Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS)
Abstract
1. Introduction
2. Methods
2.1. Study Design
- Good oral hygiene
- Full permanent dentition
- Patients with no medical history
- Patients with no history of medication
- Non-pregnant women
- Patients undergoing orthodontic treatment with clear aligners only
2.2. LIPUS Device
- A:
- Handheld electronics: It controls LIPUS treatment delivery and provides information regarding treatment procedure and status. It is powered by a rechargeable battery. The information displayed on the screen includes the current status of the device, remaining treatment time, battery charge level, and current date and time. It also maintains a complete record of treatment parameters.
- B:
- Mouthpieces: The device has two mouthpieces, one for the mandible arch treatment and the other for the maxilla arch treatment. Each mouthpiece is similar to a mouthguard and consists of 10 ultrasound emitters set inside a flexible biocompatible encapsulation. All the internal components are hermetically sealed to prevent contact with saliva. The mouthpiece is attached to the handheld electronics with a cable.
- C:
- Ultrasound coupling gel: A tasteless gel provided in single use pouches is applied to the inner walls of the mouthpiece before the start of each treatment. Patients were instructed to apply a thin layer so that LIPUS can be properly transmitted from the mouthpiece through gums to the alveolar bone surrounding the teeth roots.
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Subjects
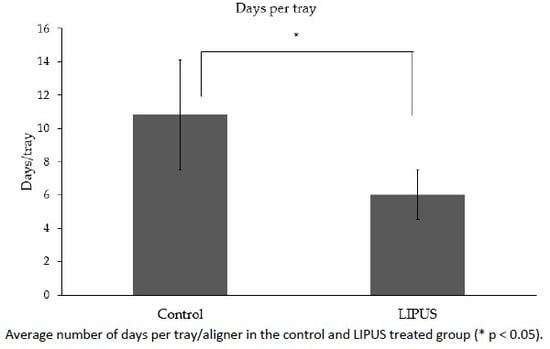
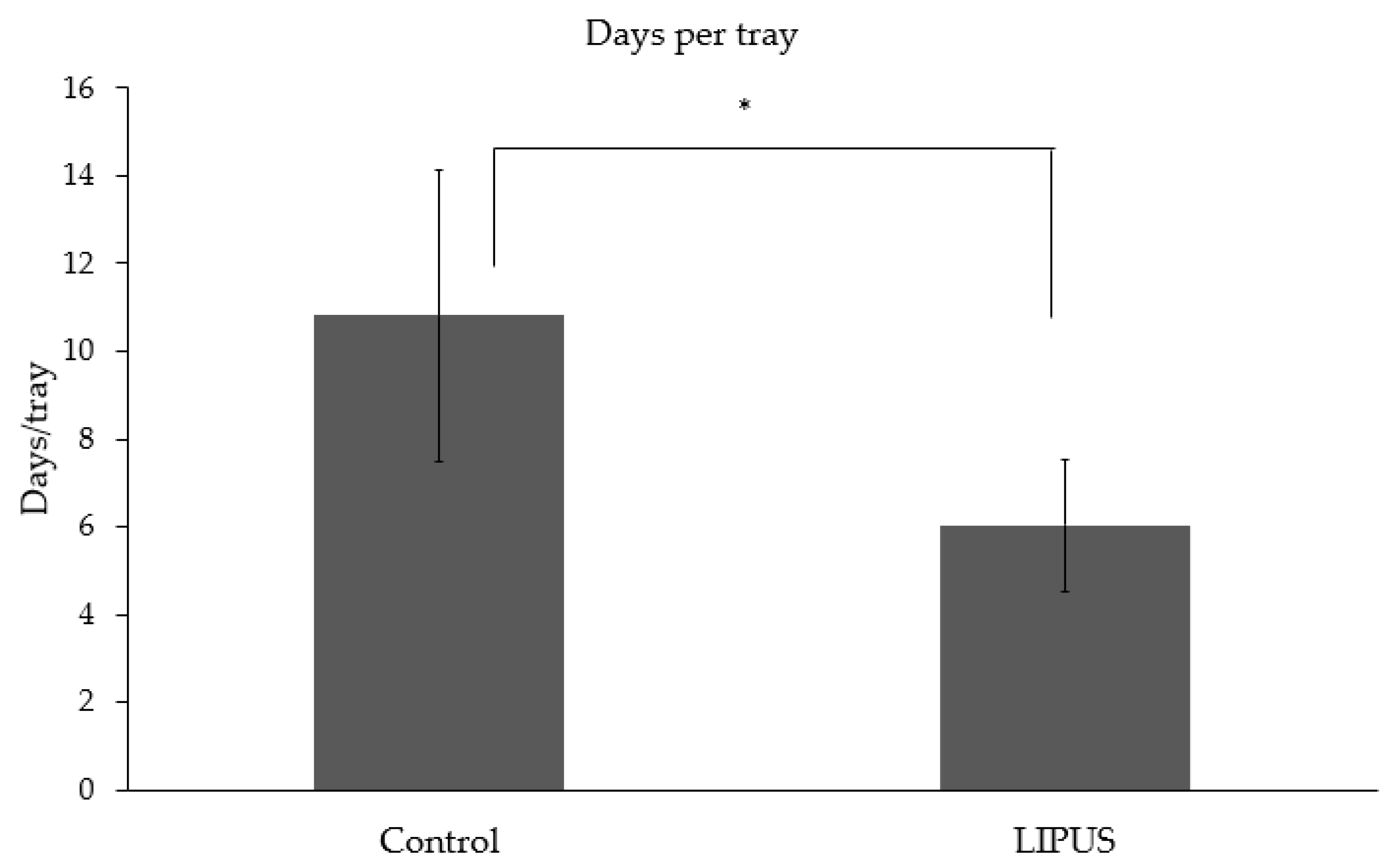
3.2. Number of Days per Tray
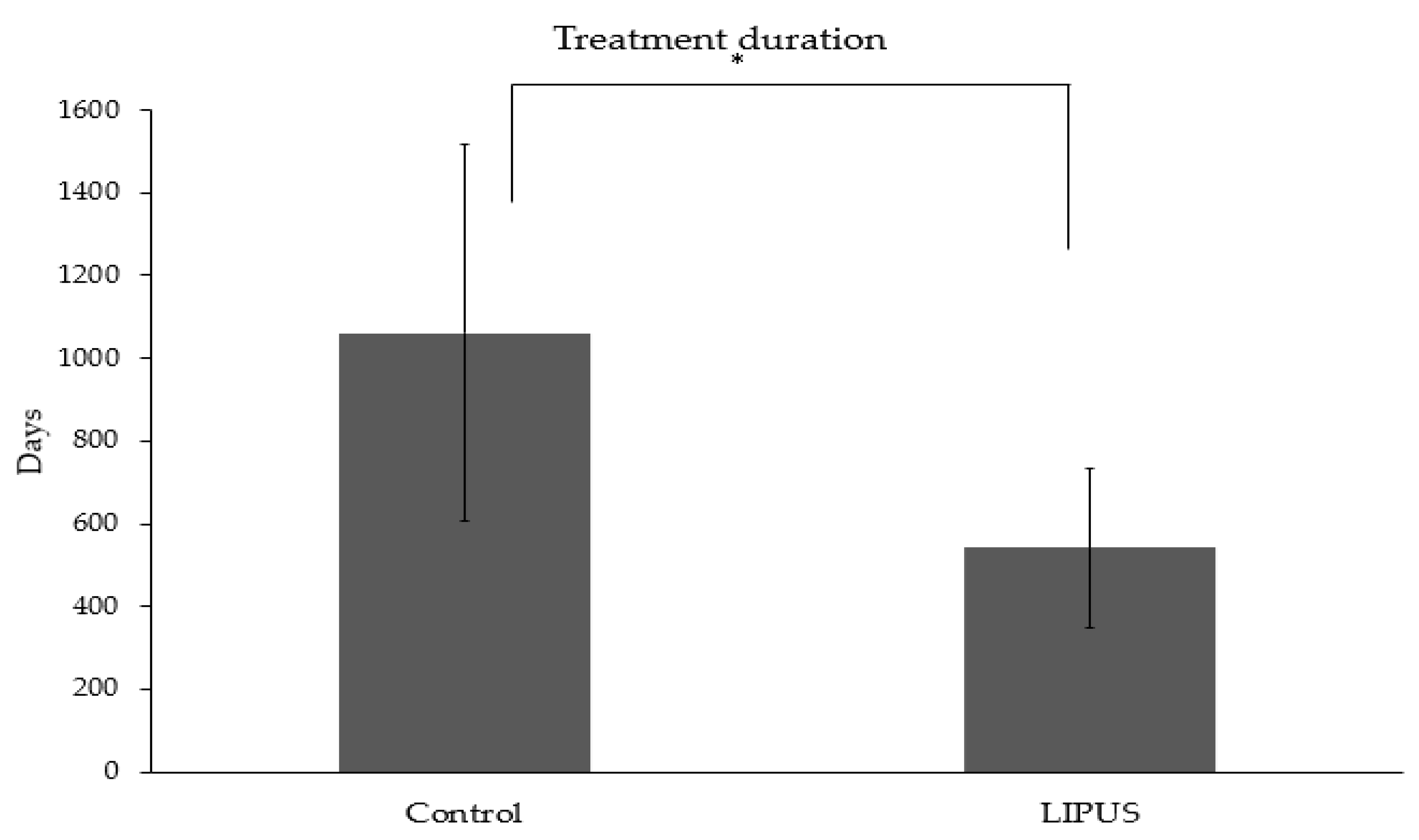
3.3. Treatment Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batista, K.B.; Thiruvenkatachari, B.; Harrison, J.E.; O’Brien, K.D. Orthodontic treatment for prominent upper front teeth (Class II malocclusion) in children and adolescents. Cochrane Database Syst. Rev. 2018, 3, 1–89. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Bona, D.A.; Tanaka, E.; Inubushi, T.; Oka, H.; Ohta, A.; Okada, H.; Miyauchi, M.; Takata, T.; Tanne, K. Cementoblast response to low- and high-intensity ultrasound. Arch. Oral Biol. 2008, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Cordasco, G.; Perillo, L.; Ramaglia, L. Mechanobiology of the tooth movement during the orthodontic treatment: A literature review. Minerva Stomatol. 2016, 65, 299–327. [Google Scholar]
