Neuroendocrine Cancer of the Breast: A Rare Entity
Abstract
1. Introduction
2. Diagnosis
2.1. Definition of Neuroendocrine Breast Cancer
- The presence of over 50% of neoplastic cells expressing neuroendocrine markers of immunohistochemistry such as chromogranin A and synaptophysin (Figure 2). Neuron-specific enolase (NSE) and CD56 appear to result in lower sensitivity and specificity, mostly because they are normally present in breast tissue [2,5,6,11]. When the neuroendocrine characteristics are shown in less than 50% of cancer cells, the tumor should be identified as a breast cancer with neuroendocrine differentiation. The focal neuroendocrine differentiation within the carcinoma of the breast is common and has no prognostic significance.
- Excluding primary extra-mammary tumors, especially of the lung and the gastroenteric tract.
- The identification of a concomitant component in situ in the breast [6].
2.2. Characteristics
2.3. Genetic Indications
3. Prognostic Factors and Prognosis
3.1. Neuroendocrine Breast Cancer and the Somatostatin Receptors
3.2. The PI3K Pathway
4. Treatment
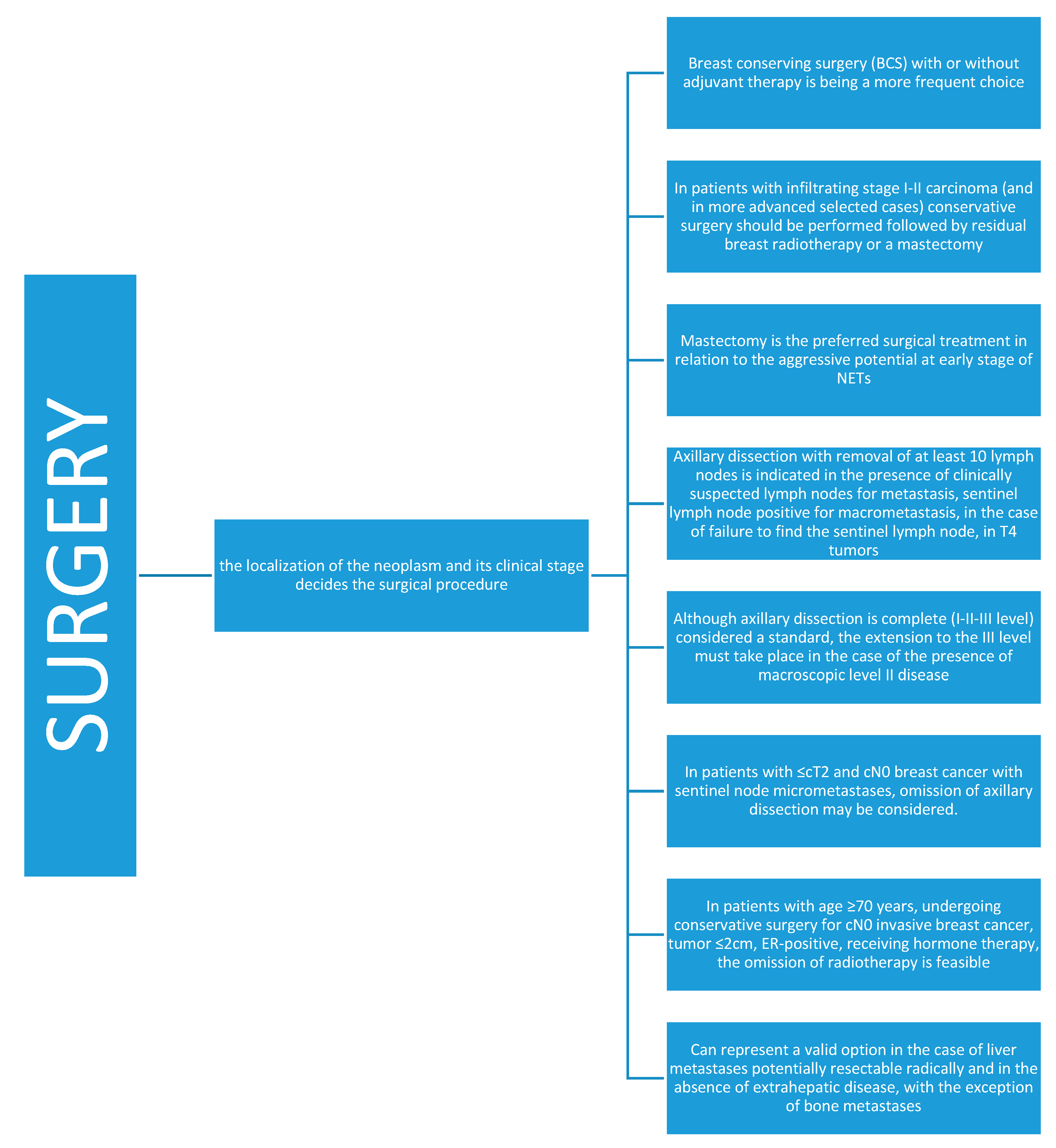
4.1. Surgery
4.2. Radiotherapy
4.3. Chemotherapy
4.4. Patient Studies
4.5. The Therapeutic Decision in NEBC
4.6. Radionuclides and Other Treatments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| STUDIES | TYPE OF STUDY | NUMBER OF CASES | |
|---|---|---|---|
| 1. | Cancers (Basel). 2020 Mar 20;12(3). pii: E733. doi: 10.3390/cancers12030733. | Clinicopathological study | 5 |
| 2. | ClinNucl Med. 2020 May;45(5):e232-e235. doi: 10.1097/RLU.0000000000003005. | Case report | 1 |
| 3. | Ann Ital Chir. 2020;91:23–26. | Clinicopathological study | 11 |
| 4. | Rev Esp Med NuclImagen Mol. 2019 May - Jun;38(3):147–153. doi: 10.1016/j.remn.2018.11.007. | Imaging histopathological study | 14 |
| 5. | Oncology. 2019;96(3):147–155. doi: 10.1159/000493348. | Immunohistochemical study | 36 |
| 6. | Clin Breast Cancer. 2019 Apr;19(2):131–136. doi: 10.1016/j.clbc.2018.09.001. | Clinicopathological and genetic study | 20 |
| 7. | Gan To Kagaku Ryoho. 2019 Dec;46(13):2222–2224. | Case report | 1 |
| 8. | Pathol Int. 2019 Aug;69(8):502–504. doi: 10.1111/pin.12832. | Case report | 1 |
| 9. | Gan To Kagaku Ryoho. 2018 Dec;45(13):2479–2481. | Case report | 1 |
| 10. | Gan To Kagaku Ryoho. 2018 Dec;45(13):2432–2434. | Case report | 1 |
| 11. | Gan To Kagaku Ryoho. 2018 Dec;45(13):1904–1906. | Case report | 1 |
| 12. | Indian J EndocrinolMetab. 2018 May-Jun;22(3):308–315. doi: 10.4103/ijem.IJEM_446_17. | Clinicopathological study | 2 |
| 13. | Ann DiagnPathol. 2018 Jun;34:122–130. doi: 10.1016/j.anndiagpath.2018.03.010 | Clinicopathological study | 36 |
| 14. | Gan To Kagaku Ryoho. 2018 Apr;45(4):682–684. | Case report | 1 |
| 15. | Breast J. 2018 Jul;24(4):644–647. doi: 10.1111/tbj.12969 | Case report | 1 |
| 16. | Gan To Kagaku Ryoho. 2017 Nov;44(12):1592–1594 | Case report | 1 |
| 17. | BMJ Case Rep. 2018 Jan 4;2018. pii: bcr-2017-223280. doi: 10.1136/bcr-2017-223280. | Case report | 1 |
| 18. | J Med Case Rep. 2017 Oct 19;11(1):290. doi: 10.1186/s13256-017-1467-0. | Case report | 1 |
| 19. | Mod Pathol. 2018 Jan;31(1):68–82. doi: 10.1038/modpathol.2017.107. | Clinicopathological and genetic study | 47 |
| 20. | Endokrynol Pol. 2017;68(5):597–602. doi: 10.5603/EP.a2017.0049. | Case report | 4 |
| 21. | Int J Surg Case Rep. 2017;38:29–31. doi: 10.1016/j.ijscr.2017.07.002. | Case report | 1 |
| 22. | Breast Dis. 2017;37(2):99–103. doi: 10.3233/BD-170283. | Case report | 1 |
| 23. | Chin J Cancer. 2017 May 11;36(1):45. doi: 10.1186/s40880-017-0211-x. | Case series and literature search | 7 + 119 |
