Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors
Abstract
1. Introduction
2. Nutritional Model Increasing the Risk of Male Infertility
2.1. Trans and Saturated Fatty Acids
2.2. Meat
2.3. Smoking and Alcohol
2.4. Caffeine
2.5. Phytoestrogens
2.6. Contaminates
2.7. Hypercaloric Diet
3. Mechanisms Associating Improper Diet and Obesity with Infertility
4. Intestinal Microbiota Disorders and Male Fertility
5. A Dietary Model Supporting Male Fertility
5.1. Mediterranean Diet
5.2. Antioxidants for Male Infertility–What is the Evidence?
5.2.1. Zinc
5.2.2. Selenium
5.2.3. Other Antioxidants
5.2.4. Omega-3 Fatty Acids
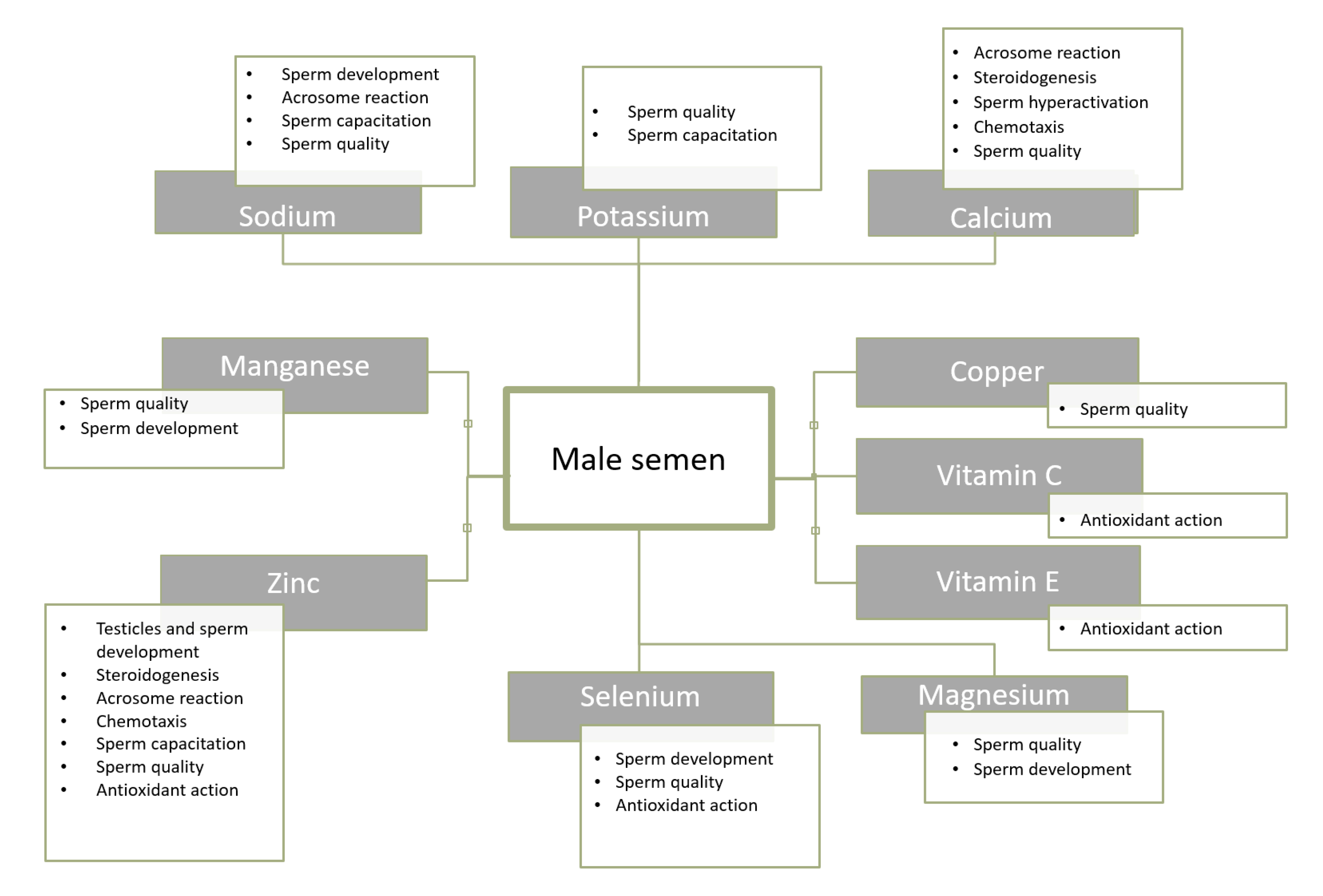
5.3. Magnesium, Calcium, Copper, Manganese
5.4. Fibre
6. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Leaver, R.B. Male infertility: An overview of causes and treatment options. Br. J. Nurs. 2016, 25, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bablok, L.; Dziadecki, W.; Szymusik, I.; Wolczynski, S.; Kurzawa, R.; Pawelczyk, L.; Jedrzejczak, P.; Hanke, W.; Kamiński, P.; Wielgos, M. Patterns of infertility in Poland—Multicenter study. Neuro. Endocrinol. Lett. 2011, 32, 799–804. [Google Scholar] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Mędraś, M.; Lwow, F.; Jóźków, P.; Szmigiero, L.; Zagrodna, A.; Zagocka, E.; Słowińska-Lisowska, M. The quality of semen among a sample of young, healthy men from Lower Silesia. Endokrynol. Pol. 2017, 68, 668–675. [Google Scholar] [CrossRef][Green Version]
- Gabrielsen, J.S.; Tanrikut, C. Chronic exposures and male fertility: The impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016, 4, 648–661. [Google Scholar] [CrossRef]
- Walczak-Jędrzejowska, R. Oxidative stress and male infertility. Part I: Factors causing oxidative stress in semen. Adv. Androl. Online 2015, 2, 5–15. [Google Scholar]
- Chiang, C.; Mahalingam, S.; Flaws, J. Environmental contaminants affecting fertility and somatic health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Christou, M.A.; Christou, P.A.; Markozannes, G.; Tsatsoulis, A.; Mastorakos, G.; Tigas, S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: A systematic review and meta-analysis. Sports Med. 2017, 47, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef]
- Hart, K.; Tadros, N.N. The role of environmental factors and lifestyle on male reproductive health, the epigenome, and resulting offspring. Panminerva Med. 2019, 61. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and male fertility: Effects of benzo-α-pyrene and resveratrol on human sperm function in vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef]
- Duca, Y.; Aversa, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Substance abuse and male hypogonadism. J. Clin. Med. 2019, 8, 732. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Varani, J. Healthful Eating, the Western Style Diet and Chronic Disease. Appro. Poult. Dairy Vet. Sci. 2017, 1, 3. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.; Anderson, R. The impact of obesity on male fertility. HJ 2015. [Google Scholar] [CrossRef] [PubMed]
