Visual Feedback and Postural Control in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
- PwMS with visual impairment: no visual impairment if visual function system was 0; with visual impairment if the visual function system was ≥1.
- PwMS with cerebellar impairment: no cerebellar impairment if cerebellar function system was 0; with cerebellar impairment if the cerebellar function system was ≥1.
- PwMS with sensory impairment: no sensory impairment if sensory function system was 0; with visual impairment if sensory function system was ≥1.
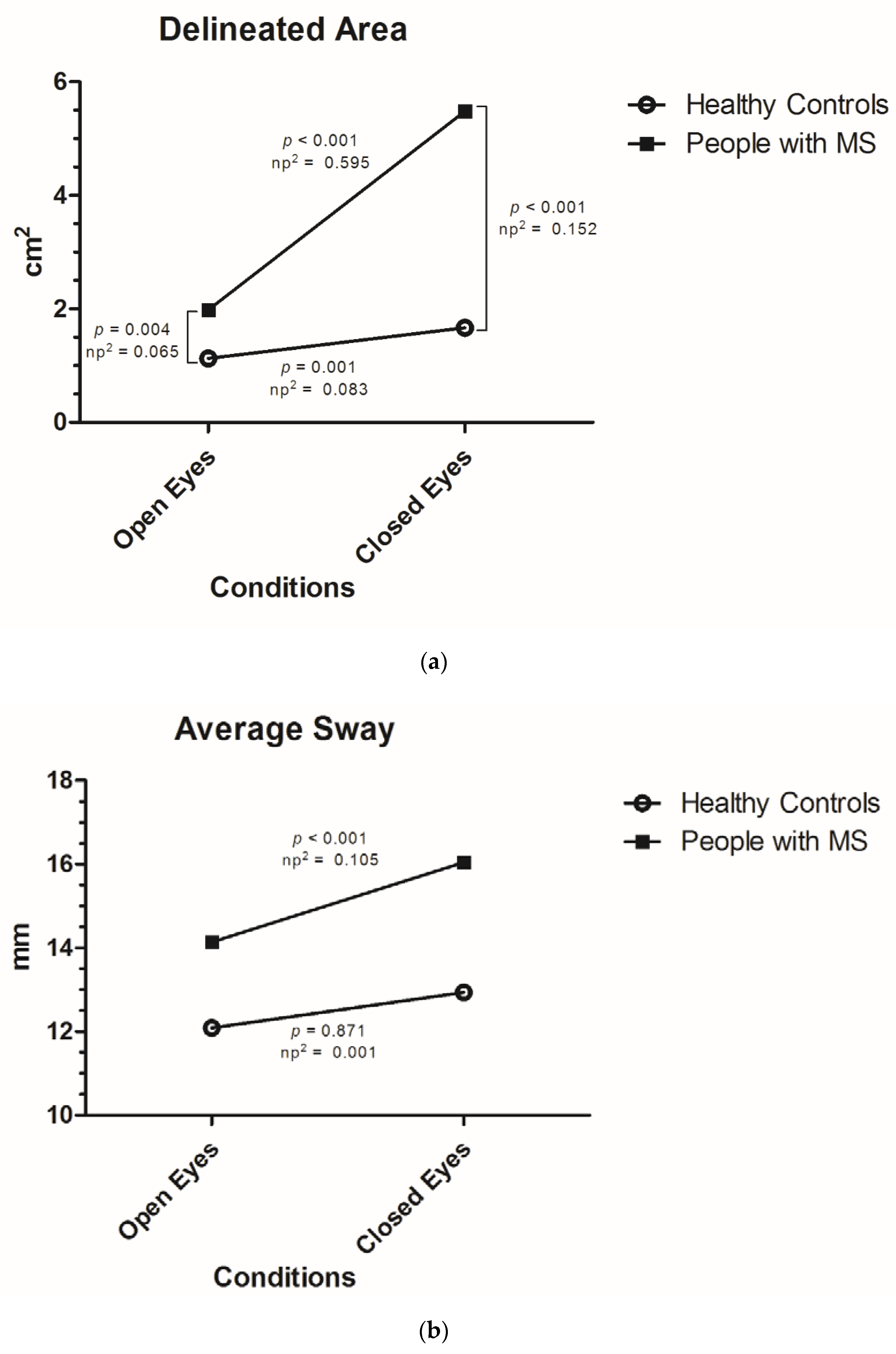
- Delineated area: total described surface during the measurement of the center of gravity of the subject calculated with 95% confidence interval (measured in cm2).