| 24. | Pan Afr Med J. 2016 Nov 29;25:205. doi: 10.11604/pamj.2016.25.205.10366. | Case report | 1 |
| 25. | Breast J. 2017 Sep;23(5):589–593. doi: 10.1111/tbj.12800. | Case report | 1 |
| 26. | Breast Care (Basel). 2016 Dec;11(6):424–426. doi: 10.1159/000453572 | Case report | 1 |
| 27. | Gan To Kagaku Ryoho. 2016 Nov;43(12):2022–2025 | Case report | 1 |
| 28. | BMC Cancer. 2017 Jan 24;17(1):72. doi: 10.1186/s12885-017-3056-4. | Clinicopathological study | 43 |
| 29. | J Pathol. 2017 Feb;241(3):405–419. doi: 10.1002/path.4837 | Genetic study | 18 |
| 30. | Ann Ital Chir. 2016 Nov 28;87. pii: S2239253X1602658X | Case report | 5 |
| 31. | Breast Dis. 2016;36(4):161–164. doi: 10.3233/BD-160226. | Case report | 1 |
| 32. | Conn Med. 2016 Oct;80(9):525–528. | Case report | 1 |
| 33. | Pan Afr Med J. 2016 May 24;24:78. doi: 10.11604/pamj.2016.24.78.8546. eCollection 2016 | Case report | 1 |
| 34. | Mod Pathol. 2016 Aug;29(8):788–98. doi: 10.1038/modpathol.2016.69. | Clinicopathological study | 22 |
| 35. | Indian J Cancer. 2015 Oct-Dec;52(4):636–7. doi: 10.4103/0019-509X.178432 | Case report | 1 |
| 36. | Int J SurgPathol. 2016 Aug;24(5):463–7. doi: 10.1177/1066896916632909. | Case report | 1 |
| 37. | Indian J Cancer. 2015 Jan-Mar;52(1):85–6. doi: 10.4103/0019-509X.175563 | Case report | 1 |
| 38. | Gan To Kagaku Ryoho. 2015 Nov;42(12):1770-2 | Case report | 1 |
| 39. | J Breast Cancer. 2015 Dec;18(4):404–8. doi: 10.4048/jbc.2015.18.4.404 | Case report | 1 |
| 40. | Breast J. 2016 Jan-Feb;22(1):113–5. doi: 10.1111/tbj.12534. Epub 2015 Nov 25 | Case report | 1 |
| 41. | Breast Care (Basel). 2015 Aug;10(4):281–3. doi: 10.1159/000431070. | Case report | 1 |
| 42. | J Med Assoc Thai. 2015 Aug;98(8):828–32. | Case report | 1 |
| 43. | CeskPatol. 2015;51(3):128–31. | Case report | 1 |
| 44. | Cancer Radiother. 2015 Aug;19(5):308–12. doi: 10.1016/j.canrad.2015.04.003. | Clinicopathological study | 21 |
| 45. | Histopathology. 2016 Feb;68(3):422–32. doi: 10.1111/his.12766. | Clinicopathological study | 128 |
| 46. | Springerplus. 2015 Mar 21;4:138. doi: 10.1186/s40064-015-0913-y. eCollection 2015. | Epidemiological study | 199 |
| 47. | Breast J. 2015 May-Jun;21(3):303–7. doi: 10.1111/tbj.12403. | Case report | 1 |
| 48. | Breast J. 2015 May-Jun;21(3):312–3. doi: 10.1111/tbj.12413. Epub 2015 Mar 23. | Case report | 1 |
| 49. | Cytojournal. 2015 Jan 22;12:1. doi: 10.4103/1742-6413.149844. | Case report | 1 |
| 50. | ApplImmunohistochemMolMorphol. 2015 Feb;23(2):97–103. doi: 10.1097/PDM.0b013e3182a40fd1. | Genetic study | 9 |
| 51. | Bull Exp Biol Med. 2015 Jan;158(3):368–70. doi: 10.1007/s10517-015-2764-5. | Histopathological study | 21 |
| 52. | Virchows Arch. 2015 Apr;466(4):479–81. doi: 10.1007/s00428-014-1704-5. | Case report | 1 |
| 53. | Breast Cancer Res Treat. 2014 Dec;148(3):637–44. doi: 10.1007/s10549-014-3207-0. | Epidemiological study | 248 |
| 54. | Histopathology. 2015 Apr;66(5):755–6. doi: 10.1111/his.12606 | Histopathological study | 59 |
| 55. | Int J Surg. 2014;12 Suppl 2:S8-S11. doi: 10.1016/j.ijsu.2014.08.392. | Case report | 1 |
| 56. | Front Med. 2015 Mar;9(1):112–6. doi: 10.1007/s11684-014-0345-z. | Case report | 1 |
| 57. | Hum Pathol. 2014 Sep;45(9):1951–6. doi: 10.1016/j.humpath.2014.06.002. | Cytogenetic study | 7 |
| 58. | J Ultrasound Med. 2014 Aug;33(8):1511–8. doi: 10.7863/ultra.33.8.1511. | Imaging and Clinicopathological study | 11 |
| 59. | AJR Am J Roentgenol. 2014 Aug;203(2):W221–30. doi: 10.2214/AJR.13.10749. | Imaging and Clinicopathological study | 87 |
| 60. | Clin Imaging. 2014 Sep-Oct;38(5):734–8. doi: 10.1016/j.clinimag.2014.05.009. | Case report | 1 |
