Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness?
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Lead Extraction Procedure
2.3. Transesophageal Echocardiographic Monitoring
2.4. Estimating the Number of Major Complications Using the SAFeTY TLE Score
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Preprocedural Data on CIED
3.3. Outcomes of Transvenous Lead Extraction
3.4. Assessment of the Risk of Major Complications Using the SAFeTY TLE Score
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Byrd, C.L.; Schwartz, S.J.; Hedin, N. Lead extraction. Indications and techniques. Cardiol. Clin. 1992, 10, 735–748. [Google Scholar] [CrossRef]
- Byrd, C.L.; Wilkoff, B.L.; Love, C.J.; Sellers, T.D.; Turk, K.T.; Reeves, R.; Young, R.; Crevey, B.; Kutalek, S.P.; Freedman, R. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994–1996. U.S. Extraction Database, MED Institute. Pacing Clin. Electrophysiol. 1999, 22, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017, 19, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Kennergren, C.; Bjurman, C.; Wiklund, R.; Gäbel, J. A single-centre experience of over one thousand lead extractions. Europace 2009, 11, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.; Epstein, L.M.; Carrillo, R.G.; Love, C.; Adler, S.W.; Riggio, D.W.; Karim, S.; Bashir, J.; Greenspon, A.J.; DiMarc, J.P.; et al. Lead extraction in the contemporary setting: The LExICon study. J. Am. Coll. Cardiol. 2010, 55, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Di Monaco, A.; Pelargonio, G.; Narducci, M.L.; Manzoli, L.; Boccia, S.; Flacco, M.E.; Capasso, L.; Barone, L.; Perna, F.; Bencardino, G.; et al. Safety of transvenous lead extraction according to centre volume: A systematic review and meta-analysis. Europace 2014, 16, 1496–1507. [Google Scholar] [CrossRef]
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; Gottipaty, V.; Shinn, T.; Dan, D.; Feldman, L.A.; et al. Complication rates associated with pacemaker or implantable cardioverter defibrillator generator replacements and upgrade procedures: Results from the REPLACE registry. Circulation 2010, 122, 1553–1561. [Google Scholar] [CrossRef]
