Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TQ-6 Synthesis and Microglia Cultivation
2.3. Cell Viability Assay
2.4. Determination of NO Production
2.5. Western Blotting Analysis
2.6. Confocal Immunofluorescence Staining Assay
2.7. Animals
2.8. Middle Cerebral Artery Occlusion-Induced Cerebral Ischemia in Mice
2.9. Measurement of Brain Infarct Volume and Edema
2.10. Platelet Aggregation
2.11. Detection of Hydroxyl Radicals through Electron Spin Resonance Spectrometry
2.12. Statistical Analysis
3. Results
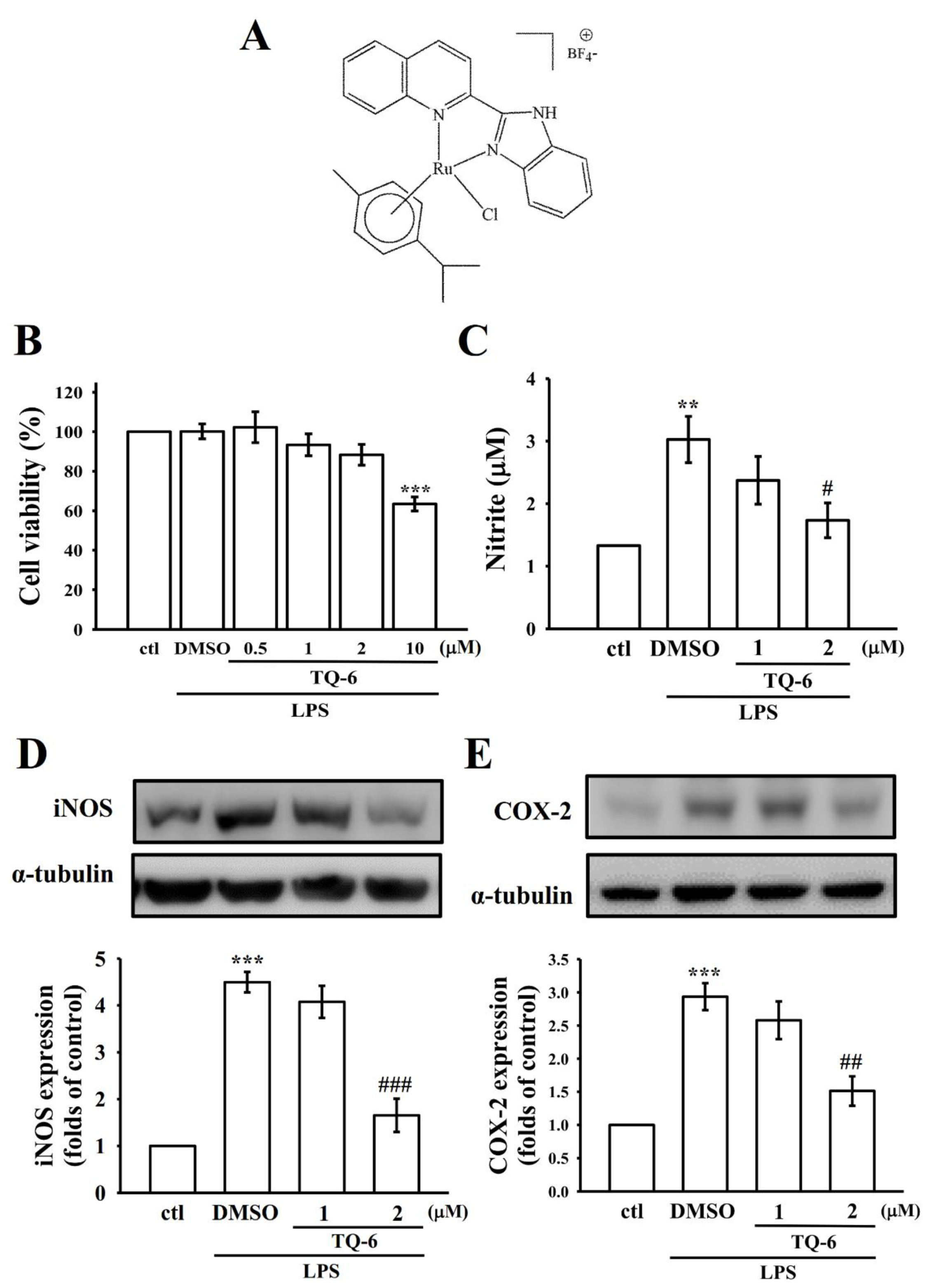
3.1. TQ-6 Reduced NO Production and Attenuated Inflammatory Markers in Microglia
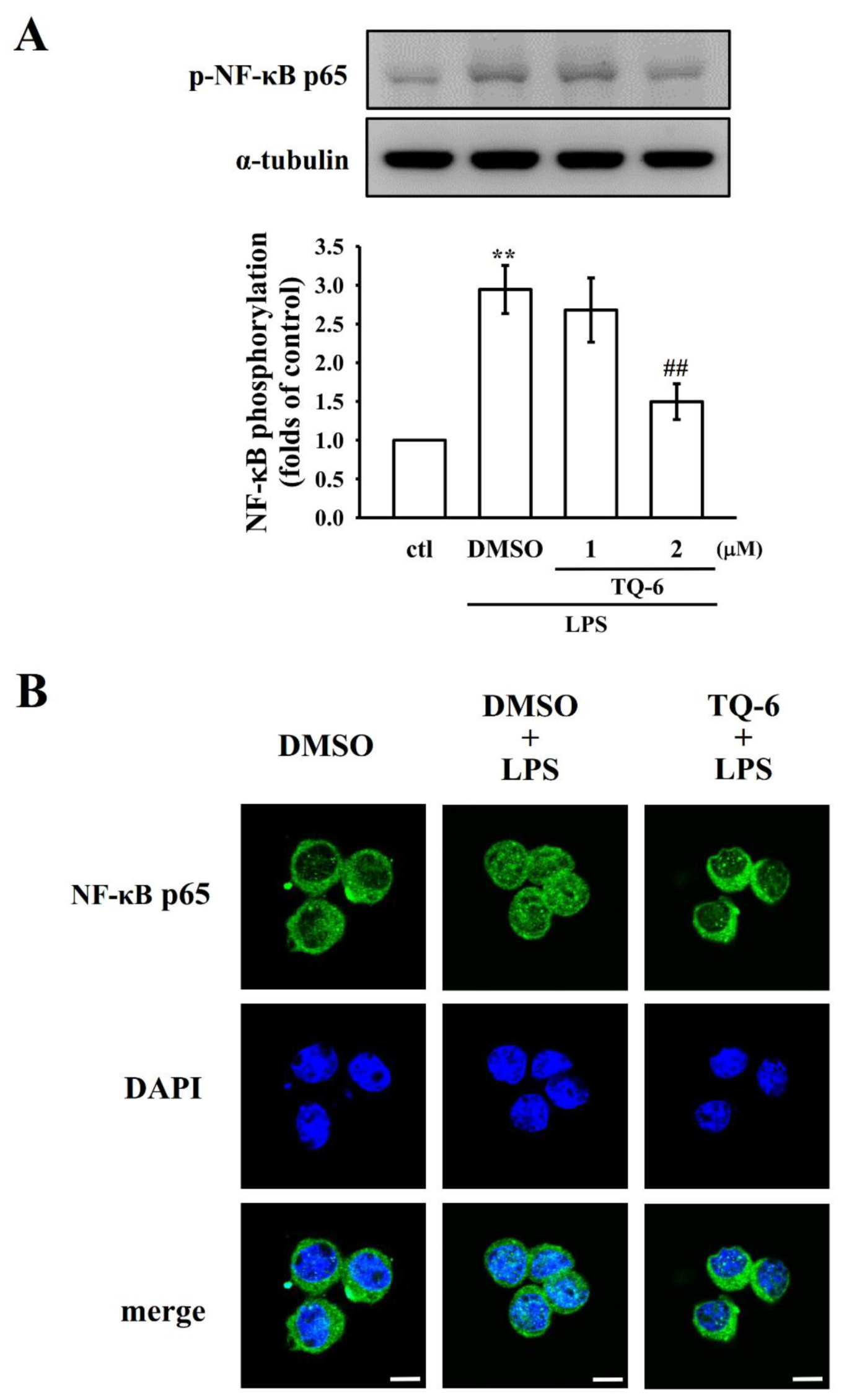
3.2. NF-κB p65 Phosphorylation and Nuclear Translocation Were Suppressed by TQ-6
3.3. TQ-6 Enhanced LPS-Mediated Nrf2/HO-1 Expression and Inhibited OH• Formation
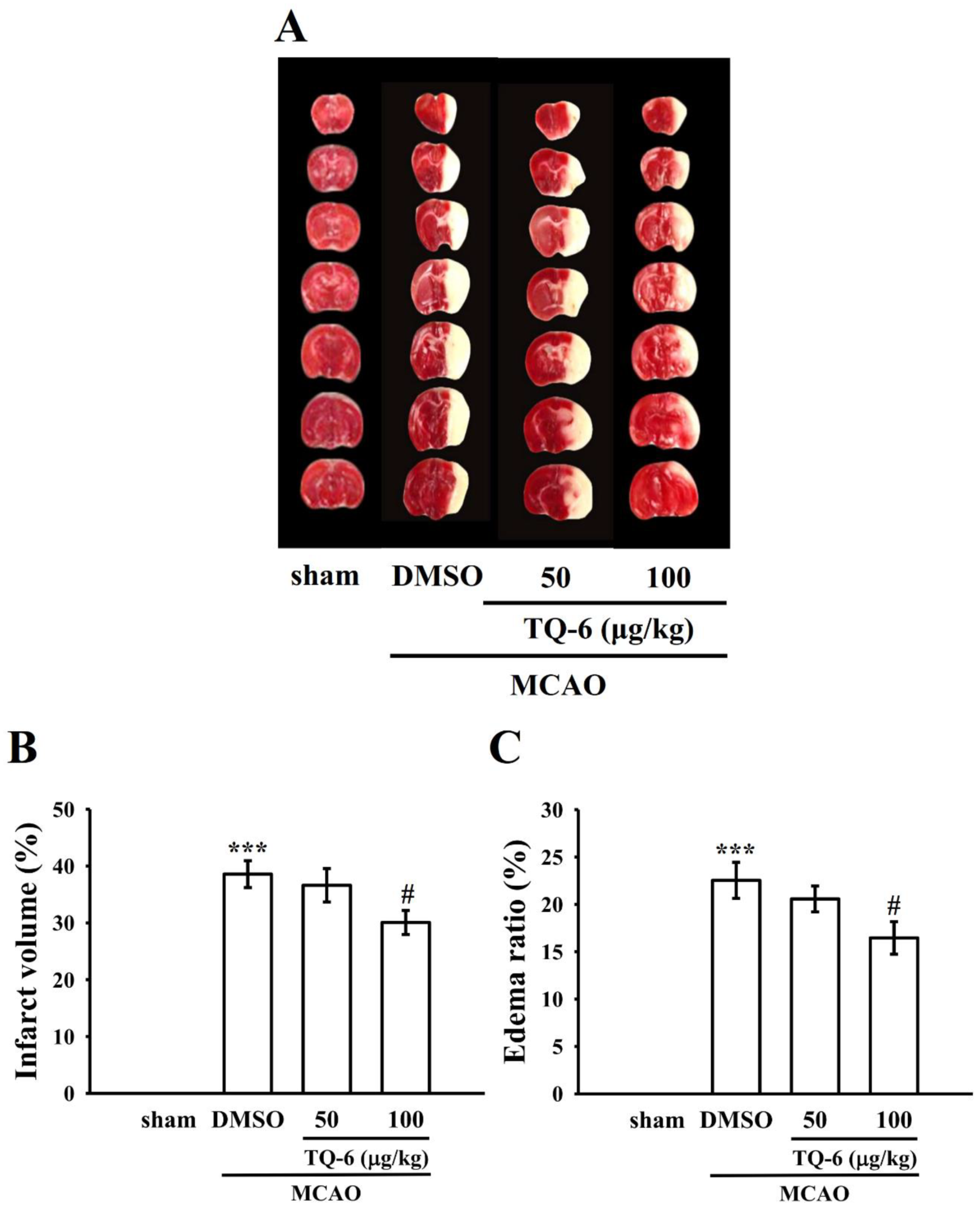
3.4. TQ-6 Diminished MCAO-Induced Brain Infarct Volume and Edema in Mice
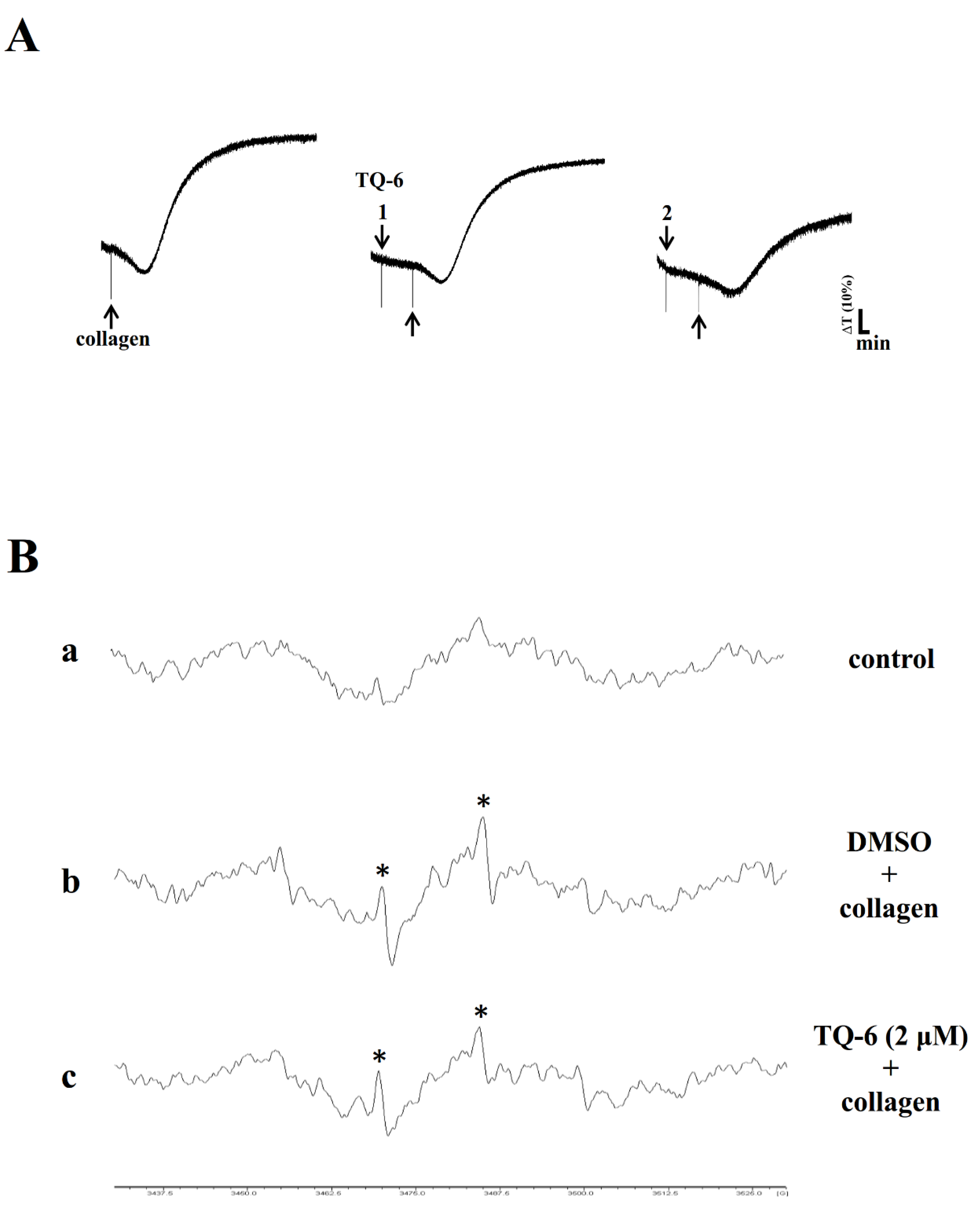
3.5. TQ-6 Inhibited Aggregation and OH• Formation in Mice Platelets
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cianciulli:, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signaling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Kim, I.S.; Koppulla, S.; Kim, B.W.; Choi, D.K. Strategic selection of neuroinflammatory models in Parkinson’s disease: Evidence from experimental studies. CNS Neurol. Disord. Drug Targets 2013, 12, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Picascia, A.; Grimaldi, V.; Iannone, C.; Soricell, A.; Napoli, C. Innate and adaptive immune response in stroke: Focus on epigenetic regulation. J. Neuroimmunol. 