| 61. | Int J Surg Case Rep. 2014;5(8):540–3. doi: 10.1016/j.ijscr.2014.05.006. | Case report | 1 |
| 62. | Eur J GynaecolOncol. 2014;35(3):325–7. | Case report | 1 |
| 63. | J BUON. 2014 Apr-Jun;19(2):419–29 | Case report | 1 |
| 64. | BMC Cancer. 2014 Mar 4;14:147. doi: 10.1186/1471-2407-14-147. | Epidemiological study | 142 |
| 65. | Clin Breast Cancer. 2014 Oct;14(5):e99-e101. doi: 10.1016/j.clbc.2014.03.001 | Case report | 1 |
| 66. | Onco Targets Ther. 2014 May 6;7:663–6. doi: 10.2147/OTT.S60782. | Case report | 1 |
| 67. | J Thorac Oncol. 2014 Jun;9(6):e40–2. doi: 10.1097/JTO.0000000000000103. | Case report | 1 |
| 68. | ClinNucl Med. 2014 Apr;39(4):396–8. doi: 10.1097/RLU.0000000000000390. | Case report | 1 |
| 69. | Gan To Kagaku Ryoho. 2013 Nov;40(12):2363–5 | Case report | 1 |
| 70. | J Clin DiagnRes. 2013 Nov;7(11):2585–6. doi: 10.7860/JCDR/2013/7051.3621 | Case report | 1 |
| 71. | Int J Surg. 2013;11 Suppl 1:S79–83. doi: 10.1016/S1743-9191(13)60023-0. | Epidemiological study | 96 |
| 72. | J Breast Health. 2014 Oct 1;10(4):242–244. doi: 10.5152/tjbh.2014.1768 | Case report | 1 |
| 73. | J Breast Health. 2014 Apr 1;10(2):125–128. doi: 10.5152/tjbh.2014.1557 | Case report | 3 |
| 74. | J Breast Health. 2014 Apr 1;10(2):119–121. doi: 10.5152/tjbh.2014.1725 | Case report | 1 |
| 75. | Breast. 2014 Apr;23(2):120–7. doi: 10.1016/j.breast.2013.11.005. | Literature search | 123 |
| 76. | APMIS. 2014 Jul;122(7):585–92. doi: 10.1111/apm.12197. | Epidemiological study | 13 |
| 77. | APMIS. 2014 Jul;122(7):593–8. doi: 10.1111/apm.12198 | Immunohistochemical study | 18 |
| 78. | EndocrPathol. 2014 Sep;25(3):219–28. doi: 10.1007/s12022-013-9277-4. | Immunohistochemical and gene expression study | 28 |
| 79. | Histopathology. 2014 Mar;64(4):597–600. doi: 10.1111/his.12276. | Case report | 1 |
| 80. | J Coll Physicians Surg Pak. 2013 Nov;23(10):820–2. doi: 11.2013/JCPSP.820822. | Case report | 1 |
| 81. | Histopathology. 2014 Apr;64(5):647–59. doi: 10.1111/his.12306. | Clinicopathological study | 59 |
| 82. | Breast Dis. 2013 Jan 1;34(2):87–93. doi: 10.3233/BD-130356. | Clinicopathological study | 15 |
| 83. | Breast Dis. 2014;34(3):95–9. doi: 10.3233/BD-130357. | Clinicopathological study | 4 |
| 84. | World J Surg Oncol. 2013 Jun 5;11:128. doi: 10.1186/1477-7819-11-128. | Case report | 1 |
| 85. | Breast J. 2013 Jul-Aug;19(4):382–7. doi: 10.1111/tbj.12121. | Clinicopathological study | 22 |
| 86. | Korean J Radiol. 2013 May-Jun;14(3):395–9. doi: 10.3348/kjr.2013.14.3.395. | Case report | 1 |
| 87. | PLoS One. 2013 Apr 22;8(4):e62487. doi: 10.1371/journal.pone.0062487. | Epidemiological study | 34 |
| 88. | Pathology. 2013 Jun;45(4):422–4. doi: 10.1097/PAT.0b013e328360dfd1. | Case report | 1 |
| 89. | J Coll Physicians Surg Pak. 2013 Apr;23(4):282–4. doi: 04.2013/JCPSP.282284. | Case report | 1 |
| 90. | Breast J. 2013 Mar-Apr;19(2):204–6. doi: 10.1111/tbj.12081 | Case report | 1 |
| 91. | ZhonghuaZhong Liu ZaZhi. 2012 Dec;34(12):917–22. doi: 10.3760/cma.j.issn.0253-3766.2012.12.008. | Imaging and clinicopathological study | 16 |
| 92. | Pan Afr Med J. 2012;13:40 | Case report | 1 |
| 93. | Clujul Med. 2013;86(2):156–9. | Case report | 1 |
| 94. | Pan Afr Med J. 2013 Nov 11;16:92. doi: 10.11604/pamj.2013.16.92.2531. | Case report | 1 |
| 95. | Neoplasma. 2013;60(2):215–22. | Clinicopathological study | 107 |
| 96. | Anticancer Res. 2012 Dec;32(12):5227–32. | Immunohistochemical study | 31 |
| 97. | CollAntropol. 2012 Sep;36(3):1053–5. | Case report | 1 |
| 98. | Anticancer Res. 2012 Nov;32(11):5079–82. | Case report | 1 |
| 99. | Am Surg. 2012 Nov;78(11):E457–8. | Case report | 1 |