- Barakat, A.F.; Wazni, O.M.; Tarakji, K.; Saliba, W.I.; Nimri, N.; Rickard, J.; Brunner, M.; Bhargava, M.; Kanj, M.; Baranowski, B.; et al. Transvenous lead extraction at the time of cardiac implantable electronic device upgrade: Complexity, safety, and outcomes. Heart Rhythm 2017, 14, 1807–1811. [Google Scholar] [CrossRef]
- Mazzone, P.; Migliore, F.; Bertaglia, E.; Facchin, D.; Daleffe, E.; Calzolari, V.; Crosato, M.; Melillo, F.; Peruzza, F.; Marzi, A.; et al. Safety and efficacy of the new bidirectional rotational Evolution® mechanical lead extraction sheath: Results from a multicentre Italian registry. Europace 2018, 20, 829–834. [Google Scholar] [CrossRef]
- Bontempi, L.; Vassanelli, F.; Cerini, M.; D’Aloia, A.; Vizzardi, E.; Gargaro, A.; Chiusso, F.; Mamedouv, R.; Lipari, A.; Curnis, A. Predicting the difficulty of a lead extraction procedure: The LED index. J. Cardiovasc. Med. 2014, 15, 668–673. [Google Scholar] [CrossRef]
- Brunner, M.P.; Yu, C.; Hussein, A.A.; Tarakji, K.G.; Wazni, O.M.; Kattan, M.W.; Wilkoff, B.L. Nomogram for predicting 30-day all-cause mortality after transvenous pacemaker and defibrillator lead extraction. Heart Rhythm 2015, 12, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Oszczygieł, E.; Kutarski, A.; Oszczygieł, A.; Mańkowska-Załuska, B.; Chudzik, M.; Wranicz, J.K.; Cygankiewicz, I. Risk score to assess mortality risk in patients undergoing transvenous lead extraction. Pacing Clin. Electrophysiol. 2017, 40, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [Green Version]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction SAFeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 28, 361. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; O’Mara, J.E.; Weiner, S.; Han, J.; Goldberger, M.H.; Gordon, G.M.; Nanna, M.; Ferrick, K.J.; Gross, J.N. Clinical utility of intraprocedural transesophageal echocardiography during transvenous lead extraction. J. Am. Soc. Echocardiogr. 2008, 21, 861–867. [Google Scholar] [CrossRef]