2015, 289, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Thored, P.; Heldmann, U.; Gomes-Leal, W.; Gisler, R.; Darsalia, V.; Taneera, J.; Nygren, J.M.; Jacobsen, S.E.; Ekdahl, C.T.; Kokaia, Z.; et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 2009, 57, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Phillip, G.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Wang, X.; Zhu, L.; Mao, L. The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012, 2012, 217580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Fumagalli, S.; DeSimoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.M.; Shu, X.D.; Li, H.Q.; Sun, Y.; Shan, H.; Sun, X.Y.; Du, R.H.; Lu, M.; Xiao, M.; Ding, J.H.; et al. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci. Ther. 2016, 22, 729–739. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Castonguay, A.; Doucet, C.; Juhas, M.; Maysinger, D. New Ruthenium (II)-Letrozole Complexes as Anticancer Therapeutics. J. Med. Chem. 2012, 55, 8799–8806. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru (II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Hsia, C.H.; Velusamy, M.; Sheu, J.R.; Khamrang, T.; Jayakumar, T.; Lu, W.J.; Lin, K.H.; Chang, C.C. A novel ruthenium (II)-derived organometallic compound, TQ-6, potently inhibits platelet aggregation: Ex vivo and in vivo studies. Sci. Rep. 2017, 7, 9556. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hsu, C.Y.; Khamrang, T.; Hsia, C.H.; Hsia, C.W.; Manubolu, M.; Sheu, J.R. Possible molecular targets of novel ruthenium complexes in antiplatelet therapy. Int. J. Mol. Sci. 2018, 19, 1818. [Google Scholar] [CrossRef]
- Sheu, J.R.; Chen, Z.C.; Hsu, M.J.; Wang, S.H.; Jung, K.W.; Wu, W.F.; Pan, S.H.; Teng, R.D.; Yang, C.H.; Hsieh, C.Y. CME-1, a novel polysaccharide, suppresses iNOS expression in lipopolysaccharide-stimulated macrophages through ceramide-initiated protein phosphatase 2A activation. J. Cell. Mol. Med. 2018, 22, 999–1013. [Google Scholar] [CrossRef]
- Yen, T.L.; Chen, R.J.; Jayakumar, T.; Lu, W.J.; Hsieh, C.Y.; Hsu, M.J.; Yang, C.H.; Chang, C.C.; Lin, Y.K.; Lin, K.H.; et al. Andrographolide stimulates p38 mitogen-activated protein kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Transl. Res. 2016, 170, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Lin, K.C.; Liu, C.P.; Lin, C.Y.; Wu, H.C.; Chou, D.S.; Geraldine, P.; Huang, S.Y.; Hsieh, C.Y.; Sheu, J.R. Prevention of arterial thrombosis by nobiletin: In vitro and in vivo studies. J. Nutr. Biochem. 2016, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Taylor, J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444. [Google Scholar] [CrossRef]
- Chou, D.S.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.F.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]