| 100. | Breast Care (Basel). 2012 Oct;7(5):408–10. doi: 10.1159/000343612. | Case report | 1 |
| 101. | BMJ Case Rep. 2012 Aug 13;2012. pii: bcr1220115343. doi: 10.1136/bcr.12.2011.5343. | Case report | 1 |
| 102. | Clin Imaging. 2012 Sep-Oct;36(5):599–601. doi: 10.1016/j.clinimag.2011.10.004. | Case report | 1 |
| 103. | J CutanPathol. 2012 Nov;39(11):1042–6. doi: 10.1111/j.1600-0560.2012.01970.x. | Case report | 1 |
| 104. | Breast Cancer. 2015 Jul;22(4):432-6. doi: 10.1007/s12282-012-0382-x. | Case report | 1 |
| 105. | Ann Ital Chir. 2013 Jan-Feb;84(1):81–5. | Case report | 1 |
| 106. | Chin J Cancer. 2012 Nov;31(11):549–56. doi: 10.5732/cjc.011.10370 | Imaging and clinicopathological study | 13 |
| 107. | J ClinPathol. 2012 Aug;65(8):699–703. doi: 10.1136/jclinpath-2012-200765. | Immunohistochemical study | 3 |
| 108. | Pathol Int. 2012 May;62(5):331–4. doi: 10.1111/j.1440-1827.2012.02786.x | Case report | 1 |
| 109. | Clin Breast Cancer. 2012 Aug;12(4):300–3. doi: 10.1016/j.clbc.2012.03.001 | Case report | 4 |
| 110. | Pathology. 2012 Apr;44(3):273–5. doi: 10.1097/PAT.0b013e3283513feb. | Case report | 1 |
| 111. | Breast. 2012 Oct;21(5):652–6. doi: 10.1016/j.breast.2012.01.016. | Clinicopathological study | 24 |
| 112. | J Korean Surg Soc. 2012 Feb;82(2):116–9. doi: 10.4174/jkss.2012.82.2.116. | Case report | 1 |
| 113. | Zhonghua Bing Li XueZaZhi. 2011 Sep;40(9):604–9 | Histopathological study | 22 |
| 114. | Surg Today. 2011 Nov;41(11):1575–8. doi: 10.1007/s00595-010-4516-5. | Case report | 1 |
| 115. | Neuro Endocrinol Lett. 2011;32(4):405–7. | Case report | 1 |
| 116. | WMJ. 2011 Jun;110(3):140–3 | Case report | 1 |
| 117. | Breast Cancer. 2014 Jul;21(4):508–13. doi: 10.1007/s12282-011-0278-1 | Case report | 1 |
| 118. | Clin Breast Cancer. 2011 Oct;11(5):342–5. doi: 10.1016/j.clbc.2011.02.006 | Case report | 1 |
| 119. | J Coll Physicians Surg Pak. 2011 Jun;21(6):371–3. doi: 07.2011/JCPSP.371373. | Case report | 1 |
| 120. | DiagnCytopathol. 2011 Jul;39(7):527–30. doi: 10.1002/dc.21494 | Case report | 1 |
| 121. | Histopathology. 2011 Jul;59(1):106–15. doi: 10.1111/j.1365-2559.2011.03880.x. | Histopathological study | 74 |
| 122. | Ann Ital Chir. 2011 Jan-Feb;82(1):61–4. | Case report | 1 |
| 123. | Surg Today. 2011 Jun;41(6):829–31. doi: 10.1007/s00595-010-4351-8. | Case report | 1 |
| 124. | Int J ImmunopatholPharmacol. 2011 Jan-Mar;24(1):251–6. | Epidemiological study | 23 |
| 125. | Hum Pathol. 2011 Aug;42(8):1169–77. doi: 10.1016/j.humpath.2010.11.014. | Clinicopathological study | 74 |
| 126. | J ClinPathol. 2011 Jun;64(6):549–51. doi: 10.1136/jcp.2011.089219 | Case report | 1 |
| 127. | J Med Dent Sci. 2010 Jun;57(2):155–63. | Histopathological study | 34 |
| 128. | Breast J. 2010 Nov-Dec;16(6):647–51. doi: 10.1111/j.1524-4741.2010.00974.x. | Case report | 1 |
| 129. | Breast Cancer Res Treat. 2011 Feb;126(1):241–5. doi: 10.1007/s10549-010-1229-9. | Clinicopathological and histopathological study | 8 |
| 130. | Ann Chir Plast Esthet. 2012 Dec;57(6):630–3. doi: 10.1016/j.anplas.2010.09.009. | Case report | 1 |
| 131. | Breast Cancer Res Treat. 2010 Nov;124(2):413–7. doi: 10.1007/s10549-010-1178-3. | Histopathological study | 9 |
| 132. | Surg Today. 2010 Sep;40(9):831–5. doi: 10.1007/s00595-009-4179-2. | Histopathological study | 13 |
| 133. | Int J ClinExpPathol. 2010 Jun 30;3(6):629–33. | Case report | 1 |
| 134. | J Med Case Rep. 2010 Jun 30;4:201. doi: 10.1186/1752-1947-4-201. | Case report | 1 |
| 135. | Cancer. 2010 Oct 1;116(19):4463–73. doi: 10.1002/cncr.25352. | Case-controlled study | 74 |
| 136. | Tunis Med. 2010 Apr;88(4):290–2 | Case report | 1 |
| 137. | Cytopathology. 2011 Feb;22(1):43–9. doi: 10.1111/j.1365-2303.2010.00742.x. | Cytopathological study | 32 |