- Hilberath, J.N.; Burrage, P.S.; Shernan, S.K.; Varelmann, D.J.; Wilusz, K.; Fox, J.A.; Eltzschig, H.K.; Epstein, L.M.; Nowak-Machen, M. Rescue transoesophageal echocardiography for refractory haemodynamic instability during transvenous lead extraction. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Regoli, F.; Caputo, M.; Conte, G.; Faletra, F.F.; Moccetti, T.; Pasotti, E.; Cassina, T.; Casso, G.; Schlotterbeck, H.; Engeler, A.; et al. Clinical utility of routine use of continuous transesophageal echocardiography monitoring during transvenous lead extraction procedure. Heart Rhythm 2015, 12, 313–320. [Google Scholar] [CrossRef]
- Oestreich, B.A.; Ahlgren, B.; Seres, T.; Zipse, M.M.; Tompkins, C.; Varosy, P.D.; Aleong, R.G. Use of Transesophageal echocardiography to improve the safety of transvenous lead extraction. JACC Clin. Electrophysiol. 2015, 1, 442–448. [Google Scholar] [CrossRef]
- Strachinaru, M.; Kievit, C.M.; Yap, S.C.; Hirsch, A.; Geleijnse, M.L.; Szili-Torok, T. Multiplane/3D transesophageal echocardiography monitoring to improve the safety and outcome of complex transvenous lead extractions. Echocardiography 2019, 36, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Kleinrok, A.; Kutarski, A. A new approach to the continuous monitoring of transvenous lead extraction using transesophageal echocardiography-Analysis of 936 procedures. Echocardiography 2020. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of over five thousand chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef] [PubMed]

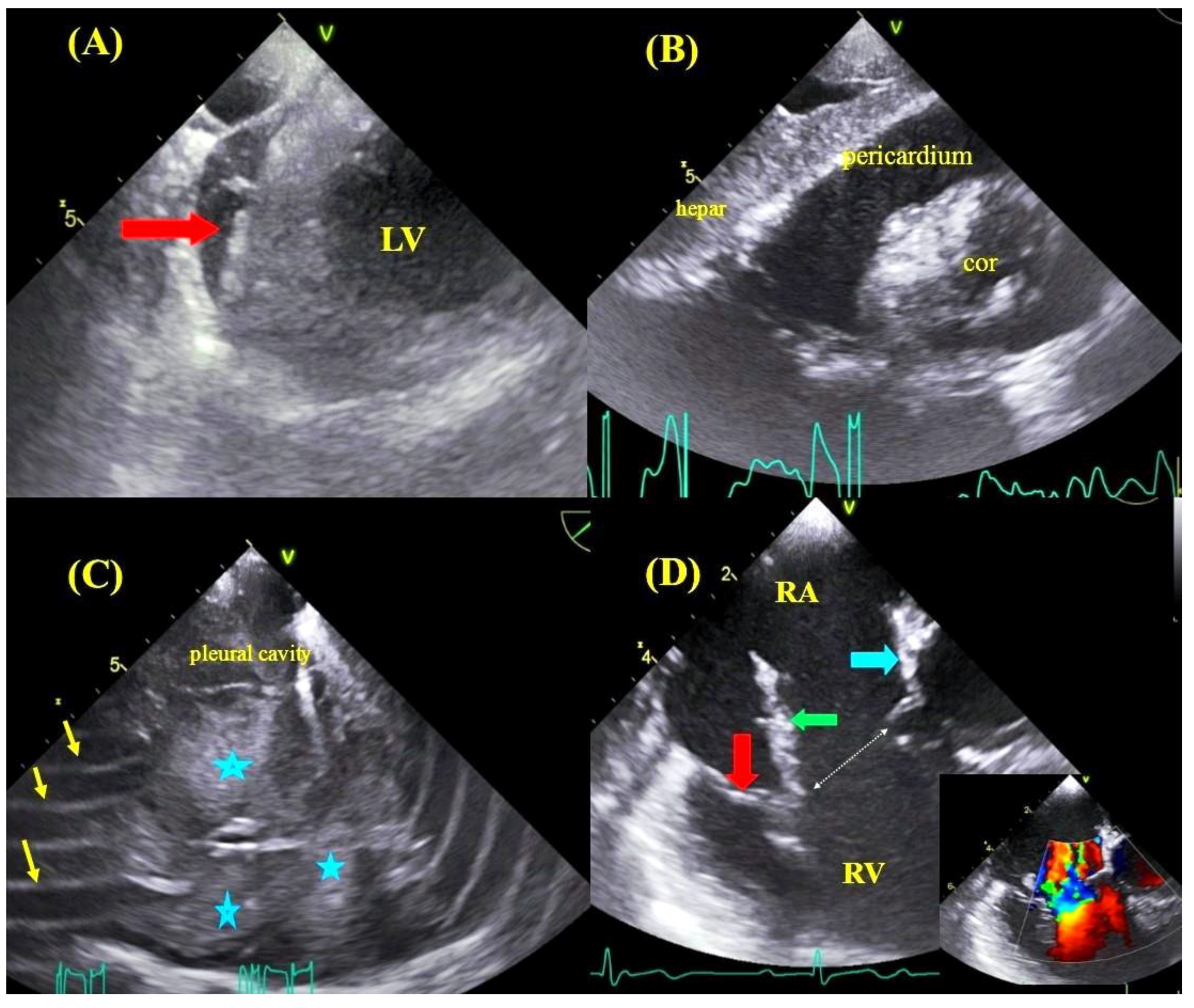


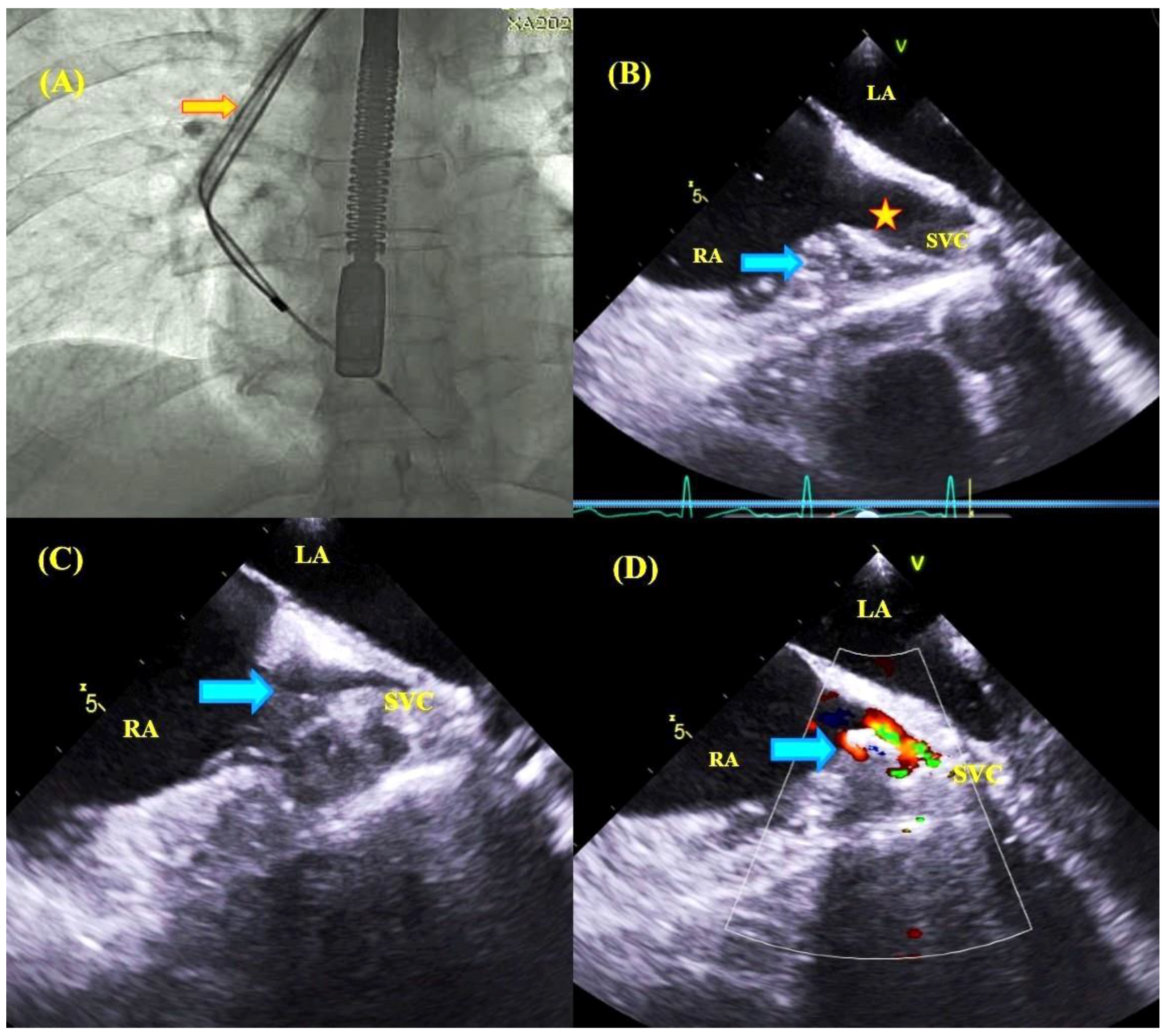


| Parameter | All Group | With TEE Monitoring | Without TEE Monitoring | Mann–Whitney “U” Test/chi2 Test |
|---|---|---|---|---|
| Number of patients | 3185 | 1079 | 2106 | |
| Patient age during TLE (years); x ± SD | 65.720 ± 15.622 N = 3185 | 67.419 ± 14.232 N = 1079 | 64.849 ± 16.223 N = 2106 | p < 0.001 |
| Patient age at first implantation (years); x ± SD | 57.601 ± 17.036 N = 3185 | 58.096 ± 15.813 N = 1079 | 57.348 ± 17.628 N = 2106 | p = 0.558 |
| NYHA class; x ± SD | 1.797 ± 0.692 N = 3185 | 2.035 ± 0.564 N = 1079 | 1.676 ± 0.719 N = 2106 | p < 0.001 |
| NYHA class III & IV; n (%) | 451 (14.160) N = 3185 | 173 (16.033) N = 1079 | 278 (13.200) N = 2106 | p < 0.05 |
| LVEF [%]; x ± SD | 48.911 ± 15.053 N = 3145 | 48.795 ± 15.852 N = 1070 | 48.971 ± 14.628 N = 2075 | p = 0.896 |
| LVEF < 40%; n (%) | 879 (27.598) N = 3185 | 331 (30.677) N = 1079 | 548 (26.021) N = 2106 | p < 0.01 |