- El Salam, M.A.A. Obesity, an enemy of male fertility: A mini review. Oman. Med. J. 2018, 33, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Schowell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Kart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, 12, CD007411. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Al-Beitawi, S.; Cipriani, S.; Alteri, A.; Chiaffarino, F.; Candiani, M.; Gerli, S.; Viganó, P.; Parazzini, F. Dietary habits and semen parameters: A systematic narrative review. Andrology 2018, 6, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Fung, T.T.; Lu, N.; Keller, S.F.; Curhan, G.C.; Choi, H.K. The dietary approaches to stop hypertension (DASH) diet, western diet, and risk of gout in men: Prospective cohort study. BMJ 2017, 357, j1794. [Google Scholar] [CrossRef]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef]
- Danielewicz, A.; Przybyłowicz, K.E.; Przybyłowicz, M. Dietary patterns and poor semen quality risk in men: A cross-sectional study. Nutrients 2018, 10, 1162. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab. J. Urol. 2017, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B. Rat testicular lipids and dietary isomeric fatty acids in essential fatty acid deficiency. Lipids 1976, 11, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Privett, O.S.; Phillips, F.; Shimasaki, H.; Nozawa, T.; Nickell, E.C. Studies of effects of trans fatty acids in the diet on lipid metabolism in essential fatty acid deficient rats. Am. J. Clin. Nutr. 1977, 30, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Hanis, T.; Zidek, V.; Sachova, J.; Klir, P.; Deyl, Z. Effects of dietary trans-fatty acids on reproductive performance of Wistar rats. Br. J. Nutr. 1989, 61, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Minguez-Alarcón, L.; Chavarro, J.E.; Mendiola, J.; Roca, M.; Tanrikut, C.; Vioque, J.; Jørgensen, N.; Torres-Cantero, A.M. Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian. J. Androl. 2017, 19, 184–190. [Google Scholar] [CrossRef]
- Veaute, C.; Andreoli, M.F.; Racca, A.; Bailat, A.; Scalerandi, M.V.; Bernal, C.; Malan Borel, I. Effects of isomeric fatty acids on reproductive parameters in mice. Am. J. Reprod. Immunol. 2007, 58, 487–496. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef]
- Jensen, T.K.; Heitmann, B.L.; Jensen, M.B.; Halldorsson, T.I.; Andersson, A.M.; Skakkebæk, N.E.; Joensen, U.N.; Lauritsen, M.P.; Christiansen, P.; Dalgård, C.; et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am. J. Clin. Nutr. 2013, 97, 411–418. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015. [Google Scholar] [CrossRef]
- Attaman, J.A.; Toth, T.L.; Furtado, J. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Willingham, E.J. Environmental review: Trenbolone and other cattle growth promoters: Need for a new risk-assessment framework. Environ. Pract. 2006, 8, 58–65. [Google Scholar] [CrossRef]