| 138. | Can J Surg. 2009 Dec;52(6):E289–90 | Case report | 1 |
| 139. | Breast Care (Basel). 2009 Nov;4(5):324–327. doi: 10.1159/000236226. | Case report | 1 |
| 140. | Indian J Cancer. 2009 Oct-Dec;46(4):352–4. doi: 10.4103/0019-509X.55566 | Case report | 1 |
| 141. | Cancer Radiother. 2009 Dec;13(8):775–7. doi: 10.1016/j.canrad.2009.06.021 | Case report | 1 |
| 142. | Breast Cancer. 2012 Oct;19(4):360–4. doi: 10.1007/s12282-009-0160-6 | Case report | 1 |
| 143. | Mod Pathol. 2009 Nov;22(11):1401–14. doi: 10.1038/modpathol.2009.112 | Genetic study | 6 |
| 144. | Chir Ital. 2009 Mar-Apr;61(2):265–7 | Case report | 1 |
| 145. | ClinNucl Med. 2009 Feb;34(2):74–5. doi: 10.1097/RLU.0b013e318192c343 | Case report | 1 |
| 146. | Med MolMorphol. 2009 Mar;42(1):58–61. doi: 10.1007/s00795-008-0432-9. | Case report | 1 |
| 147. | Breast J. 2009 Mar-Apr;15(2):205–6. doi: 10.1111/j.1524-4741.2009.00700.x | Case report | 1 |
| 148. | Int J Surg. 2008;6 Suppl 1:S113–5. doi: 10.1016/j.ijsu.2008.12.007. | Observational study | 35 |
| 149. | Indian J PatholMicrobiol. 2009 Jan-Mar;52(1):71–3. | Case report | 1 |
| 150. | J Korean Med Sci. 2008 Dec;23(6):1118–20. doi: 10.3346/jkms.2008.23.6.1118. | Case report | 1 |
| 151. | Indian J Med PaediatrOncol. 2009 Jan;30(1):31–4. doi: 10.4103/0971-5851.56334. | Case report | 1 |
| 152. | Malays J Pathol. 2008 Jun;30(1):57–61. | Case report | 1 |
| 153. | Oncol Rep. 2008 Dec;20(6):1369–74. | Clinicopathological study | 12 |
| 154. | Anal Quant CytolHistol. 2008 Oct;30(5):306–8. | Case report | 1 |
| 155. | Pathologica. 2008 Jun;100(3):176–80 | Case report | 2 |
| 156. | J Ultrasound Med. 2008 Sep;27(9):1401–5. | Case report | 1 |
| 157. | Surg Today. 2008;38(8):734–8. doi: 10.1007/s00595-007-3716-0 | Case report | 1 |
| 158. | Histopathology. 2008 Sep;53(3):288–98. doi: 10.1111/j.1365-2559.2008.03093.x | Clinicopathological study | 20 |
| 159. | Pathol Res Pract. 2008;204(11):851–5. doi: 10.1016/j.prp.2008.04.006. | Case report | 1 |
| 160. | G Chir. 2008 May;29(5):203–6 | Case series | 10 |
| 161. | Cir Esp. 2008 May;83(5):273–4 | Case report | 1 |
| 162. | Clin Breast Cancer. 2007 Dec;7(11):892–4. doi: 10.3816/CBC.2007.n.056. | Case report | 1 |
| 163. | Radiat Med. 2008 Jan;26(1):28–32. doi: 10.1007/s11604-007-0182-y. | Case report | 1 |
| 164. | DiagnCytopathol. 2008 Jan;36(1):54–7. | Case report | 1 |
| 165. | Am J Clin Dermatol. 2007;8(6):379–83. | Case report | 1 |
| 166. | Tumori. 2007 Sep-Oct;93(5):496–8. | Case report | 1 |
| 167. | Breast Cancer. 2007;14(4):414–9. | Case report | 1 |
| 168. | Breast. 2008 Feb;17(1):111–4. | Case report | 1 |
| 169. | Breast Cancer. 2007;14(2):250–3. | Case report | 1 |
| 170. | Eur J GynaecolOncol. 2007;28(2):142–4. | Case report | 1 |
| 171. | Indian J PatholMicrobiol. 2007 Jan;50(1):65–8. | Case report | 1 |
| 172. | Int J Cancer. 2007 Jul 1;121(1):127–35. | Epidemiological study | 76 |
| 173. | J ClinPathol. 2006 Aug;59(8):888 | Case report | 2 |
| 174. | Am Surg. 2006 Feb;72(2):185–7. | Case report | 1 |
| 175. | Breast J. 2005 Nov-Dec;11(6):487 | Case report | 1 |
| 176. | J ClinPathol. 2005 Jul;58(7):775–8. | Case report | 3 |
| 177. | J Exp Clin Cancer Res. 2004 Dec;23(4):691–6. | Case report | 1 |
| 178. | Breast. 2004 Dec;13(6):527–9. | Case report | 1 |
| 179. | Breast. 2004 Aug;13(4):329–33. | Clinicopathological study | 9 |
| 180. | Eur J ObstetGynecolReprod Biol. 2004 Aug 10;115(2):231–3. | Case report | 1 |
| 181. | J Nippon Med Sch. 2004 Jun;71(3):203–8. | Case report | 1 |
| 182. | Ann Pathol. 2004 Jun;24(3):278–83 | Case report | 1 |