| Hemoglobin concentration; (g/dL 0 x ± SD | 12.901 ± 1.946 N = 3121 | 12.589 ± 2.007 N = 1059 | 13.062 ± 1.894 N = 2062 | p < 0.001 |
| Renal failure, creatinine concentration > 1.3 mg/dL; n (%) | 680 (21.601) N = 3148 | 262 (24.764) N = 1058 | 418 (20.000) N = 2090 | p < 0.01 |
| Diabetes mellitus; n (%) | 624 (19.592) N = 3185 | 229 (21.223) N = 1079 | 395 (18.756) N = 2106 | p = 0.107 |
| Arterial hypertension; n (%) | 1852 (58.148) N = 3185 | 572 (53.012) N = 1079 | 1280 (60.779) N = 2106 | p < 0.001 |
| Coronary artery disease/stroke/peripheral artery disease n; n (%) | 1328 (41.695) N = 3185 | 455 (42.169) N = 1079 | 873 (41.453) N = 2106 | p = 0.720 |
| Permanent atrial fibrillation; n (%) | 724 (22.732) N = 3185 | 257 (23.818) N = 1079 | 467 (22.175) N = 2106 | p = 0.316 |
| Prior sternotomy; n (%) | 478 (15.008) N = 3185 | 145 (13.438) N = 1079 | 333 (15.812) N = 2106 | p = 0.085 |
| Charlson comorbidity index; x ± SD | 4.616 ± 3.621 N = 3178 | 4.972 ± 3.756 N = 1072 | 4.434 ± 3.538 N = 2106 | p < 0.001 |
| Parameter | All Group | With TEE Monitoring | Without TEE Monitoring | Mann–Whitney “U” Test/chi2 Test |
|---|---|---|---|---|
| Number of patients | 3185 | 1079 | 2106 | |
| Indications for TLE | ||||
| LRIE certain or probable with or without pocket infection; n (%) | 749 (23.516) N = 3185 | 167 (15.477) N = 1079 | 582 (27.635) N = 2106 | p <0.001 |
| Local (pocket) infection (only); n (%) | 324 (10.173) N = 3185 | 71 (6.580) N = 1079 | 253 (12.013) N = 2106 | p < 0.001 |
| Non-infectious indications, all; n (%) | 2112 (66.311) N = 3185 | 841 (77.943) N = 1079 | 1271 (60.351) N = 2106 | p < 0.001 |
| * Mechanical lead damage (electric failure) n (%) | 816 (38.636) N = 2112 | 337 (40.071) N = 841 | 479 (37.687) N = 1271 | p = 0.291 |
| * Lead dysfunction (exit/entry block, dislodgement, extracardiac pacing) n (%) | 387 (18.324) N = 2112 | 159 (18.906) N = 841 | 228 (17.939) N = 1271 | p = 0.614 |
| * Lead dysfunction caused by (usually dry)—perforation n (%) | 350 (16.572) N = 2112 | 143 (17.004) N = 841 | 207 (16.286) N = 1271 | p = 0.708 |
| * Change of pacing mode/upgrading, downgrading n (%) | 168 (7.955) N = 2112 | 66 (7.848) N = 841 | 102 (8.025) N = 1271 | p = 0.948 |
