The Marker of Tubular Injury, Kidney Injury Molecule-1 (KIM-1), in Acute Kidney Injury Complicating Acute Pancreatitis: A Preliminary Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Sarr, M.G.; Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Tsiotos, G.G.; Vege, S.S. The new revised classification of acute pancreatitis 2012. Surg. Clin. N. Am. 2013, 93, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drożdż, R.; Kuśnierz-Cabala, B. The interplay between inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: Clinical implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with Acute Pancreatitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Wajda, J.; Dumnicka, P.; Maraj, M.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. Potential prognostic markers of acute kidney injury in the early phase of acute pancreatitis. Int. J. Mol. Sci. 2019, 20, 3714. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [Green Version]
- Gougol, A.; Dugum, M.; Dudekula, A.; Greer, P.; Slivka, A.; Whitcomb, D.C.; Yadav, D.; Papachristou, G.I. Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J. Gastroenterol. 2017, 23, 5431–5437. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Q.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. High urinary excretion of kidney injury molecule-1 predicts adverse outcomes in acute kidney injury: A case control study. Crit. Care 2016, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hsu, R.K.; Hsu, C.Y. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 283–292. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute kidney injury: Diagnostic approaches and controversies. Clin. Biochem. Rev. 2016, 27, 153–175. [Google Scholar]
- Schley, G.; Köberle, C.; Manuilova, E.; Rutz, S.; Forster, C.; Weyand, M.; Formentini, I.; Kientsch-Engel, R.; Eckardt, K.-U.; Willam, C. Comparison of Plasma and Urine Biomarker Performance in Acute Kidney Injury. PLoS ONE 2015, 10, e0145042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moresco, R.N.; Bochi, G.V.; Stein, C.S.; De Carvalho, J.A.M.; Cembranel, B.M.; Bollick, Y.S. Urinary kidney injury molecule-1 in renal disease. Clin. Chim. Acta 2018, 487, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wang, N. Kidney injury molecule-1 in kidney disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar] [PubMed]