| 183. | Chin Med J (Engl). 2004 Oct;117(10):1536–40 | Clinicopathological study | 81 |
References
- De Santis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.; Menet, E.; Tille, J.C.; Lae, M.; Fuhrmann, L.; Bonneau, C.; Deniziaut, G.; Melaabi, S.; Ng, C.C.K.; Marchiò, C.; et al. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod. Pathol. 2018, 31, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Palazzo, J.; Sanati, S.; Florento, E.; Contreras, E.; Xiu, J.; Swensen, J.; Gatalica, Z. Potential Novel Therapy Targets in Neuroendocrine Carcinomas of the Breast. Clin. Breast Cancer 2019, 19, 131–136. [Google Scholar] [CrossRef]
- Cheymol, C.; Abramovici, O.; Do Cao, C.; Dumont, A.; Robin, Y.M.; El Hajbi, F.; Dansin, E.; Bonneterre, J.; Lauridant, G. Neuroendocrine tumors of the breast: Myth or reality? A systematic review. Bull. Cancer 2018, 105, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Bogina, G.; Turazza, M.; Bortesi, L.; Duranti, S.; Massocco, A.; Zamboni, G.; Carbognin, G.; Alongi, F.; Salgarello, M.; et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist 2016, 21, 28–32. [Google Scholar] [CrossRef] [PubMed]
- AbouDalle, I.; Abbas, J.; Boulos, F.; Salem, Z.; Assi, H.I. Primary small cell carcinoma of the breast: A case report. J. Med. Case Rep. 2017, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.W.; Dyson, P.; Barthelmes, L. Neuroendocrine breast tumours: Breast cancer or neuroendocrine cancer presenting in the breast? Breast 2014, 23, 120–127. [Google Scholar] [CrossRef]
- Bussolati, G.; Gugliotta, P.; Sapino, A.; Eusebi, V.; Lloyd, R.V. Chromogranin-reactive endocrine cells in argyrophilic carcinomas (“carcinoids”) and normal tissue of the breast. Am. J. Pathol. 1985, 120, 186–192. [Google Scholar]
- Feyrter, F.; Hartmann, G. On the carcinoid growth form of the carcinoma mammae, specially the carcinoma solidum (Gelatinosum) mammae. Frankf. Z. Pathol. 1963, 73, 24–39. [Google Scholar]
- Cubilla, A.L.; Woodruff, J.M. Primary carcinoid tumor of the breast. A report of eight patients. Am. J. Surg. Pathol. 1977, 1, 283–292. [Google Scholar] [CrossRef]
- KeltenTalu, C.; Leblebici, C.; KilicaslanOzturk, T.; Hacihasanoglu, E.; Baykal Koca, S.; Gucin, Z. Primary breast carcinomas with neuroendocrine features: Clinicopathological features and analysis of tumor growth patterns in 36 cases. Ann. Diagn. Pathol. 2018, 34, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.M.; Ma, T.K.; Chu, W.C.; Lam, W.W.; Poon, C.S.; Chan, W.C. Neuroendocrine differentiation in pure type mammary mucinous carcinoma is associated with favorable histologic and immunohistochemical parameters. Mod. Pathol. 2004, 17, 568–572. [Google Scholar] [CrossRef]
- Park, Y.M.; Wu, Y.; Wei, W.; Yang, W.T. Primary neuroendocrine carcinoma of the breast: Clinical, imaging, and histologic features. AJR Am. J. Roentgenol. 2014, 203, W221–W230. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Yang, R.L.; Allison, K.H.; Norton, J.A.; Hernandez-Boussard, T.; Wapnir, I.L. Impact of histological subtype on long-term outcomes of neuroendocrine carcinoma of the breast. Breast Cancer Res. Treat. 2014, 148, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.T.; van der Zwan, J.M.; de Herder, W.W.; van Velthuysen, M.F. Importance of complete pathology reporting for neuroendocrine carcinoma: WHO guidelines are a good start but not enough. Neuroendocrinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Gori, S.; Dieci, M.V.; Biganzoli, L.; Calabrese, M.; Cortesi, L.; Criscitiello, C.; Del Mastro, L.; Dellepiane, C.; Fortunato, L.; Franco, P.; et al. AIOM Guidelines. 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Mammella.pdf (accessed on 16 March 2020).