- Stoll, G.; Kleinschnitz, C.; Nieswandt, B. Molecular mechanisms of thrombus formation in ischemic stroke: Novel insights and targets for treatment. Blood 2008, 112, 3555–3562. [Google Scholar] [CrossRef]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and systemic endotoxin challenges exacerbate the local infl ammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef] [PubMed]
- Jordán, J.; Segura, T.; Brea, D.; Galindo, M.F.; Castillo, J. Inflammation as therapeutic objective in stroke. Curr. Pharm. Des. 2008, 14, 3549–3564. [Google Scholar] [CrossRef]
- Rock, R.B.; Peterson, P.K. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J. Neuroimmune Pharmacol. 2006, 1, 117–126. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Boje, K.M.; Arora, P.K. Microglia-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Hsiao, H.Y.; Mak, O.T. Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Kapoor, S.; Singh, S.; Chatterji, B.P.; Panda, D. Transcription factor NF-jB associates with microtubules and stimulates apoptosis in response to suppression of microtubule dynamics in MCF-7 cells. Biochem. Pharmacol. 2015, 93, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.W.; Cheon, S.Y.; Park, S.Y.; Song, J.; Lee, J.H. Tryptanthrin suppresses the activation of the LPS-treated BV2 microglial cell line via Nrf2/HO-1 antioxidant signaling. Front. Cell Neurosci. 2017, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Lin, S.Z.; Chang, N.C. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/ HO-1 signaling. Free Radic. Biol. Med. 2014, 77, 71–81. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; Garcia-Yague, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef]