- Average sway: average distance or fluctuation from the center of all measurements (in mm).
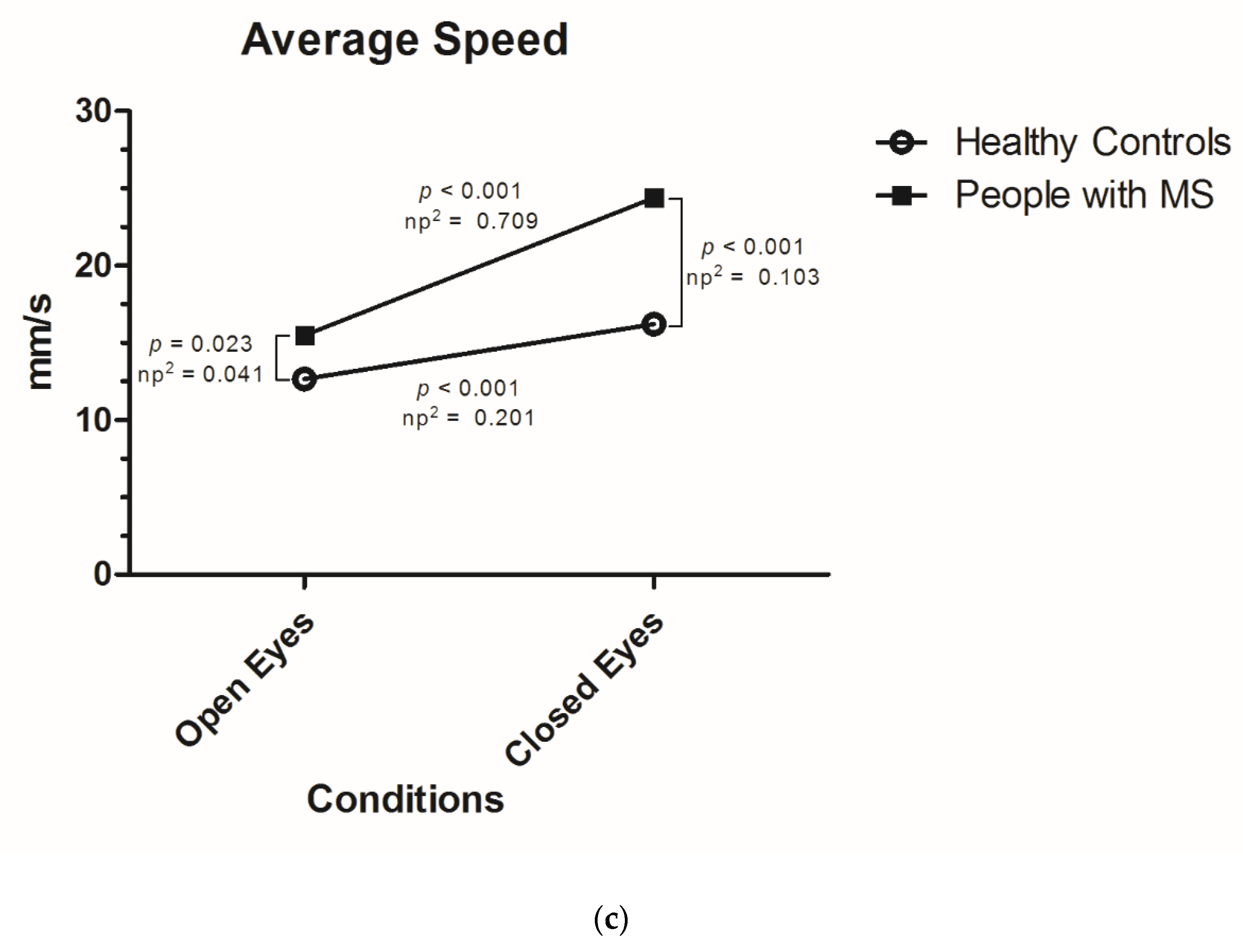
- Average speed: average speed at which the central pressure point of the subjects moves on the platform (measured in mm/s).