- Brask, J.B.; Talman, M.L.; Wielenga, V.T. Tissue microarray analysis as a screening tool for neuroendocrine carcinoma of the breast. APMIS 2014, 122, 593–598. [Google Scholar] [CrossRef]
- Arslan, E.; FikretÇermik, T.; Didem Can Trabulus, F.; KeltenTalu, C.; Başaran, Ş. Diagnostic impact of 18F-FDG PET/CT on the management of rare breast carcinomas: Apocrine and neuroendocrine carcinomas. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 147–153. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Kim, S.A.; DeLair, D.F.; Bose, S.; Laury, A.R.; Chopra, S.; Mertens, R.B.; Dhall, D. Comparison of metastatic neuroendocrine neoplasms to the breast and primary invasive mammary carcinomas with neuroendocrine differentiation. Mod. Pathol. 2016, 29, 788–798. [Google Scholar] [CrossRef]
- Gevorgyan, A.; Bregni, G.; Galli, G.; Zanardi, E.; de Braud, F.; Di Cosimo, S. HER2-Positive Neuroendocrine Breast Cancer: Case Report and Review of Literature. Breast Care 2016, 11, 424–426. [Google Scholar] [CrossRef]
- Tremelling, A.; Samuel, S.; Murray, M. Primary small cell neuroendocrine carcinoma of the breast—A case report and review of the literature. Int. J. Surg. Case Rep. 2017, 38, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Bogina, G.; Munari, E.; Brunelli, M.; Bortesi, L.; Marconi, M.; Sommaggio, M.; Lunardi, G.; Gori, S.; Massocco, A.; Pegoraro, M.C.; et al. Neuroendocrine differentiation in breast carcinoma: Clinicopathological features and outcome. Histopathology 2016, 68, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Geyer, F.C.; Ng, C.K.; Piscuoglio, S.; De Filippo, M.R.; Cupo, M.; Schultheis, A.M.; Lim, R.S.; Burke, K.A.; Guerini-Rocco, E.; et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J. Pathol. 2017, 241, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Roininen, N.; Takala, S.; Haapasaari, K.M.; Jukkola-Vuorinen, A.; Mattson, J.; Heikkilä, P.; Karihtala, P. Primary neuroendocrine breast carcinomas are associated with poor local control despite favourable biological profile: A retrospective clinical study. BMC Cancer 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiang, D.B.; Wei, B.; Abraham, S.C.; Huo, L.; Albarracin, C.T.; Zhang, H.; Babiera, G.; Caudle, A.S.; Akay, C.L.; Rao, P.; et al. Molecular cytogenetic characterization of mammary neuroendocrine carcinoma. Hum. Pathol. 2014, 45, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Ding, T.; Xing, Y.; Wei, W.; Tian, Z.; Tang, F.; Abraham, S.; Nayeemuddin, K.; Hunt, K.; Wu, Y. Invasive neuroendocrine carcinoma of the breast: A distinctive subtype of aggressive mammary carcinoma. Cancer 2010, 116, 4463–4473. [Google Scholar] [CrossRef]
- Tian, Z.; Wei, B.; Tang, F.; Wei, W.; Gilcrease, M.Z.; Huo, L.; Albarracin, C.T.; Resetkova, E.; Middleton, L.; Sahin, A.; et al. Prognostic significance of tumor grading and staging in mammary carcinomas with neuroendocrine differentiation. Hum. Pathol. 2011, 42, 1169–1177. [Google Scholar] [CrossRef]
- López-Bonet, E.; Alonso-Ruano, M.; Barraza, G.; Vazquez-Martin, A.; Bernadó, L.; Menendez, J.A. Solid neuroendocrine breast carcinomas: Incidence, clinico-pathological features and immunohistochemical profiling. Oncol. Rep. 2008, 20, 1369–1374. [Google Scholar]
- Rovera, F.; Masciocchi, P.; Coglitore, A.; La Rosa, S.; Dionigi, G.; Marelli, M.; Boni, L.; Dionigi, R. Neuroendocrine carcinomas of the breast. Int. J. Surg. 2008, 6 (Suppl. 1), S113–S115. [Google Scholar] [CrossRef]
- Roininen, N.; Takala, S.; Haapasaari, K.M.; Jukkola-Vuorinen, A.; Mattson, J.; Heikkilä, P.; Karihtala, P. NeuroendocrineBreast Carcinomas Share Prognostic Factors with GastroenteropancreaticNeuroendocrine Tumors: A Putative Prognostic Role of Menin, p27, and SSTR-2A. Oncology 2019, 96, 147–155. [Google Scholar] [CrossRef]
- Terlević, R.; PerićBalja, M.; Tomas, D.; Skenderi, F.; Krušlin, B.; Vranic, S.; Demirović, A. Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrinebreast cancer. Ann. Diagn. Pathol. 2019, 38, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Del Rio-Moreno, M.; Alors-Perez, E.; Borges de Souza, P.; Prados-Gonzalez, M.E.; CastaÑo, J.P.; Luque, R.M.; Gahete, M.D. Peptides derived from the extracellular domain of the somatostatin receptor splicing variant SST5TMD4 increase malignancy in multiple cancer cell types. Transl. Res. 2019, 211, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Irelli, A.; Sirufo, M.M.; Scipioni, T.; De Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. mTOR Links Tumor Immunity and Bone Metabolism: What are the Clinical Implications? Int. J. Mol. Sci. 2019, 20, 5841. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Ceccarelli, C.; Brighi, N.; Maggio, I.; Santini, D.; Mosconi, C.; Ricci, C.; Biasco, G.; Campana, D. Determination of Mammalian Target of Rapamycin Hyperactivation as Prognostic Factor in Well-Differentiated Neuroendocrine Tumors. Gastroenterol. Res. Pract. 2017, 7872519. [Google Scholar] [CrossRef]
- Massimini, M.; Palmieri, C.; De Maria, R.; Romanucci, M.; Malatesta, D.; De Martinis, M.; Maniscalco, L.; Ciccarelli, A.; Ginaldi, L.; Buracco, P.; et al. 17-AAG and Apoptosis, Autophagy and Mitophagy in Canine Osteosarcoma Cell Lines. Vet. Pathol. 2017, 54, 405–412. [Google Scholar] [CrossRef]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int. J. Mol. Sci. 2018, 19, 747. [Google Scholar] [CrossRef]
- Lu, C.S.; Huang, S.H.; Ho, C.L.; Chen, J.H.; Chao, T.Y. Primaryneuroendocrine carcinoma of the breast. J. BUON 2014, 19, 419–429. [Google Scholar]
- National Comprehensive Cancer Network. Breast Cancer (Version 3). 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 16 March 2020).