- Velagapudi, R.; Kumar, A.; Bhatia, H.S.; El-Bakoush, A.; Lepiarz, I.; Fiebich, B.L.; Olajide, O.A. Inhibition of neuroinflammation by thymoquinone requires activation of Nrf2/ARE signalling. Int. Immunopharmacol. 2017, 48, 17–29. [Google Scholar] [CrossRef]
- Campanella, M.; Sciorati, C.; Tarozzo, G.; Beltramo, M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke 2002, 33, 586–592. [Google Scholar] [CrossRef]
- Lalancette-Hébert, M.; Swarup, V.; Beaulieu, J.M.; Bohacek, I.; Abdelhamid, E.; Weng, Y.C.; Sato, S.; Kriz, J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 2012, 32, 10383–10395. [Google Scholar] [CrossRef]
- Hoonur, R.S.; Patil, B.R.; Badiger, D.S.; Vadavi, R.S.; Gudasi, K.B.; Dandawate, P.R.; Ghaisas, M.M.; Padhye, S.B.; Nethaji, M. Transition metal complexes of 3-aryl-2-substituted 1,2-dihydroquinazolin-4(3H)-one derivatives: New class of analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2010, 45, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Choi, K.H.; Park, M.S.; Lee, J.S.; Saver, J.L.; Cho, K.H. Clinical significance of acute and serial platelet function testing in acute ischemic stroke. J. Am. Heart Assoc. 2018, 7, e008313. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomized trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- Hackam, D.G.; Spence, J.D. Antiplatelet Therapy in Ischemic Stroke and Transient Ischemic Attack. Stroke 2019, 50, 773–778. [Google Scholar] [CrossRef]