Statistical Analysis
3. Results
3.1. Postural Stability According to Visual Stimulus in People with MS and Healthy Controls
3.2. Correlation of Balance Outcomes According to Visual Conditions with Established MS Disability and Function Tests
3.3. Influence of Visual Impairment in Postural Control According to EDSS Function Systems
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Cameron, M.H.; Lord, S. Postural control in multiple sclerosis: Implications for fall prevention. Curr. Neurol. Neurosci. Rep. 2010, 10, 407–412. [Google Scholar] [CrossRef]
- Deliagina, T.G.; Orlovsky, G.N.; Zelenin, P.V.; Beloozerova, I.N. Neural Bases of Postural Control. Physiology 2006, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Bethoux, F.; Bennett, S. Evaluating walking in patients with multiple sclerosis: Which assessment tools are useful in clinical practice? Int. J. MS Care 2011, 13, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018, 159, 237–250. [Google Scholar] [CrossRef]
- Doty, R.L.; MacGillivray, M.R.; Talab, H.; Tourbier, I.; Reish, M.; Davis, S.; Cuzzocreo, J.L.; Shepard, N.T.; Pham, D.L. Balance in multiple sclerosis: Relationship to central brain regions. Exp. Brain Res. 2018, 236, 2739–2750. [Google Scholar] [CrossRef]
- Prosperini, L.; Kouleridou, A.; Petsas, N.; Leonardi, L.; Tona, F.; Pantano, P.; Pozzilli, C. The relationship between infratentorial lesions, balance deficit and accidental falls in multiple sclerosis. J. Neurol. Sci. 2011, 304, 55–60. [Google Scholar] [CrossRef]
- Fling, B.W.; Dutta, G.G.; Schlueter, H.; Cameron, M.H.; Horak, F.B. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front. Hum. Neurosci. 2014, 8, 814. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.H.; Horak, F.B.; Herndon, R.R.; Bourdette, D. Imbalance in multiple sclerosis: A result of slowed spinal somatosensory conduction. Somatosens. Mot. Res. 2008, 25, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Corradini, M.L.; Fioretti, S.; Leo, T.; Piperno, R. Early recognition of postural disorders in multiple sclerosis through movement analysis: A modeling study. IEEE Trans. Bio-Med Eng. 1997, 44, 1029–1038. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B.; Nashner, L.M.; Diener, H.C. Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 1990, 82, 167–177. [Google Scholar] [CrossRef]
- Khasnis, A.; Gokula, R.M. Romberg’s test. J. Postgrad. Med. 2003, 49, 169–172. [Google Scholar]
- Daley, M.L.; Swank, R.L. Changes in postural control and vision induced by multiple sclerosis. Agressologie 1983, 24, 327–329. [Google Scholar]
- Rougier, P.; Faucher, M.; Cantalloube, S.; Lamotte, D.; Vinti, M.; Thoumie, P. How proprioceptive impairments affect quiet standing in patients with multiple sclerosis. Somatosens. Mot. Res. 2007, 24, 41–51. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X. Relative importance of vision and proprioception in maintaining standing balance in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 39, 101901. [Google Scholar] [CrossRef]