- Nowak, N.; Skupien, J.; Niewczas, M.A.; Yamanouchi, M.; Major, M.; Croall, S.; Smiles, A.; Warram, J.H.; Bonventre, J.V.; Krolewski, A.S. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016, 89, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perco, P.; Oberbauer, R. Kidney injury molecule-1 as a biomarker of acute kidney injury in renal transplant recipients—Commentary. Nat. Clin. Pract. Nephrol. 2008, 4, 362–363. [Google Scholar] [CrossRef]
- Huang, Y.; Don-Wauchope, A.C. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: A systematic review. Inflamm. Allergy Drug Targets 2011, 10, 260–271. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, P.A. Acute Pancreatitis: Landmark Studies, Management Decisions, and the Future. Pancreas 2016, 45, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Tu, Y.; Wang, H.; Sun, R.; Ni, Y.; Ma, L.; Xv, F.; Hu, X.; Jiang, L.; Wu, A.; Chen, X.; et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren. Fail. 2014, 36, 1559–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khreba, N.A.; Abdelsalam, M.; Wahab, A.M.; Sanad, M.; Elhelaly, R.; Adel, M.; El-Kannishy, G. Kidney Injury Molecule 1 (KIM-1) as an Early Predictor for Acute Kidney Injury in Post-Cardiopulmonary Bypass (CPB) in Open Heart Surgery Patients. Int. J. Nephrol. 2019, 2019, 6265307. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Shao, X.; Xie, Y.; Wang, Q.; Che, X.; Zhang, M.; Xu, W.; Xu, Y.; Mou, S.; Ni, Z. Kidney Injury Molecule-1 is Elevated in Nephropathy and Mediates Macrophage Activation via the Mapk Signalling Pathway. Cell. Physiol. Biochem. 2017, 41, 769–783. [Google Scholar] [CrossRef]
- Xue, W.; Xie, Y.; Wang, Q.; Xu, W.; Mou, S.; Ni, Z. Diagnostic performance of urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin for acute kidney injury in an obstructive nephropathy patient. Nephrology 2014, 19, 186–194. [Google Scholar] [CrossRef]
- Lei, L.; Li, L.P.; Zeng, Z.; Mu, J.X.; Yang, X.; Zhou, C.; Wang, Z.L.; Zhang, H. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Van Timmeren, M.; van den Heuvel, M.; Bailly, V.; Bakker, S.; van Goor, H.; Stegeman, C. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. 2007, 212, 209–217. [Google Scholar] [CrossRef]