| * Abandoned lead/prevention of abandonment (AF, overmuch of leads) n (%) | 99 (4.688) N = 2112 | 28 (3.329) N = 841 | 71 (5.586) N = 1271 | p < 0.05 |
| * Threatened/potentially threatened lead (loops, free ending, left heart, LDTD) n (%) | 89 (4.214) | 36 (4.281) N = 841 | 53 (4.170) N = 1271 | p = 0.989 |
| * Other (MRI indication, cancer, pain of pocket, loss of indication for pacing / ICD) n (%) | 72 (3.409) N = 2112 | 31 (3.686) N = 841 | 41 (3.226) N = 1271 | p = 0.654 |
| * Recapture venous access (symptomatic. occlusion, SVC syndrome, lead replacement/upgrading) n (%) | 131 (6.203) N = 2112 | 41 (4.875) N = 841 | 90 (7.081) N = 1271 | p < 0.05 |
| Preoperative data on CIED | ||||
| Dwell time of oldest lead in patients before TLE (months); x ± SD | 98.212 ± 72.773 N = 3185 | 112.665 ± 75.955 N = 1079 | 90.807 ± 69.959 N = 2106 | p < 0.001 |
| Mean implant duration before TLE (months); x ± SD | 90.050 ± 63.656 N = 3185 | 105.235 ± 67.991 N = 1079 | 82.271 ± 59.858 N = 2106 | p < 0.001 |
| CIED with ICD or CS lead; n (%) | 1011 (31.743) N = 3185 | 379 (35.125) N = 1079 | 632 (30.009) N = 2106 | p < 0.01 |
| Number of abandoned leads before TLE; x ± SD | 0.119 ± 0.323 N = 3185 | 0.087 ± 0.282 N = 1079 | 0.135 ± 0.342 N = 2106 | p < 0.05 |
| Presence of abandoned lead(s) before TLE; n (%) | 379 (11.900) N = 3185 | 95 (8.804) N = 1079 | 284 (13.485) N = 2106 | p < 0.001 |
| Abandoned leads only (EPM. EHV); n (%) | 32 (1.004) N = 3185 | 4 (0.371) N = 1079 | 28 (1.330) N = 2106 | p < 0.05 |
| Number of leads in the system before TLE; x ± SD | 1.817 ± 0.634 N = 3161 | 1.829 ± 0.622 N = 1076 | 1.812 ± 0.640 N = 2085 | p = 0.400 |
| Number of leads in the heart before TLE; x ± SD | 1.962 ± 0.758 N = 3185 | 1.930 ± 0.715 N = 1079 | 1.978 ± 0.779 N = 2106 | p = 0.324 |
| Parameter | All Group | With TEE Monitoring | Without TEE Monitoring | Mann–Whitney “U” Test/chi2 Test |
|---|---|---|---|---|
| Number of patients | 3185 | 1079 | 2106 | |
| Number of extracted leads | 5258 | 1760 | 3498 | |
| Oldest extracted lead (years); x ± SD | 8.021 ± 5.962 N = 3185 | 9.290 ± 6.307 N = 1079 | 7.372 ± 5.670 N = 2106 | p < 0.001 |
| Sum of dwell times of extracted leads (years); x ± SD | 13.091 ± 12.202 N = 3185 | 15.466 ± 13.960 N = 1079 | 11.876 ± 11.004 N = 2106 | p < 0.001 |