- Henricks, D.M.; Gray, S.L.; Owenby, J.J.; Lackey, B.R. Residues from anabolic preparations after good veterinary practice. APMIS 2001, 109, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Toth, T.L.; Wright, D.L.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J. Nutr. 2014, 144, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Williams, P.L.; Gaskins, A.J.; Mendiola, J.; Jørgensen, N.; Swan, S.H.; Chavarro, J.E. Meat intake and reproductive parameters among young men. Epidemiology 2014, 25, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, H.; Li, Y.; Cao, J. Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil. Steril. 2011, 95, 116–123. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef]
- Ricci, E.; Viganò, P.; Cipriani, S. Coffee and caffeine intake and male infertility: A systematic review. Nutr. J. 2017, 16, 37. [Google Scholar] [CrossRef]
- Cooper, A.R. To eat soy or to not eat soy: The ongoing look at phytoestrogens and fertility. Fertil. Steril. 2019, 112, 825–826. [Google Scholar] [CrossRef]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Desmawati, D.; Sulastri, D. A Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Cawood, E.; Kinniburgh, D.; Provan, A.; Collins, A.R.; Irvine, D.S. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin. Sci. 2001, 100, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Beaton, L.K.; McVeigh, B.L.; Dillingham, B.L.; Lampe, J.W.; Duncan, A.M. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertil. Steril. 2009, 94, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Watanabe, S.; Setchell, K.D. Report on the 8th international symposium on the role of soy in health promotion and chronic disease prevention and treatment. J. Nutr. 2009, 139, 796S–802S. [Google Scholar] [CrossRef]
- Casini, M.L.; Gerli, S.; Unfer, V. An infertile couple suffering from oligospermia by partial sperm maturation arrest: Can phytoestrogens play a therapeutic role? A case report study. Gynecol. Endocrinol. 2006, 22, 399–401. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Hauser, R.; Gaskins, A.J. Effects of bisphenol A on male and couple reproductive health: A review. Fertil. Steril. 2016, 106, 864–870. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Marques, A.; Peralta, M.; Naia, A.; Loureiro, N.; de Matos, M.G. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur. J. Public Health 2017, 28, 295–300. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproductio 2017, 154, 123–131. [Google Scholar] [CrossRef]