- Tang, Y.; Mak, S.K.; Xu, A.P.; Lan, H.Y. Role of C-reactive protein in the pathogenesis of acute kidney injury. Nephrology 2018, 23, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Castellani, M.L.; De Lutiis, M.A.; Toniato, E.; Conti, F.; Felaco, P.; Fulcheri, M.; Theoharides, T.C.; Caraffa, A.; Antinolfi, P.; Conti, P.; et al. Impact of RANTES, MCP-1 and IL-8 in mast cells. J. Biol. Regul. Homeost. Agents 2010, 24, 1–6. [Google Scholar] [PubMed]
- Baer, P.C.; Gauer, S.; Wegner, B.; Schubert, R.; Geiger, H. C-reactive protein induced activation of MAP-K and RANTES in human renal distal tubular epithelial cells in vitro. Clin. Nephrol. 2006, 66, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chung, A.C.K.; Zhou, L.; Huang, X.R.; Liu, F.; Fu, P.; Fan, J.M.; Szalai, A.J.; Lan, H.Y. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab. Investig. 2011, 91, 837–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeeha, R.; Skinner, D.L.; De Vasconcellos, K.; Magula, N.P. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology 2018, 23, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Nie, X.; Cai, B.; Tang, J.-T.; He, Y.; Miao, Q.; Song, H.-L.; Luo, T.-X.; Gao, B.-X.; Wang, L.-L.; et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: A prospective study. PLoS ONE 2013, 8, e82250. [Google Scholar] [CrossRef] [Green Version]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar]
- Ishani, A.; Nelson, D.; Clothier, B.; Schult, T.; Nugent, S.; Greer, N.; Slinin, Y.; Ensrud, K.E. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch. Intern. Med. 2011, 171, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Mao, Y.; Fu, X.; Feng, Z.; Xu, J. Comparison of biomarkers in rat renal ischemia-reperfusion injury. Int. J. Clin. Exp. Med. 2015, 8, 7577–7584. [Google Scholar]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Devarajan, P. Biomarkers for the early detection of acute kidney injury. Curr. Opin. Pediatr. 2011, 23, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, X.; Huang, H.-B.; Feng, G.; Cao, Y.-H.; Cheng, Q.-S.; Li, S.-H.; He, C.-Y.; Lu, W.-H.; Qin, M.-M. Baseline Serum Cystatin C Is a Potential Predictor for Acute Kidney Injury in Patients with Acute Pancreatitis. Dis. Markers 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, P.K.; Kochhar, R.; Sarotra, P.; Medhi, B.; Jha, V.; Gupta, V. Neutrophil gelatinase-associated lipocalin: An early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open 