- Xie, W.; Zheng, F.; Zhong, B.; Song, X. Long-Term Antiplatelet Mono- and Dual Therapies after Ischemic Stroke or Transient Ischemic Attack: Network Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002259. [Google Scholar] [CrossRef]
- Oliver, C.N.; Starke-Reed, P.E.; Stadtman, E.R.; Liu, G.J.; Carney, J.M.; Floyd, R.A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion induced injury to gerbil brain. Proc. Natl. Acad. Sci. USA 1990, 87, 5144–5147. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, C.-H.; Jayakumar, T.; Sheu, J.-R.; Hsia, C.-W.; Huang, W.-C.; Velusamy, M.; Lien, L.-M. Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation. J. Clin. Med. 2020, 9, 996. https://doi.org/10.3390/jcm9040996
Hsia C-H, Jayakumar T, Sheu J-R, Hsia C-W, Huang W-C, Velusamy M, Lien L-M. Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation. Journal of Clinical Medicine. 2020; 9(4):996. https://doi.org/10.3390/jcm9040996
Chicago/Turabian StyleHsia, Chih-Hsuan, Thanasekaran Jayakumar, Joen-Rong Sheu, Chih-Wei Hsia, Wei-Chieh Huang, Marappan Velusamy, and Li-Ming Lien. 2020. "Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation" Journal of Clinical Medicine 9, no. 4: 996. https://doi.org/10.3390/jcm9040996
APA StyleHsia, C.-H., Jayakumar, T., Sheu, J.-R., Hsia, C.-W., Huang, W.-C., Velusamy, M., & Lien, L.-M. (2020). Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation. Journal of Clinical Medicine, 9(4), 996. https://doi.org/10.3390/jcm9040996