| Number of extracted leads in one patient; x ± SD | 1.651 ± 0.747 N = 3185 | 1.631 ± 0.719 N = 1079 | 1.661 ± 0.761 N = 2106 | p = 0.477 |
| HV therapy (ICD) lead was extracted; n (%) | 874 (27.441) N = 3185 | 341 (31.603) N = 1079 | 533 (25.309) N = 2106 | p < 0.001 |
| CS (LV pacing) lead was extracted; n (%) | 417 (13.093) N = 3185 | 122 (11.307) N = 1079 | 295 (14.008) N = 2106 | p < 0.05 |
| Extraction of abandoned lead(s) (any); n (%) | 422 (13.250) N = 3185 | 95 (8.804) N = 1079 | 327 (15.527) N = 2106 | p < 0.001 |
| Lead fracture during extraction; n (%) | 137 (4.301) N = 3185 | 51 (4.727) N = 1079 | 86 (4.084) N = 2106 | p = 0.451 |
| Loss of free lead fragment; n (%) | 15 (0.471) N = 3185 | 7 (0.648) N = 1079 | 8 (0.380) N = 2106 | p = 0.438 |
| Technical difficulty during TLE (any); n (%) | 592 (18.587) N = 3185 | 257 (23.818) N = 1079 | 335 (15.907) N = 2106 | p < 0.001 |
| Two or more technical difficulties; n (%) | 127 (3.987) N = 3185 | 75 (6.951) N = 1079 | 52 (2.469) N = 2106 | p < 0.001 |
| Necessity to change venous approach; n (%) | 135 (4.240) N = 3184 | 18 (1.668) N = 1079 | 117 (5.558) N = 2105 | p < 0.001 |
| Use of other than extracted lead venous approach; n (%) | 173 (5.432) N = 3185 | 28 (2.595) N = 1079 | 145 (6.885) N = 2106 | p < 0.001 |
| Necessity to use other than Byrd dilator tools (Evo, TightR, lassos, basket catheters); n (%) | 183 (5.746) N = 3185 | 75 (6.951) N = 1079 | 108 (5.128) N = 2106 | p < 0.05 |
| Procedure duration (skin-to-skin); minutes x ± SD | 59.952 ± 25.717 N = 3185 | 62.747 ± 27.138 N = 1079 | 58.520 ± 24.842 N = 2106 | p < 0.001 |
| Procedure duration (sheath-to-sheath time); minutes x ± SD | 15.125 ± 23.274 N = 3185 | 15.374 ± 24.396 N = 1079 | 14.997 ± 22.682 N = 2106 | p < 0.001 |
| Procedure duration average single lead extraction time; minutes x ± SD | 8.965 ± 12.418 N = 3182 | 9.111 ± 14.260 N = 1079 | 8.891 ± 11.363 N = 2103 | p < 0.01 |
| Partial radiological success (retained tip or < 4 cm lead fragment); n (%) | 136 (4.270) N = 3185 | 34 (3.151) N = 1079 | 102 (4.843) N = 2106 | p < 0.05 |
| Complete clinical success; n (%) | 3116 (97.834) N = 3185 | 1056 (97.868) N = 1079 | 2060 (97.816) N = 2106 | p = 0.975 |