- Wade, P.M., Jr.; Mills, S.E.; Read, M.; Cloud, W.; Lambert, M.J., III; Smith, R.E. Small cell neuroendocrine (oat cell) carcinoma of the breast. Cancer 1983, 52, 121–125. [Google Scholar] [CrossRef]
- Richter-Ehrenstein, C.; Arndt, J.; Buckendahl, A.C.; Eucker, J.; Weichert, W.; Kasajima, A.; Schneider, A.; Noske, A. Solid neuroendocrine carcinomas of the breast: Metastases or primary tumors? Breast Cancer Res. Treat. 2010, 124, 413–417. [Google Scholar] [CrossRef]
- Hare, F.; Giri, S.; Patel, J.K.; Hahn, A.; Martin, M.G. A population-based analysis of outcomes for small cell carcinoma of the breast by tumor stage and the use of radiation therapy. Springerplus 2015, 4, 138. [Google Scholar] [CrossRef]
- Alkaied, H.; Harris, K.; Azab, B.; Dai, Q. Primary neuroendocrine breast cancer, how much do we know so far? Med. Oncol. 2012, 29, 2613–2618. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Ahmed, M. Rare epithelial breast cancer: Surgery and adjuvant therapy. Transl. Cancer Res. 2019, 8, S479–S492. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Perez, E.A.; Olson, J.A., Jr. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef]
- Yildirim, Y.; Elagoz, S.; Koyuncu, A.; Aydin, C.; Karadayi, K. Management of neuroendocrine carcinomas of the breast: A rare entity. Oncol. Lett. 2011, 2, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, F.; Zhu, W.; Xu, B. Neuroendocrine carcinoma of the breast: A review of 126 cases in China. Chin. J. Cancer 2017, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Canbak, T.; Acar, A.; Tolan, H.K.; Ozbagriacik, M.; Ezberci, F. Primary neuroendocrine carcinoma of the breast: A 5-year experiences. Ann. Ital. Chir. 2020, 91, 23–26. [Google Scholar] [PubMed]
- Irelli, A.; Sirufo, M.M.; Scipioni, T.; De Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. Breast cancer patients receiving denosumab during adjuvant aromatase inhibitors treatment: Who are the “inadequate responders” patients to denosumab? J. BUON 2020, 25, 2, in press. [Google Scholar]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Savelli, G.; Zaniboni, A.; Bertagna, F.; Bosio, G.; Nisa, L.; Rodella, C.; Biasiotto, G.; Bettinsoli, G.; Migliorati, E.; Peli, A.; et al. Peptide Receptor Radionuclide Therapy (PRRT) in a Patient Affected by Metastatic Breast Cancer with Neuroendocrine Differentiation. Breast Care 2012, 7, 408–410. [Google Scholar] [CrossRef]
- Scaramuzzi, G.; Murgo, R.M.; Cuttitta, A.; Ciuffreda, L. Neuroendocrine carcinoma of the breast. Our experience and a proposal of a therapeutic algorithm for a rare tumor. G. Chir. 2008, 29, 203–206. [Google Scholar]
- Abdel-Rahman, O. Vascular endothelial growth factor (VEGF) pathway and neuroendocrine neoplasms (NENs): Prognostic and therapeutic considerations. Tumour Biol. 2014, 35, 10615–10625. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.A.; Minucci, S.; Spada, F.; Galdy, S.; Elgendy, M.; Ravenda, P.S.; Zampino, M.G.; Murgioni, S.; Fazio, N. Dual inhibition of mTOR pathway and VEGF signalling in neuroendocrine neoplasms: From bench to bedside. Cancer Treat. Rev. 2015, 41, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.E.; Gattuso, P. Neuroendocrine Tumors of the Breast. Arch. Pathol. Lab. Med. 2017, 141, 1577–1581. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irelli, A.; Sirufo, M.M.; Morelli, L.; D’Ugo, C.; Ginaldi, L.; De Martinis, M. Neuroendocrine Cancer of the Breast: A Rare Entity. J. Clin. Med. 2020, 9, 1452. https://doi.org/10.3390/jcm9051452
Irelli A, Sirufo MM, Morelli L, D’Ugo C, Ginaldi L, De Martinis M. Neuroendocrine Cancer of the Breast: A Rare Entity. Journal of Clinical Medicine. 2020; 9(5):1452. https://doi.org/10.3390/jcm9051452
Chicago/Turabian StyleIrelli, Azzurra, Maria Maddalena Sirufo, Luca Morelli, Carlo D’Ugo, Lia Ginaldi, and Massimo De Martinis. 2020. "Neuroendocrine Cancer of the Breast: A Rare Entity" Journal of Clinical Medicine 9, no. 5: 1452. https://doi.org/10.3390/jcm9051452
APA StyleIrelli, A., Sirufo, M. M., Morelli, L., D’Ugo, C., Ginaldi, L., & De Martinis, M. (2020). Neuroendocrine Cancer of the Breast: A Rare Entity. Journal of Clinical Medicine, 9(5), 1452. https://doi.org/10.3390/jcm9051452