- Maleeh, I.; Robinson, J.; Wadhwa, S. Role of alveolar bone in mediating orthodontic tooth movement and relapse. In Biology of Orthodontic Tooth Movement: Current Concepts and Applications in Orthodontic Practice; Shroff, B., Ed.; Springer: Cham/Basel, Switzerland, 2016; pp. 1–12. [Google Scholar]
- Oppenheim, A. Tissue changes, particularly of the bone, incident to tooth movement. Eur. J. Orthod. 2007, 29, i2–i15. [Google Scholar] [CrossRef]
- Schwarz, A.M. Tissue changes incidental to orthodontic tooth movement. Int. J. Orthod. Oral Surg. Radiogr. 1932, 18, 331–352. [Google Scholar] [CrossRef]
- Rygh, P. Ultrastructural changes of the periodontal fibers and their attachment in rat molar periodontium incident to orthodontic tooth movement. Eur. J. Oral Sci. 1973, 81, 467–480. [Google Scholar] [CrossRef]
- Reitan, K. The initial tissue reaction incident to orthodontic tooth movement as related to the influence of function, an experimental histologic study on animal and human material. Acta Odontol. Scand. Suppl. 1951, 6, 1–240. [Google Scholar]
- Huang, H.; Williams, R.C.; Kyrkanides, S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 620–632. [Google Scholar] [CrossRef]
- Almpani, K.; Kantarci, A. Nonsurgical methods for the acceleration of the orthodontic tooth movement. Front Oral Biol. 2016, 18, 80–91. [Google Scholar]
- Showkatbakhsh, R.; Jamilian, A.; Showkatbakhsh, M. The effect of pulsed electromagnetic fields on the acceleration of tooth movement. World J. Orthod. 2010, 11, e52–e56. [Google Scholar]
- Hassan, A.H.; Al-Fraidi, A.A.; Al-Saeed, S.H. Corticotomy-assisted orthodontic treatment: Review. Open Dent. J. 2010, 13, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Işeri, H.; Kişnişci, R.; Bzizi, N.; Tüz, H. Rapid canine retraction and orthodontic treatment with dentoalveolar distraction osteogenesis. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hayashi, M.; Fujita, S.; Yoshida, T.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur. J. Orthod. 2010, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Chiba, M.; Ohashi, T.; Sato, M.; Shimizu, Y.; Igarashi, K.; Mitani, H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 572–583. [Google Scholar] [CrossRef]
- Ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Dinno, M.A.; Dyson, M.; Young, S.R.; Mortimer, A.J.; Hart, J.; Crum, L.A. The significance of membrane changes in the safe and effective use of therapeutic and diagnostic ultrasound. Phys. Med. Biol. 1989, 34, 1543–1552. [Google Scholar] [CrossRef]
- Inubushi, T.; Tanaka, E.; Rego, E.B.; Ohtani, J.; Kawazoe, A.; Tanne, K.; Miyauchi, M.; Takata, T. Ultrasound stimulation attenuates resorption of tooth root induced by experimental force application. Bone 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Long term effect of low intensity pulsed ultrasound on a human tooth slice organ culture. Arch. Oral Biol. 2012, 57, 760–768. [Google Scholar] [CrossRef]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Effect of low-intensity pulsed ultrasound on orthodontically induced root resorption in beagle dogs. Ultrasound Med. Biol. 2014, 40, 1187–1196. [Google Scholar] [CrossRef]
- Raza, H.; Major, P.; Dederich, D.; El-Bialy, T. Effect of low-intensity pulsed ultrasound on orthodontically induced root resorption caused by torque: A prospective, double-blind, controlled clinical trial. Angle Orthod. 2016, 86, 550–557. [Google Scholar] [CrossRef]
- El-Bialy, T.; El-Shamy, I.; Graber, T.M. Repair of orthodontically induced root resorption by ultrasound in humans. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 186–193. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, T.; Farouk, K.; Carlyle, T.D.; Wiltshire, W.; Drummond, R.; Dumore, T.; Knowlton, K.; Tompson, B. Effect of low intensity pulsed ultrasound (LIPUS) on tooth movement and root resorption: A prospective multi-center randomized controlled trial. J. Clin. Med. 2020, 16, 804. [Google Scholar] [CrossRef]
- Xue, H.; Zheng, J.; Cui, Z.; Bai, X.; Li, G.; Zhang, C.; He, S.; Li, W.; Lajud, S.A.; Duan, Y.; et al. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS ONE 2013, 8, e68926. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Shimo, T.; Kurio, N.; Okui, T.; Ibaragi, S.; Kunisada, Y.; Obata, K.; Masui, M.; Pai, P.; Horikiri, Y.; et al. Low-intensity pulsed ultrasound stimulation promotes osteoblast differentiation through hedgehog signaling. J. Cell Biochem. 2018, 119, 4352–4360. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Sasaki, T. Differentiation and functions of osteoclasts and odontoclasts in mineralized tissue resorption. Microsc. Res. Tech. 2003, 61, 483–495. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D. Root resorption and the OPG/RANKL/RANK system: A mini review. J. Oral Sci. 2008, 50, 367–376. [Google Scholar] [CrossRef]
- Street, J.; Lenehan, B. Vascular endothelial growth factor regulates osteoblast survival-evidence for an autocrine feedback mechanism. J. Orthop. Surg. Res. 2009, 4, 19. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Poynter, J.A.; Manukyan, M.C.; Herrmann, J.L.; Abarbanell, A.M.; Weil, B.R.; Meldrum, D.R. Pretreating mesenchymal stem cells with interleukin-1β and transforming growth factor-β synergistically increases vascular endothelial growth factor production and improves mesenchymal stem cell-mediated myocardial protection after acute ischemia. Surgery 2012, 151, 353–363. [Google Scholar] [CrossRef]
- Alikhani, M.; Alyami, B.; Lee, I.S.; Almoammar, S.; Vongthongleur, T.; Alikhani, M.; Alansari, S.; Sangsuwon, C.; Chou, M.Y.; Khoo, E.; et al. Saturation of the biological response to orthodontic forces and its effect on the rate of tooth movement. Orthod. Craniofac. Res. 2015, 18, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Ohnishi, T.; Bandow, K.; Kakimoto, K.; Chiba, N.; Maeda, A.; Fukunaga, T.; Miyawaki, S.; Matsuguchi, T. Reduction of orthodontic tooth movement by experimentally induced periodontal inflammation in mice. Eur. J. Oral Sci. 2009, 117, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Chou, M.Y.; Khoo, E.; Alansari, S.; Kwal, R.; Elfersi, T.; Almansour, A.; Sangsuwon, C.; Al-Jearah, M.; Nervina, J.M.; et al. Age-dependent biologic response to orthodontic forces. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 632–644. [Google Scholar] [CrossRef]
- Pounder, N.M.; Harrison, A.J. Low intensity pulsed ultrasound for fracture healing: A review of the clinical evidence and the associated biological mechanism of action. Ultrasonics 2008, 48, 330–338. [Google Scholar] [CrossRef] [PubMed]