- Jedrzejczak, P.; Fraczek, M.; Szumala-Kakol, A.; Taszarek-Hauke, G.; Pawelczyk, L.; Kurpisz, M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in in vitro conditions. Int. J. Androl. 2005, 28, 275–283. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Khodamoradi, K.; Parmar, M.; Khosravizadeh, Z.; Kuchakulla, M.; Manoharan, M.; Arora, H. The role of leptin and obesity on male infertility. Curr. Opin. Urol. 2020, 30, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, M. Review of the role of leptin in the regulation of male reproductive function. Andrologia 2018, 50, e12965. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, M.; Yadav, A.K.; Hemalatha, R.; Yadav, H.; Marotta, F.; Yamashiro, Y. Gut microbiota in health and disease: An overview focused on metabolic inflammation. Benef. Microbes 2017, 7, 181–194. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.; Skoracka, K.; Skrypnik, D. The influence of western-style diet on the permeability of the intestinal barrier. Metab. Disord. Forum 2019, 10, 88–97. [Google Scholar]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to brain dysbiosis:mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral Supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in ldlr mice—Role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2019. [Google Scholar] [CrossRef]
- Radko, M.; Bogdanowicz, M.; Syryło, T. Bakteriospermia and its influence on human semen parametrs. Post. Androl. Online 2018, 5, 62–69. [Google Scholar]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef]
- Szkodziak, P.; Wozniak, S.; Czuczwar, P.; Wozniakowska, E.; Milart, P.; Mroczkowski, A.; Paszkowski, T. Infertility in the light of new scientific reports—focus on male factor. Ann. Agric. Environ. Med. 2016, 23, 227–230. [Google Scholar] [CrossRef]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An overview on role of some trace elements in human reproductive health, sperm function and fertilization proces. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of trace elements for oxidative status and quality of human sperm. Balkan Med. J. 2017, 34, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Babio, N.; Carrell, D.T.; Bulló, M.; Salas-Salvadó, J. Adherence to the Mediterranean diet is positively associated with sperm motility: A cross-sectional analysis. Sci. Rep. 2019, 9, 3389. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The effect of nutrients and dietary supplements on sperm quality parameters: A systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Moraleda, R.; Giardina, S.; Anton, E.; Blanco, J.; Salas-Salvadó, J.; Bulló, M. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.A.; Xun, L.; FitzGerald, L.Z.; Esguerra, S.; Henning, S.M.; Carpenter, C.L. Walnuts improve semen quality in men consuming a Western-style diet: Randomized control dietary intervention trial. Biol. Reprod. 2012, 87, 1–8. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Ferrari, S.; De Cosmi, V.; La Vecchia, I.; Cavadini, M.; La Vecchia, C.; Parazzini, F. Mediterranean diet and the risk of poor semen quality: Cross-sectional analysis of men referring to an Italian Fertility Clinic. Andrology 2019, 7, 156–162. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2016, 32, 215–222. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Somigliana, E.; Cipriani, S.; Castiglioni, M.; Chiaffarino, F.; Vignali, M.; Gallotti, B.; Parazzini, F. Mediterranean diet and outcomes of assisted reproduction: An Italian cohort study. Am. J. Obstet. Gynecol. 2019, 221, 627.e1–627.e14. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and male fertility: From molecular studies to clinical evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019. [CrossRef] [PubMed]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance—Challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.P.; Cocuzza, M.; Elterman, D. Optimizing male fertility: Oxidative stress and the use of antioxidants. World J. Urol. 2019, 37, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.P. Zinc levels in seminal fluid in infertile males and its relation with serum free testosterone. JCDR 2016, 10, CC05–CC08. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Miura, C.; Kikuchi, K.; Celino, F.T.; Agusa, T.; Tanabe, S.; Miura, T. Zinc is an essential trace element for spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 10859–10864. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A necessary ion for mammalian sperm fertilization competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef]
- Lerda, D. Study of sperm characteristics in persons occupationally exposed to lead. Am. J. Ind. Med. 1992, 22, 567–571. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar]
- Hawkes, W.C.; Turek, P.J. Effects of dietary selenium on sperm motility in healthy men. J. Androl. 2001, 22, 764–772. [Google Scholar] [PubMed]
- Hurst, R.; Bao, Y.P.; Ridley, S. Phospholipid hydroperoxide cysteinę peroxidase activity of human serum albumin. Biochem. J. 1999, 338, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The effect of oral selenium supplementation on human sperm motility. Br. J. Urol. 1998, 82, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.; Arowololu, A.O.; Shittu, O.B.; Adejuwon, C.A.; Osotimehin, B. Selenium status of idiopathic infertile Nigerian males. Biol. Trace Elem. Res. 2005, 104, 9–18. [Google Scholar] [CrossRef]
- Mintziori, G.; Mousiolis, A.; Duntas, L.H.; Goulis, D.G. Evidence for a manifold role of selenium in infertility. Hormones 2020, 19, 55–59. [Google Scholar] [CrossRef]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohe, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef]
- Rao, M.; VSharma, P.S. Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod. Toxicol. 2001, 15, 705–712. [Google Scholar] [CrossRef]
- Irani, M.; Amirian, M.; Sadeghi, R.; Lez, J.L.; Latifnejad Roudsari, R. The effect of folate and folate plus zinc supplementation on endocrine parameters and sperm characteristics in sub-fertile men: A systematic review and meta-analysis. Urol. J. 2017, 14, 4069–4078. [Google Scholar]