| Complete procedural success; n (%) | 3064 (96.201) N = 3185 | 1054 (97.683) N = 1079 | 2010 (95.442) N = 2106 | p < 0.01 |
| Procedure-related death (intra-, post-procedural); n (%) | 6 (0.188) N = 3185 | 0 (0.000) N = 1079 | 6 (0.285) N = 2106 | p = 0.186 |
| Died within 6 months; n (%) | 166 5.212 N = 3185 | 48 (4.449) N = 1079 | 118 (5.603) N = 2106 | p = 0.193 |
| Died within one year; n (%) | 263 (8.257) N = 3185 | 83 (7.692) N = 1079 | 180 (8.547) N = 2106 | p = 0.446 |
| Simulation of Major Complications According to SAFeTY TLE Score | All Group | With TEE Monitoring | Without TEE Monitoring | Mann–Whitney “U” Test/chi2 Test | |
|---|---|---|---|---|---|
| Number of patients | 3185 | 1079 | 2106 | ||
| Sum of dwell times of extracted leads ≥ 16.5 years; n (%) | S | 864 (27.127) N = 3185 | 360 (33.364) N = 1079 | 504 (23.932) (N = 2106 | p < 0.001 |
| Hemoglobin concentration ≤ 11.5 g/dL; n (%) | A | 710 (22.749) N = 3121 | 299 (27.711) N = 1059 | 411 (19.932) N = 2062 | p < 0.001 |
| Female; n (%) | Fe | 1231 (38.650) N = 3185 | 410 (37.998) N = 1079 | 821 (38.984) N = 2106 | p = 0.616 |
| Number of previous CIED procedures (all); x ± SD | T | 1.822 ± 1.081 N = 3185 | 1.779 ± 0.965 N = 1079 | 1.844 ± 1.136 N = 2106 | p = 0.852 |
| Patients aged at first implantation below 30 years; n (%) | Y | 275 (8.634) N = 3185 | 82 (7.600) N = 1079 | 193 (9.164) N = 2106 | p = 0.155 |
| Number of SAFeTY TLE score points; x ± SD | 5.777 ± 4.226 N = 3165 | 6.143 ± 4.395 N = 1079 | 5.593 ± 4.127 N = 2106 | 0.004 | |
| Probability of perforation (SVC, RA, RV) according to SAFeTY-TLE score; x (95% CI) | 1.721 95% CI (1.590–1.852) | 1.898 95% CI (1.664–2.132) | 1.630 95% CI (1.472–1.788) | 0.002 | |
| Calculated number of major complications (perforations; SVC, RA, RV) according to SAFeTY-TLE score; n (%) | 55 (1.721) N = 3185 | 21 (1.898) N = 1079 | 34 (1.898) N = 2106 | 0.592 |
| Major Complication | All Group | With TEE Monitoring | Without TEE Monitoring | Mann–Whitney “U” Test/chi2 Test |
|---|---|---|---|---|
| Number of patients | 3185 | 1079 | 2106 | |
| Real major complication (any); n (%) | 60 (1.884) N = 3185 | 21 (1.946) N = 1079 | 39 (1.852) N = 2106 | 0.962 |