- Klatt, B.N.; Sparto, P.J.; Terhorst, L.; Winser, S.; Heyman, R.; Whitney, S.L. Relationship between subjective visual vertical and balance in individuals with multiple sclerosis. Physiother. Res. Int. 2019, 24, e1757. [Google Scholar] [CrossRef] [Green Version]
- Melillo, F.; Di Sapio, A.; Martire, S.; Malentacchi, M.; Matta, M.; Bertolotto, A. Computerized posturography is more sensitive than clinical Romberg Test in detecting postural control impairment in minimally impaired Multiple Sclerosis patients. Mult. Scler. Relat. Disord. 2017, 14, 51–55. [Google Scholar] [CrossRef]
- Prosperini, L.; Pozzilli, C. The clinical relevance of force platform measures in multiple sclerosis: A review. Mult. Scler. Int. 2013, 2013, 756564. [Google Scholar] [CrossRef] [Green Version]
- Kalron, A.; Achiron, A. Postural control, falls and fear of falling in people with multiple sclerosis without mobility aids. J. Neurol. Sci. 2013, 335, 186–190. [Google Scholar] [CrossRef]
- Kalron, A.; Givon, U.; Frid, L.; Dolev, M.; Achiron, A. Static Posturography and Falls According to Pyramidal, Sensory and Cerebellar Functional Systems in People with Multiple Sclerosis. PLoS ONE 2016, 11, e0164467. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Klöditz, A.; Trentzsch, K.; Ziemssen, T. Balance Testing in Multiple Sclerosis—Improving Neurological Assessment With Static Posturography? Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Prosperini, L.; Petsas, N.; Raz, E.; Sbardella, E.; Tona, F.; Mancinelli, C.R.; Pozzilli, C.; Pantano, P. Balance deficit with opened or closed eyes reveals involvement of different structures of the central nervous system in multiple sclerosis. Mult. Scler. J. 2013, 20, 81–90. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Cutter, G.R.; Baier, M.L.; Rudick, R.A.; Cookfair, D.L.; Fischer, J.S.; Petkau, J.; Syndulko, K.; Weinshenker, B.G.; Antel, J.P.; Confavreux, C.; et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999, 122, 871–882. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Social Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Li, L.; Zhang, S.; Dobson, J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J. Sport Health Sci. 2019, 8, 218–227. [Google Scholar] [CrossRef]
- Speers, R.A.; Kuo, A.D.; Horak, F.B. Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture 2002, 16, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Creath, R.; Kiemel, T.; Horak, F.; Jeka, J.J. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J. Vestib. Res. Equilib. Orientat. 2008, 18, 39–49. [Google Scholar]
- Bergin, P.S.; Bronstein, A.M.; Murray, N.M.; Sancovic, S.; Zeppenfeld, D.K. Body sway and vibration perception thresholds in normal aging and in patients with polyneuropathy. J. Neurol. Neurosurg. Psychiatry 1995, 58, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.; Chan, A.; Dansereau, R.; Remaud, A.; Bilodeau, M. Performance of center-of-pressure measures. In Proceedings of the International Conference on Electrical and Computer Systems (ICECS), Ottawa, ON, Canada, 22–24 August 2012. [Google Scholar]