- Gvozdjáková, A.; Kucharská, J.; Dubravicky, J.; Mojto, V.; Singh, R.B. Coenzyme Q 10, α -tocopherol, and oxidative stress could be important metabolic biomarkers of male infertility. Disease Markers 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Miguel, A.C. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, B.C.; Gaskins, A.J.; Hauser, R.; Chavarro, J.E.; Tanrikut, C.; EARTH Study Team. Coenzyme Q10 intake from food and semen parameters in a subfertile population. Urology 2017, 102, 100–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radigue, C.; Es-Slami, S.; Soufir, J.C. Relationship of carnitine transport across the epididymis to blood carnitine and androgens in rats. Arch. Androl. 1996, 37, 27–31. [Google Scholar] [CrossRef]
- Johansen, L.; Bøhmer, T. Carnitine-binding related supressed oxygen uptake by spermatozoa. Arch. Androl. 1978, 1, 321–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sigman, M.; Glass, S.; Campagnone, J.; Pryor, J.L. Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2006, 85, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420. [Google Scholar] [CrossRef]
- Zareba, P.; Colaci, D.S.; Afeiche, M.; Gaskins, A.J.; Jørgensen, N.; Mendiola, J.; Swan, S.H.; Chavarro, J.E. Semen quality in relation to antioxidant intake in a healthy male population. Fertil. Steril. 2013, 100, 1572–1579. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Safarinejad, S. The roles of omega-3 and omega-6 fatty acids in idiopathic male infertility. Asian J. Androl. 2012, 14, 514–515. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind, placebo-controlled, randomised study. Andrologia 2011, 43, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Nourmohamadi, M.; Hajipour, S.; Taghizadeh, M.; Asemi, Z.; Keshavarz, S.A.; Jafarnejad, S. The effect of omega-3 fatty acids, EPA, and/or DHA on male infertility: A systematic review and meta-analysis. J. Diet. Suppl. 2019, 16, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Falsig, A.L.; Gleerup, C.S.; Knudsen, U.B. The influence of omega-3 fatty acids on semen quality markers: A systematic PRISMA review. Andrology 2019, 7, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Flik, G.; Groenen, P.M.; Swinkels, D.W.; Thomas, C.M.; Copius-Peereboom, J.H.; Merkus, H.M.; Steegers-Theunissen, R.P. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001, 15, 131–136. [Google Scholar] [CrossRef]
- Machal, L.; Chládek, G.; Straková, E. Copper, phosphorus and calcium in bovine blood and seminal plasma in relation to semen quality. J. Anim. Feed Sci. 2002, 11, 425–435. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Zhou, W.; Gao, E. Effects of manganese on routine semen quality parameters: Results from a population-based study in China. BMC Publ. Health 2012, 12, 919. [Google Scholar] [CrossRef]
- Cheema, R.S.; Bansal, A.K.; Bilaspuri, G.S. Manganese provides antioxidant protection for sperm cryopreservation that may offer new consideration for clinical fertility. Oxid. Med. Cell. Longev. 2009, 2, 152–159. [Google Scholar] [CrossRef]