- De Gusmão, C.V.B.; Pauli, J.R.; Saad, M.J.A.; Alves, J.M.; Belangero, W.D. Low-intensity ultrasound increases FAK, ERK-1/2, and IRS-1 expression of intact rat bones in a noncumulative manner. Clin. Orthop. Relat. Res. 2010, 468, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Siraki, A.G.; Uludağ, H.; Dederich, D.N.; Flood, P.; El-Bialy, T. Role of reactive oxygen species during low-intensity pulsed ultrasound application in MC-3 T3 E1 pre-osteoblast cell culture. Ultrasound Med. Biol. 2017, 43, 2699–2712. [Google Scholar] [CrossRef]
- Kusuyama, J.; Nakamura, T.; Ohnishi, T.; Eiraku, N.; Noguchi, K.; Matsuguchi, T. Low-intensity pulsed ultrasound (LIPUS) promotes BMP9-induced osteogenesis and suppresses inflammatory responses in human periodontal ligament derived stem cells. J. Orthop. Trauma 2017, 31, S4. [Google Scholar] [CrossRef]
- Doan, N.; Reher, P.; Meghji, S.; Harris, M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J. Oral Maxillofac. Surg. 1999, 57, 409–419. [Google Scholar] [CrossRef]
- Young, S.R.; Dyson, M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med. Biol. 1990, 16, 261–269. [Google Scholar] [CrossRef]
- Naruse, K.; Mikuni-Takagaki, Y.; Azuma, Y.; Ito, M.; Oota, T.; Kameyama, K.; Itoman, M. Anabolic response of mouse bone-marrow-derived stromal cell clone ST2 cells to low-intensity pulsed ultrasound. Biochem. Biophys. Res. Commun. 2000, 268, 216–220. [Google Scholar] [CrossRef]
- Suzuki, A.; Takayama, T.; Suzuki, N.; Sato, M.; Fukuda, T.; Ito, K. Daily low-intensity pulsed ultrasound-mediated osteogenic differentiation in rat osteoblasts. Acta Biochim. Biophys. Sin. 2009, 41, 108–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, W.C.; Pang, J.H.S.; Hsu, C.C.; Chu, N.K.; Lin, M.S.; Hu, C.F. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor beta. J. Orthop. Res. 2006, 24, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Cheung, W.H.; Zhang, C.; Lee, K.M.; Lo, H.K. Low intensity pulsed ultrasound stimulates osteogenic activity of human periosteal cells. Clin. Orthop. Relat. Res. 2004, 418, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Harle, J.; Salih, V.; Knowles, J.C.; Mayia, F.; Olsen, I. Effects of therapeutic ultrasound on osteoblast gene expression. J. Mater. Sci. Mater. Med. 2001, 12, 1001–1004. [Google Scholar] [CrossRef]
- Cheung, W.H.; Chow, S.K.H.; Sun, M.H.; Qin, L.; Leung, K.S. Low-intensity pulsed ultrasound accelerated callus formation, angiogenesis and callus remodeling in osteoporotic fracture healing. Ultrasound Med. Biol. 2011, 37, 231–238. [Google Scholar] [CrossRef]