- Gillette, J.C.; Quick, N.E.; Abbas, J.J. Center of pressure measures to assess standing performance. Biomed. Sci. Instrum. 2002, 38, 239–244. [Google Scholar]
- Prosperini, L.; Castelli, L. Spotlight on postural control in patients with multiple sclerosis. Degener. Neurol. Neuromuscul. Dis. 2018, 8, 25–34. [Google Scholar] [CrossRef] [Green Version]
| PwMS (n = 99) | HC (n = 30) | ||
|---|---|---|---|
| Mean age in years | 35.01 (SD 8.21) | 34.03 (SD 7.98) | p = 0.892 |
| Females | 68 (68.69%) | 21 (70%) | p = 0.563 |
| Years Since Diagnosis (mean, SD) | 5.48 (SD 4.62) | n.a. | |
| MS Subtype | |||
| RRMS | 91.9% | n.a. | |
| Transition to SPMS | 7.1% | n.a. | |
| SPMS | 1.0% | n.a. | |
| EDSS (median, IQR) | 2.0 (IQR 1.50–3.0) | n.a. | |
| Visual Function System (median, IQR) | 0 (IQR 0–1) | n.a. | |
| PwMS with visual impairment (n, %) | 35 (35.3%) | n.a. | |
| Cerebellar Function System (median, IQR) | 1 (IQR 0–1) | n.a. | |
| PwMS with cerebellar impairment (n, %) | 57 (57.8%) | n.a. | |
| Sensory Function System (median, IQR) | 1 (IQR 0–2) | n.a. | |
| PwMS with sensory impairment (n, %) | 70 (70.7%) | n.a. | |
| MSFC Z-score (mean, SD) | 0.602 (SD 0.421) | 0.913 (SD 0.164) | p < 0.001 |
| Balance Outcome | Total (n = 129) | PwMS (n = 99) | HC (n = 30) |
|---|---|---|---|
| Delineated Area (cm2) (mean, SD) | |||
| Open eyes | 1.78 (SD 1.47) | 1.98 (SD 1.61) | 1.13 (SD 0.54) |
| Closed eyes | 4.59 (SD 6.90) | 5.48 (SD 7.65) | 1.67 (SD 0.98) |
| Increase | +2.81 (SD 6.06); +158% | +3.50 (SD 6.76); +177% | +0.54 (SD 0.88); +48% |
| Average Sway (mm) (mean, SD) | |||
| Open eyes | 13.66 (SD 7.33) | 14.14 (SD 7.85) | 12.09 (SD 5.07) |
| Closed eyes | 15.31 (SD 7.49) | 16.04 (SD 7.59) | 12.94 (SD 6.75) |
| Increase | +1.65 (SD 5.32); +12% | +1.90 (SD 5.62); +13% | +0.84 (SD 4.18); +7% |
| Average Speed of Sway (mm/s) (mean, SD) | |||
| Open eyes | 14.83 (SD 6.33) | 15.49 (SD 7.00) | 12.66 (SD 2.30) |
| Closed eyes | 22.49 (SD 13.42) | 24.40 (SD 14.66) | 16.22 (SD 3.97) |
| Increase | +7.67 (SD 8.94); +52% | +8.91 (SD 9.77); +45% | +3.56 (SD 2.75); +28% |
| Source | df | Mean Square | F | p | ŋp2 | |
|---|---|---|---|---|---|---|
| Delineated Area | Vision | 1 | 0.454 | 14.102 | <0.001 | 0.101 |
| MS Diagnosis | 1 | 3.303 | 18.140 | <0.001 | 0.127 | |
| Vision * MS Diagnosis | 1 | 0.415 | 12.890 | <0.001 | 0.093 | |
| Average Sway | Vision | 1 | 0.069 | 4.014 | 0.047 | 0.031 |
| MS Diagnosis | 1 | 0.225 | 2.678 | 0.104 | 0.021 | |
| Vision * MS Diagnosis | 1 | 0.050 | 2.911 | 0.090 | 0.023 | |
| Average Speed of Sway | Vision | 1 | 0.119 | 23.628 | <0.001 | 0.159 |
| MS Diagnosis | 1 | 0.451 | 10.892 | 0.001 | 0.080 | |
| Vision * MS Diagnosis | 1 | 0.062 | 12.277 | 0.001 | 0.089 |
| Balance Parameter | EDSS | Visual Function System | Cerebellar Function System | Sensory Function System | MSFC |
|---|---|---|---|---|---|
| Open Eyes | |||||