| Real hemopericardium; n (%) | 43 (1.350) N = 3185 | 13 (1.205) N = 1079 | 30 (1.425) N = 2106 | 0.729 |
| Real hemopericardium or hemothorax; n (%) | 48 (1.507) N = 3185 | 15 (1.390) N = 1079 | 33 (1.567) N = 2106 | 0.815 |
| Real tricuspid valve damage during TLE; n (%) | 12 (0.377) N = 3185 | 6 (0.556) N = 1079 | 6 (0.285) N = 2106 | 0.381 |
| Rescue cardiac surgery; n (%) | 37 (1.162) N = 3185 | 16 (1.483) N = 1079 | 21 (0.997) N = 2106 | p = 0.300 |
| Number of Patients | Calculated Number of Major Complications (Perforations; SVC, RA, RV) According to SAFeTY-TLE Score; n (%) | Real Number of Major Complications (Perforations; SVC, RA, RV) n (%) | Change N (%) | chi2 Test | |
|---|---|---|---|---|---|
| All group | N = 3185 | 55 (1.721) N = 3185 | 48 (1.507) N = 3185 | −7 (−12.727) | p < 0.05 |
| With TEE monitoring | N = 1079 | 21 (1.898) N = 1079 | 15 (1.390) N = 1079 | −6 (−28.571) | p < 0.05 |
| Without TEE monitoring | N = 2106 | 34 (1.898) N = 2106 | 33 (1.567) N = 2106 | −1 (2.941) | p = 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Tomaszewski, A.; Brzozowski, W.; Szcześniak-Stańczyk, D.; Kleinrok, A.; et al. Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness? J. Clin. Med. 2020, 9, 1382. https://doi.org/10.3390/jcm9051382
Nowosielecka D, Jacheć W, Polewczyk A, Tułecki Ł, Tomków K, Stefańczyk P, Tomaszewski A, Brzozowski W, Szcześniak-Stańczyk D, Kleinrok A, et al. Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness? Journal of Clinical Medicine. 2020; 9(5):1382. https://doi.org/10.3390/jcm9051382
Chicago/Turabian StyleNowosielecka, Dorota, Wojciech Jacheć, Anna Polewczyk, Łukasz Tułecki, Konrad Tomków, Paweł Stefańczyk, Andrzej Tomaszewski, Wojciech Brzozowski, Dorota Szcześniak-Stańczyk, Andrzej Kleinrok, and et al. 2020. "Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness?" Journal of Clinical Medicine 9, no. 5: 1382. https://doi.org/10.3390/jcm9051382
APA StyleNowosielecka, D., Jacheć, W., Polewczyk, A., Tułecki, Ł., Tomków, K., Stefańczyk, P., Tomaszewski, A., Brzozowski, W., Szcześniak-Stańczyk, D., Kleinrok, A., & Kutarski, A. (2020). Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness? Journal of Clinical Medicine, 9(5), 1382. https://doi.org/10.3390/jcm9051382