- Gleizal, A.; Li, S.; Pialat, J.B.; Beziat, J.L. Transcriptional expression of calvarial bone after treatment with low-intensity ultrasound: An in vitro study. Ultrasound Med. Biol. 2006, 32, 1569–1574. [Google Scholar] [CrossRef]
- Reher, P.; Doan, N.; Bradnock, B.; Meghji, S.; Harris, M. Therapeutic ultrasound for osteoradionecrosis: An in vitro comparison between 1 MHz and 45 kHz machines. Eur. J. Cancer 1998, 34, 1962–1968. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Lingling, E.; Wang, D. Ultrasound enhances the healing of orthodontically induced root resorption in rats. Angle Orthod. 2012, 82, 48–55. [Google Scholar] [CrossRef]
- Bandow, K.; Nishikawa, Y.; Ohnishi, T.; Kakimoto, K.; Soejima, K.; Iwabuchi, S.; Kuroe, K.; Matsuguchi, T. Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J. Cell Physiol. 2007, 211, 392–398. [Google Scholar] [CrossRef]
- Feres, M.F.N.; Kucharski, C.; Diar-Bakirly, S.; El-Bialy, T. Effect of low-intensity pulsed ultrasound on the activity of osteoclasts: An in vitro study. Arch. Oral Biol. 2016, 70, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Kuroda, S.; Horiuchi, S.; Tabata, A.; El-Bialy, T. Low-intensity pulsed ultrasound in dentofacial tissue engineering. Ann. Biomed. Eng. 2015, 43, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L. Safety assurance in obstetrical ultrasound. Semin. Ultrasound CT MR 2008, 29, 156–164. [Google Scholar] [CrossRef] [PubMed]



| Control | LIPUS | |
|---|---|---|
| Class I | 7 | 7 |
| Class II | 13 | 13 |
| Class III | 14 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; El-Bialy, T. Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS). J. Clin. Med. 2020, 9, 1303. https://doi.org/10.3390/jcm9051303
Kaur H, El-Bialy T. Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS). Journal of Clinical Medicine. 2020; 9(5):1303. https://doi.org/10.3390/jcm9051303
Chicago/Turabian StyleKaur, Harmanpreet, and Tarek El-Bialy. 2020. "Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS)" Journal of Clinical Medicine 9, no. 5: 1303. https://doi.org/10.3390/jcm9051303
APA StyleKaur, H., & El-Bialy, T. (2020). Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS). Journal of Clinical Medicine, 9(5), 1303. https://doi.org/10.3390/jcm9051303