. Cardiovascular Side Effects
3.2.7. Infection Risk
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- State Medical Marijuana Laws. National Conference of State Legislatures (NCSL). Available online: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed on 9 June 2019).
- Denham, B.E. Attitudes toward legalization of marijuana in the United States, 1986–2016: Changes in determinants of public opinion. Int. J. Drug Policy 2019, 71, 78–90. [Google Scholar] [CrossRef]
- Cerda, M.; Wall, M.; Keyes, K.M.; Galea, S.; Hasin, D. Medical marijuana laws in 50 states: Investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012, 120, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.; Renstrom, M.; Poznyak, V. (Eds.) The Health and Social Effects of Nonmedical Cannabis Use; World Health Organization: Geneve, Switzerland, 2016. [Google Scholar]
- Hall, W.; Weier, M. Assessing the public health impacts of legalizing recreational cannabis use in the USA. Clin. Pharmacol. Ther. 2015, 97, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.D.; Resko, S.M.; Szechy, K.; Smith, R.; Early, T.J. Characteristics Associated with Attitudes toward Marijuana Legalization in Michigan. J. Psychoact. Drugs 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Casarett, D. Medical Marijuana Use in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sativex(R) (delta-9-tetrahydrocannabinol and cannabidiol). GW Pharma Ltd. Available online: https://www.bayer.ca/omr/online/sativex-pm-en.pdf (accessed on 9 June 2019).
- Drug Approval Package: Epidiolex (Cannabidiol). GW Research Ltd. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000TOC.cfm (accessed on 9 June 2019).
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef]
- Yamaori, S.; Koeda, K.; Kushihara, M.; Hada, Y.; Yamamoto, I.; Watanabe, K. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab. Pharmacokinet. 2012, 27, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Yamaori, S.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Characterization of the structural determinants required for potent mechanism-based inhibition of human cytochrome P450 1A1 by cannabidiol. Chem. Biol. Interact. 2014, 215, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Yamaori, S.; Kushihara, M.; Yamamoto, I.; Watanabe, K. Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem. Pharmacol. 2010, 79, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab. Dispos. 2011, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamaschi, M.M.; Queiroz, R.H.; Zuardi, A.W.; Crippa, J.A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Lindblad, C.I.; Hanlon, J.T.; Gross, C.R.; Sloane, R.J.; Pieper, C.F.; Hajjar, E.R.; Ruby, C.M.; Schmader, K.E.; Multidisciplinary Consensus, P. Clinically important drug-disease interactions and their prevalence in older adults. Clin. Ther. 2006, 28, 1133–1143. [Google Scholar] [CrossRef]
- Mallet, L.; Spinewine, A.; Huang, A. The challenge of managing drug interactions in elderly people. Lancet 2007, 370, 185–191. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Semla, T.P.; Schmader, K.E. Alternative Medications for Medications in the Use of High-Risk Medications in the Elderly and Potentially Harmful Drug-Disease Interactions in the Elderly Quality Measures. J. Am. Geriatr. Soc. 2015, 63, e8–e18. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Public Law No: 115-334. Agriculture Improvement Act of 2018. In Proceedings of the 115th Congress of the United States of America (2017–2018); 20 December 2018.
- SYNDROS (Dronabinol) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205525s003lbl.pdf (accessed on 20 February 2020).
- MARINOL (Dronabinol) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 20 February 2020).
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Zhang, J.; Fan, N.; Teng, Z.Q.; Wu, Y.; Yang, H.; Tang, Y.P.; Sun, H.; Song, Y.; Chen, C. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 2013, 155, 1154–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [Green Version]
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 2009, 55, 2180–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemberger, L.; Martz, R.; Rodda, B.; Forney, R.; Rowe, H. Comparative pharmacology of Delta9-tetrahydrocannabinol and its metabolite, 11-OH-Delta9-tetrahydrocannabinol. J. Clin. Investig. 1973, 52, 2411–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wang, J.; Huang, S.Q.; Su, H.H.; Zhou, S.F. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr. Drug Metab. 2009, 10, 781–834. [Google Scholar] [CrossRef]
- Lee, C.R.; Goldstein, J.A.; Pieper, J.A. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharmacogenetics 2002, 12, 251–263. [Google Scholar] [CrossRef]
- Tournier, N.; Chevillard, L.; Megarbane, B.; Pirnay, S.; Scherrmann, J.M.; Decleves, X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int. J. Neuropsychopharmacol. 2010, 13, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Stott, C.; White, L.; Wright, S.; Wilbraham, D.; Guy, G. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus 2013, 2, 236. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, J.C.; Ji, X.; Guo, H.L.; Wang, X.; Zhang, B.; Wang, T.; Chen, F. Potential Pharmacokinetic Herb-Drug Interactions: Have we Overlooked the Importance of Human Carboxylesterases 1 and 2? Curr. Drug Metab. 2019, 20, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, X.; Markowitz, J.S. In Vitro Inhibition of Carboxylesterase 1 by Major Cannabinoids and Selected Metabolites. Drug Metab. Dispos. 2019, 47, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Hall, W.D.; Peacock, A.; Lintzeris, N.; Bruno, R.; Larance, B.; Nielsen, S.; Cohen, M.; Chan, G.; Mattick, R.P.; et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018, 3, e341–e350. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Cuttler, C.; Spradlin, A.; McLaughlin, R.J. A naturalistic examination of the perceived effects of cannabis on negative affect. J. Affect. Disord. 2018, 235, 198–205. [Google Scholar] [CrossRef]
- EPIDIOLEX (Cannabidiol) Prescribing Information. Available online: https://www.epidiolex.com/sites/default/files/EPIDIOLEX_Full_Prescribing_Information.pdf (accessed on 9 June 2019).