2018, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ebert, N.; Delanaye, P.; Shlipak, M.; Jakob, O.; Martus, P.; Bartel, J.; Gaedeke, J.; van der Giet, M.; Schuchardt, M.; Cavalier, E.; et al. Cystatin C standardization decreases assay variation and improves assessment of glomerular filtration rate. Clin. Chim. Acta 2016, 456, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Wajda, J.; Dumnicka, P.; Sporek, M.; Maziarz, B.; Kolber, W.; Ząbek-Adamska, A.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis? J. Clin. Med. 2020, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Sporek, M.; Gala-Błądzińska, A.; Dumnicka, P.; Mazur-Laskowska, M.; Kielczewski, S.; Walocha, J.; Ceranowicz, P.; Kuźniewski, M.; Mituś, J.; Kuśnierz-Cabala, B. Urine NGAL is useful in the clinical evaluation of renal function in the early course of acute pancreatitis. Folia Med. Crac. 2016, 56, 13–25. [Google Scholar]
- Liu, X.; Foster, M.C.; Tighiouart, H.; Anderson, A.H.; Beck, G.J.; Contreras, G.; Coresh, J.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in CKD. Am. J. Kidney Dis. 2016, 68, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Van de Vrie, M.; Deegens, J.K.; van der Vlag, J.; Hilbrands, L.B. Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL). Am. J. Kidney Dis. 2014, 63, 573–576. [Google Scholar] [CrossRef]
- Schuh, M.P.; Nehus, E.; Ma, Q.; Haffner, C.; Bennett, M.; Krawczeski, C.D.; Devarajan, P. Long-term Stability of Urinary Biomarkers of Acute Kidney Injury in Children. Am. J. Kidney Dis. 2016, 67, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Moledina, D.G.; Hall, I.E.; Thiessen-Philbrook, H.; Reese, P.P.; Weng, F.L.; Schröppel, B.; Doshi, M.D.; Wilson, F.P.; Coca, S.G.; Parikh, C.R. Performance of Serum Creatinine and Kidney Injury Biomarkers for Diagnosing Histologic Acute Tubular Injury. Am. J. Kidney Dis. 2017, 70, 807–816. [Google Scholar] [CrossRef]
- De Geus, H.R.; Fortrie, G.; Betjes, M.G.; Van Schaik, R.H.; Groeneveld, A.J. Time of injury affects urinary biomarker predictive values for acute kidney injury in critically ill, non-septic patients. BMC Nephrol. 2013, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Zhou, X.; Dai, D.; Liu, X.; Wang, L.; Zhou, Y.; Luo, X.; Cai, Q. Assessment of urinary kidney injury molecule-1 and interleukin-18 in the early post-burn period to predict acute kidney injury for various degrees of burn injury. BMC Nephrol. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