- Wirth, J.J.; Rossano, M.G.; Daly, D.C.; Paneth, N.; Puscheck, E.; Potter, R.C.; Diamond, M.P. Ambient manganese exposure is negatively associated with human sperm motility and concentration. Epidemiology 2007, 18, 270–273. [Google Scholar] [CrossRef]
- Knazicka, Z.; Tvrda, E.; Bardos, L.; Lukac, N. Dose- and time-dependent effect of copper ions on the viability of bull spermatozoa in different media. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2012, 47, 1294–1300. [Google Scholar] [CrossRef]
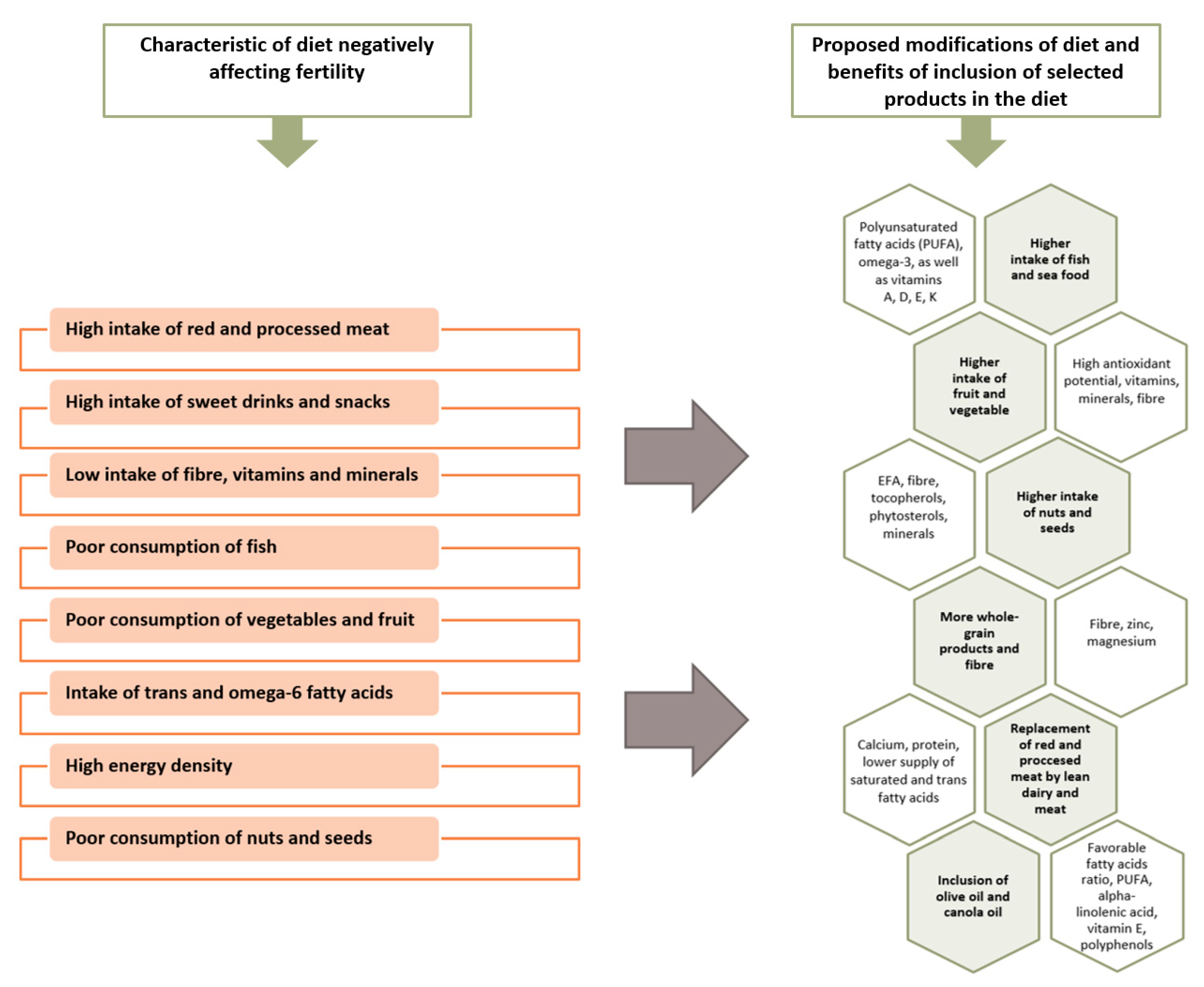
| Dietary Component/Items | Active Substances | Comments/Remarks |
|---|---|---|
| Oily sea fish | PUFA, omega-3 Fat-soluble vitamins A, D, E, K | Fish and seafood represent the main sources of DHA and EPA in the diet, therefore their incorporation in the diet may be associated with the improvement of semen quality [44]. Fish are often contaminated with mercury and other neurotoxic substances [44]. |
| Vegetables and fruit | Antioxidants, folic acid, fibre, minerals | Vegetables and fruits provide the basis for pro-healthy nutrition models, which are associated with the improvement of semen quality and fertility [6,7,20,25,83]. It is worth choosing raw vegetables and fruits. Research suggests that pesticide residues may modify the beneficial effect of fruit and vegetable consumption on the quality of semen [29]. |
| Nuts, seeds | EFAs, fibre, tocopherols, phytosterols, polyphenols, minerals | It is important to choose nuts and unroasted and unsalted seeds. The use of nuts in the diet may have a beneficial effect on the quality of sperm [84,85,86]. |
| Whole-grain products | Fibre, zinc, magnesium | It is recommended to limit the consumption of refined flour products and choose whole-grain products, which are rich in fibre [6,20]. |
| Lean dairy | Calcium, a wholesome protein | It is beneficial to choose low-fat dairy products, due to a lower saturated fat content [20]. |
| Olive oil, rapeseed oil | PUFA, alpha-linolenic acid, vitamin E, polyphenols | It is advisable to substitute saturated fats with vegetable oils containing unsaturated acid residues [6,7]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors. J. Clin. Med. 2020, 9, 1400. https://doi.org/10.3390/jcm9051400
Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors. Journal of Clinical Medicine. 2020; 9(5):1400. https://doi.org/10.3390/jcm9051400
Chicago/Turabian StyleSkoracka, Kinga, Piotr Eder, Liliana Łykowska-Szuber, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2020. "Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors" Journal of Clinical Medicine 9, no. 5: 1400. https://doi.org/10.3390/jcm9051400
APA StyleSkoracka, K., Eder, P., Łykowska-Szuber, L., Dobrowolska, A., & Krela-Kaźmierczak, I. (2020). Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors. Journal of Clinical Medicine, 9(5), 1400. https://doi.org/10.3390/jcm9051400