- Fassberg, M.M.; Cheung, G.; Canetto, S.S.; Erlangsen, A.; Lapierre, S.; Lindner, R.; Draper, B.; Gallo, J.J.; Wong, C.; Wu, J.; et al. A systematic review of physical illness, functional disability, and suicidal behaviour among older adults. Aging Ment. Health 2016, 20, 166–194. [Google Scholar] [CrossRef]
- Ferro, M.A. Major depressive disorder, suicidal behaviour, bipolar disorder, and generalised anxiety disorder among emerging adults with and without chronic health conditions. Epidemiol. Psychiatr. Sci. 2016, 25, 462–474. [Google Scholar] [CrossRef]
- Brzozowska, N.I.; de Tonnerre, E.J.; Li, K.M.; Wang, X.S.; Boucher, A.A.; Callaghan, P.D.; Kuligowski, M.; Wong, A.; Arnold, J.C. The Differential Binding of Antipsychotic Drugs to the ABC Transporter P-Glycoprotein Predicts Cannabinoid-Antipsychotic Drug Interactions. Neuropsychopharmacology 2017, 42, 2222–2231. [Google Scholar] [CrossRef]
- Bonar, E.E.; Cranford, J.A.; Arterberry, B.J.; Walton, M.A.; Bohnert, K.M.; Ilgen, M.A. Driving under the influence of cannabis among medical cannabis patients with chronic pain. Drug Alcohol Depend. 2019, 195, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Goodin, A.J. Letter to the editor: The prevalence of drivers under the influence of medical cannabis must be considered within proper context. Res. Soc. Adm. Pharm. 2019. [Google Scholar] [CrossRef]
- Byrne, C.J.; Walsh, C.; Cahir, C.; Ryan, C.; Williams, D.J.; Bennett, K. Anticholinergic and sedative drug burden in community-dwelling older people: A national database study. BMJ Open 2018, 8, e022500. [Google Scholar] [CrossRef]
- Lunn, C.A.; Reich, E.P.; Bober, L. Targeting the CB2 receptor for immune modulation. Expert Opin. Ther. Targets 2006, 10, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; Friedman, H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003, 74, 486–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, C.S. Cannabinoids and Viral Infections. Pharmaceuticals 2010, 3, 1873–1886. [Google Scholar] [CrossRef] [PubMed]


| Product (Approval Date) | Active Ingredient(s) | Dosage Form | Route | Recommended Dose | Indication(s) |
|---|---|---|---|---|---|
| SATIVEX a (2011–12) | Delta-9-THC and cannabidiol | Solution, spray | Buccal Spray | Titrated up to 12 sprays per day (patient median is 4–8 sprays). 2.7 mg THC and 2.5 mg CBD per spray. | Adjunctive treatment of spasticity and neuropathic pain in MS |
| Adjunctive analgesic for moderate to severe pain in advanced cancer | |||||
| MARINOL (1985) | Dronabinol b | Capsules | Oral | 2.5 mg 2× daily; max 5 mg 2× daily | Anorexia associated with AIDS |
| 5 mg/m2 4–6× daily; max 15 mg/m2 4–6× daily | Nausea and vomiting with chemotherapy in patients for whom conventional treatment failed | ||||
| SYNDROS (1985) | Dronabinol b | Solution | Oral | 2.1 mg 2× daily; max 8.4 mg daily | Anorexia associated with AIDS |
| 4.2 mg/m2 4–6× per day; max 12.6 mg/m2 4–6× per day | Nausea and vomiting with chemotherapy in patients for whom conventional treatment failed |
| Product | Population Studied | Interacting Drugs | Results |
|---|---|---|---|
| THC+CBD mucosal spray (Sativex), 4 sprays | Healthy Adults (n = 36) | Rifampicin 600 mg (CYP3A, CYP2C19 inducer) | THC: 36% decrease 11-OH-THC: 87% decrease |
| Ketoconazole 400 mg (CYP3A inhibitor) | THC: 27% increase 11-OH-THC: 204% increase | ||
| Omeprazole 40 mg (CYP2C19 inhibitor) | No change in THC or 11-OH-THC |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, J.D. Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug–Drug Interactions. J. Clin. Med. 2020, 9, 919. https://doi.org/10.3390/jcm9040919
Brown JD. Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug–Drug Interactions. Journal of Clinical Medicine. 2020; 9(4):919. https://doi.org/10.3390/jcm9040919
Chicago/Turabian StyleBrown, Joshua D. 2020. "Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug–Drug Interactions" Journal of Clinical Medicine 9, no. 4: 919. https://doi.org/10.3390/jcm9040919
APA StyleBrown, J. D. (2020). Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug–Drug Interactions. Journal of Clinical Medicine, 9(4), 919. https://doi.org/10.3390/jcm9040919