| Delineated Area | 0.327 | 0.113 | 0.262 | 0.287 | −0.358 |
| p < 0.001 | p = 0.269 | p = 0.009 | p = 0.004 | p < 0.001 | |
| Average Sway | 0.266 | 0.116 | 0.060 | 0.166 | −0.342 |
| p = 0.008 | p = 0.255 | p = 0.555 | p = 0.101 | p < 0.001 | |
| Average Speed of Sway | 0.285 | 0.207 | 0.275 | 0.299 | −0.299 |
| p = 0.004 | p = 0.041 | p = 0.006 | p = 0.022 | p = 0.003 | |
| Closed Eyes | |||||
| Delineated Area | 0.427 | 0.132 | 0.396 | 0.334 | –0.422 |
| p < 0.001 | p = 0.194 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Average Sway | 0.330 | 0.130 | 0.160 | 0.286 | −0.384 |
| p < 0.001 | p = 0.200 | p = 0.114 | p = 0.004 | p < 0.001 | |
| Average Speed of Sway | 0.334 | 0.120 | 0.343 | 0.306 | –0.293 |
| p < 0.001 | p = 0.241 | p = 0.001 | p = 0.002 | p = 0.003 | |
| Difference | |||||
| Delineated Area | 0.405 | 0.091 | 0.379 | 0.361 | −0.385 |
| p < 0.001 | p = 0.375 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Average Sway | 0.042 | –0.034 | 0.118 | 0.090 | 0.0003 |
| p = 0.683 | p = 0.741 | p = 0.244 | p = 0.375 | p = 0.997 | |
| Average Speed of Sway | 0.329 | 0.002 | 0.284 | 0.297 | −0.259 |
| p < 0.001 | p = 0.987 | p = 0.004 | p = 0.003 | p = 0.010 |
| Difference after Withdrawal of Visual Stimulus | No Visual Impairment (n = 63) | With Visual Impairment (n = 35) | p (Effect Size) |
|---|---|---|---|
| Difference Delineated Area (cm2) | 3.18 (SD 5.40) | 4.06 (SD 8.82) | p = 0.963 (0.120) |
| Difference Average Sway (mm) | 2.09 (SD 5.53) | 1.53 (SD 5.91) | p = 0.649 (0.097) |
| Difference Average Speed of Sway (mm/s) | 8.65 (7.67) | 9.52 (SD 12.93 | p = 0.876 (0.100) |
| No cerebellar impairment (n = 42) | With cerebellar impairment (n = 57) | ||
| Difference Delineated Area (cm2) | 2.28 (SD 4.18) | 4.40 (SD 8.07) | p = 0.057 (0.329) |
| Difference Average Sway (mm) | 1.22 (SD 6.02) | 2.40 (SD 5.30) | p = 0.307 (0.208) |
| Difference Average Speed of Sway (mm/s) | 7.39 (SD 8.15) | 10.04 (SD 10.75) | p = 0.031 (0.277) |
| No Sensory impairment (n = 29) | With Sensory impairment (n = 70) | ||
| Difference Delineated Area (cm2) | 1.55 (SD 2.39) | 4.31 (SD 7.76) | p = 0.004 (0.480) |
| Difference Average Sway (mm) | −0.01 (SD 3.98) | 2.69 (SD 6.02) | p = 0.011 (0.851) |
| Difference Average Speed of Sway (mm/s) | 6.32 (SD 7.41) | 9.99 (SD 10.46) | p = 0.026 (0.404) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inojosa, H.; Schriefer, D.; Trentzsch, K.; Klöditz, A.; Ziemssen, T. Visual Feedback and Postural Control in Multiple Sclerosis. J. Clin. Med. 2020, 9, 1291. https://doi.org/10.3390/jcm9051291
Inojosa H, Schriefer D, Trentzsch K, Klöditz A, Ziemssen T. Visual Feedback and Postural Control in Multiple Sclerosis. Journal of Clinical Medicine. 2020; 9(5):1291. https://doi.org/10.3390/jcm9051291
Chicago/Turabian StyleInojosa, Hernan, Dirk Schriefer, Katrin Trentzsch, Antonia Klöditz, and Tjalf Ziemssen. 2020. "Visual Feedback and Postural Control in Multiple Sclerosis" Journal of Clinical Medicine 9, no. 5: 1291. https://doi.org/10.3390/jcm9051291