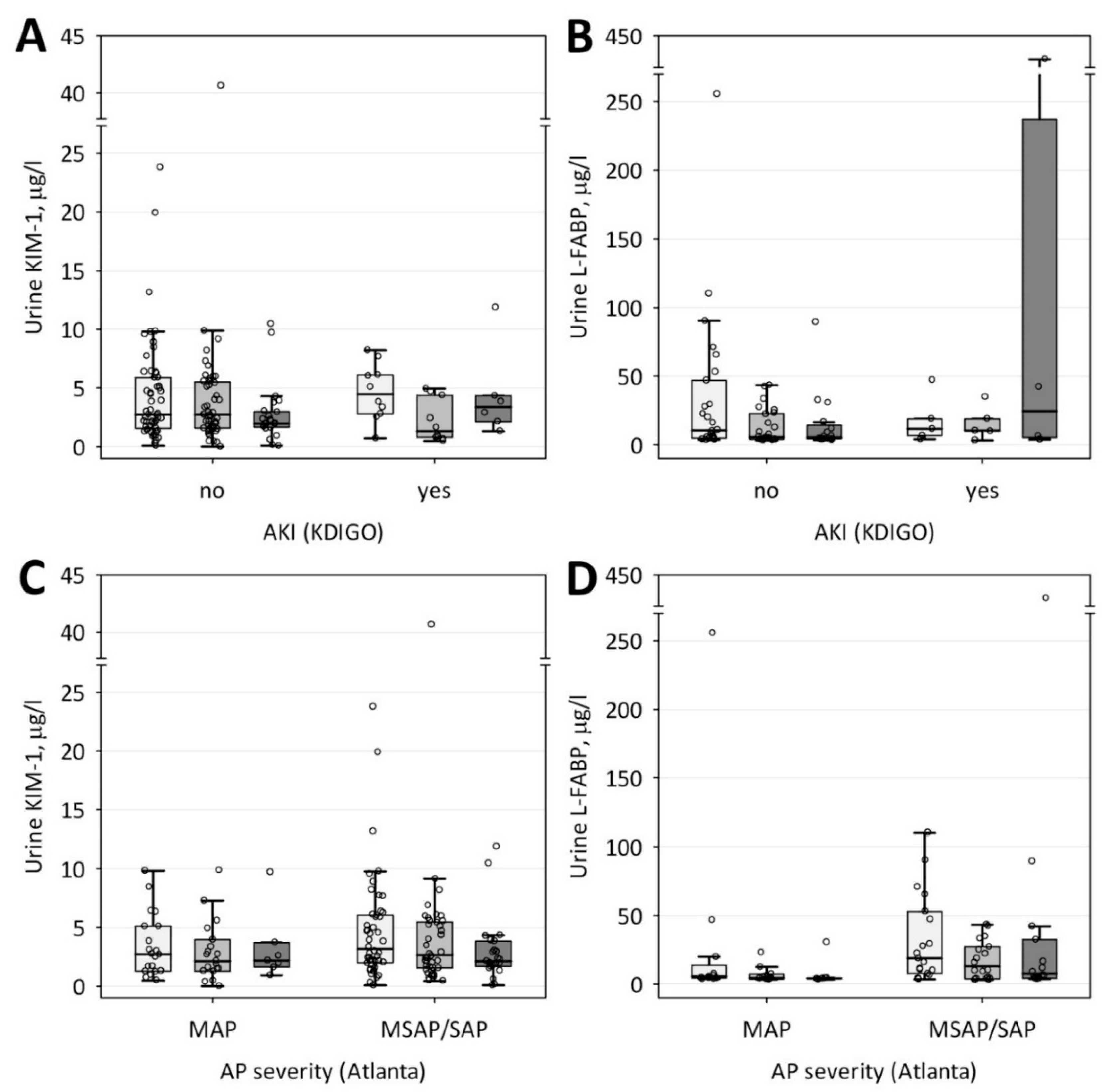
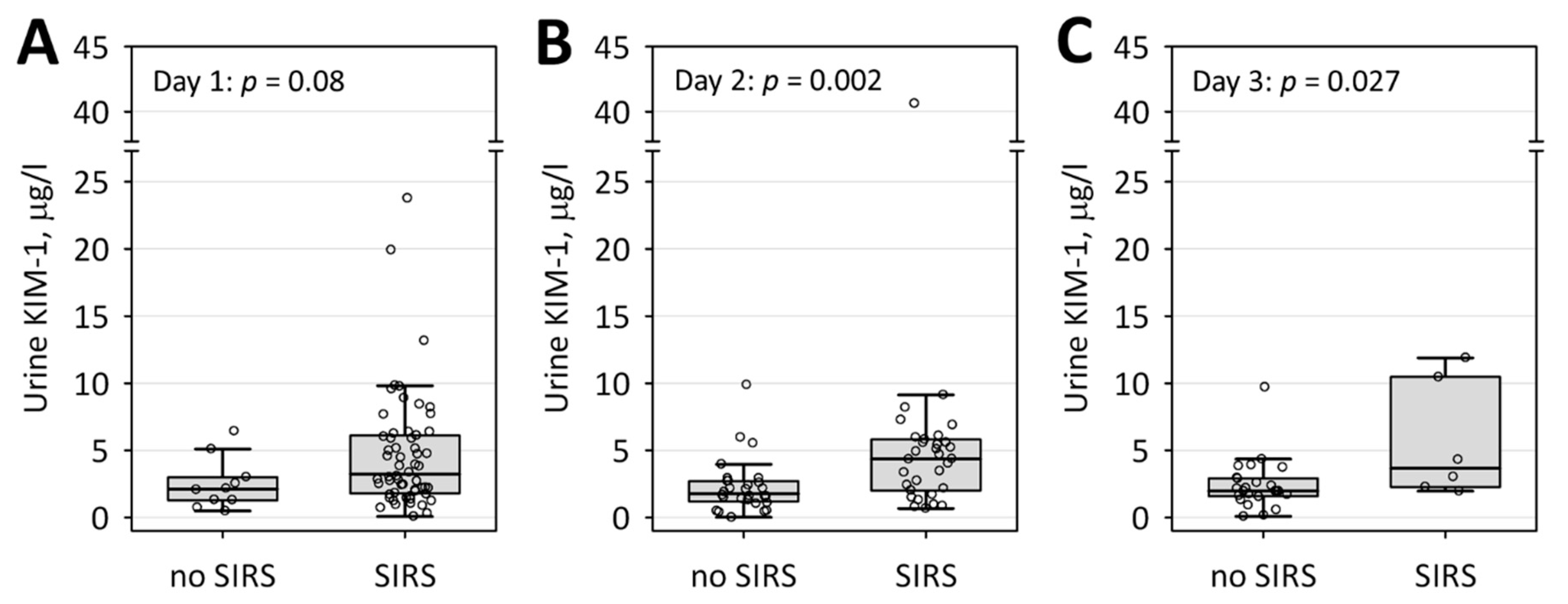
| Characteristic | AKI (n = 10) | No AKI (n = 59) | p-Value |
|---|---|---|---|
| Mean age ± SD, years | 55.0 ± 16.9 | 46.9 ± 16.0 | 0.1 |
| Male sex, n (%) | 10 (100) | 41 (69) | 0.042 |
| Comorbidities: | |||
| any, n (%) | 6 (60) | 22 (37) | 0.2 |
| cardiovascular disease, n (%) | 4 (40) | 15 (25) | 0.3 |
| diabetes, n (%) | 0 | 4 (7) | 0.4 |
| AP etiology: | |||
| biliary, n (%) | 4 (40) | 15 (25) | 0.7 |
| alcohol, n (%) | 2 (20) | 20 (34) | |
| hyperlipidemia, n (%) | 0 | 4 (7) | |
| idiopathic, n (%) | 4 (40) | 18 (31) | |
| other, n (%) | 0 | 2 (3) | |
| Ranson’s score at first 48 h >3 points, n (%) | 4 (40) | 15 (25) | 0.3 |
| BISAP on day 1 ≥3 points, n (%) | 4 (40) | 13 (22) | 0.2 |
| Systemic inflammatory response syndrome on day 1, n (%) | 9 (90) | 49 (83) | 0.6 |
| Necrotizing AP, n (%) | 1 (10) | 7 (12) | 0.9 |
| AP severity: | |||
| mild, n (%) | 1 (10) | 20 (34) | 0.056 |
| moderately severe, n (%) | 7 (70) | 37 (63) | |
| severe, n (%) | 2 (20) | 2 (3) | |
| Treatment in ICU, n (%) | 2 (20) | 2 (3) | 0.037 |
| Surgery, n (%) | 1 (10) | 3 (5) | 0.5 |
| Median length of hospital stay (lower; upper quartile), days | 12 (10; 15) | 12 (9; 15) | 0.6 |
| Mortality, n (%) | 2 (20) | 1 (2) | 0.009 |
| Laboratory Test | AKI (n = 10) | No AKI (n = 59) | p-Value |
|---|---|---|---|
| Amylase, U/L | 443 (84; 677) | 546 (172; 1812) | 0.4 |
| Lactate dehydrogenase, U/L | 854 (661; 1027) | 553 (472; 744) | 0.010 |
| Albumin, g/L | 35 (31; 35) | 36 (33; 40) | 0.5 |
| Total calcium, mmol/L | 2.04 ± 0.21 | 2.16 ± 0.19 | 0.1 |
| Bilirubin, µmol/L | 39.2 (19.1; 68.7) | 24.8 (13.7; 36.9) | 0.2 |
| Glucose, mmol/L | 7.22 (6.78; 10.72) | 7.33 (5.94; 8.78) | 0.3 |
| Creatinine, µmol/L | 98.1 (91.9; 113.2) | 68.1 (59.7; 74.3) | <0.001 |
| Urea, mmol/L | 7.50 (6.08; 9.00) | 4.08 (3.17; 5.33) | 0.002 |
| Urine KIM-1, µg/L | 4.47 (2.80; 6.10) | 2.73 (1.57; 5.88) | 0.2 |
| Urine L-FABP, µg/L * | 11.7 (6.8; 18.9) | 10.5 (4.84; 46.83) | 0.8 |
| Hematocrit, % | 46.3 (38.1; 48.7) | 43.7 (40.7; 46.1) | 0.3 |
| White blood cells, ×103/µL | 14.1 (9.6; 19.2) | 13.6 (11.4; 15.7) | 0.9 |
| Neutrophils, ×103/µL | 12.6 (11.2; 17.5) | 11.0 (7.4; 14.7) | 0.5 |
| CRP, mg/L | 122.0 (12.2; 253.4) | 23.8 (8.70; 89.0) | 0.3 |
| uPAR, µg/L | 5.34 (3.46; 6.28) | 3.71 (2.82; 4.82) | 0.042 |
| Procalcitonin, µg/L | 0.38 (0.23; 1.68) | 0.14 (0.05; 0.36) | 0.014 |
| D-dimer, mg/L | 1.80 (1.00; 6.06) | 1.75 (0.87; 2.88) | 0.5 |
| sFlt-1, ng/mL | 160 (108; 204) | 136 (120; 161) | 0.6 |
| Variable | Day 1 (n = 69) | Day 2 (n = 69) | Day 3 (n = 30) |
|---|---|---|---|
| Creatinine | R = 0.13; p = 0.3 | R = − 0.20; p = 0.1 | R = − 0.18; p = 0.4 |
| Urea | R = 0.23; p = 0.07 | R = 0.07; p = 0.6 | R = 0.21; p = 0.3 |
| Urine L-FABP | R = 0.12; p = 0.5 | R = 0.54; p = 0.002 * | R =0.49; p = 0.028 * |
| Lactate dehydrogenase | R = 0.20; p = 0.1 | R = 0.32; p = 0.029 * | R = 0.54; p = 0.005 * |
| Albumin | R = − 0.20; p = 0.2 | R = − 0.22; p = 0.2 | R = − 0.57; p = 0.020 * |
| Hematocrit | R = 0.08; p = 0.5 | R = − 0.06; p = 0.7 | R = − 0.38; p = 0.040 * |
| Neutrophils | R = 0.40; p = 0.021 * | R = 0.04; p = 0.8 | R = 0.18; p = 0.4 |
| CRP | R = 0.36; p = 0.004 * | R = 0.27; p = 0.045 * | R = 0.33; p = 0.08 |
| uPAR | R = 0.30; p = 0.020 * | R = 0.20; p = 0.2 | R = 0.29; p = 0.1 |
| Procalcitonin | R = 0.32; p = 0.012 * | R = 0.13; p = 0.3 | R = 0.39; p = 0.042 * |
| D-dimer | R = 0.06; p = 0.6 | R = 0.31; p = 0.020 * | R = 0.33; p = 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajda, J.; Dumnicka, P.; Kolber, W.; Sporek, M.; Maziarz, B.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. The Marker of Tubular Injury, Kidney Injury Molecule-1 (KIM-1), in Acute Kidney Injury Complicating Acute Pancreatitis: A Preliminary Study. J. Clin. Med. 2020, 9, 1463. https://doi.org/10.3390/jcm9051463
Wajda J, Dumnicka P, Kolber W, Sporek M, Maziarz B, Ceranowicz P, Kuźniewski M, Kuśnierz-Cabala B. The Marker of Tubular Injury, Kidney Injury Molecule-1 (KIM-1), in Acute Kidney Injury Complicating Acute Pancreatitis: A Preliminary Study. Journal of Clinical Medicine. 2020; 9(5):1463. https://doi.org/10.3390/jcm9051463
Chicago/Turabian StyleWajda, Justyna, Paulina Dumnicka, Witold Kolber, Mateusz Sporek, Barbara Maziarz, Piotr Ceranowicz, Marek Kuźniewski, and Beata Kuśnierz-Cabala. 2020. "The Marker of Tubular Injury, Kidney Injury Molecule-1 (KIM-1), in Acute Kidney Injury Complicating Acute Pancreatitis: A Preliminary Study" Journal of Clinical Medicine 9, no. 5: 1463. https://doi.org/10.3390/jcm9051463






