The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status
Abstract
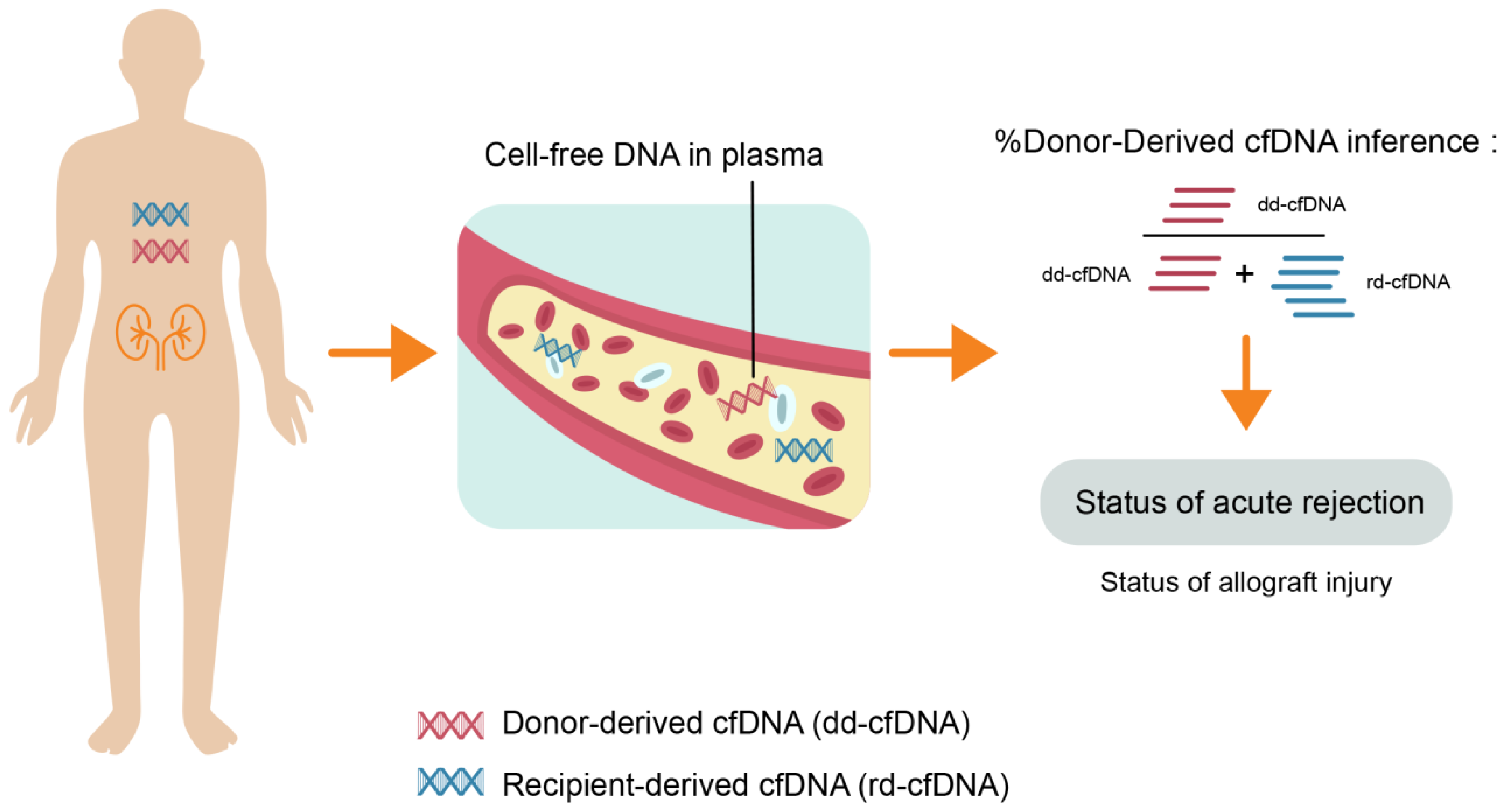
:1. Introduction
2. The Evolution of Donor-Derived Cell-Free DNA Assays
3. dd-cfDNA and Renal Allograft Rejection
4. Potential Directions and Future Scope
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Schaubel, D.E.; Jia, X.; Li, S.; Port, F.K.; Saran, R. Survival on dialysis post-kidney transplant failure: Results from the Scientific Registry of Transplant Recipients. Am. J. Kidney Dis. 2007, 49, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.R.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. J. Clin. Med. 2020, 9, 1193. [Google Scholar] [CrossRef] [Green Version]
- Thongprayoon, C.; Kaewput, W.; Kovvuru, K.; Hansrivijit, P.; Kanduri, S.R.; Bathini, T.; Chewcharat, A.; Leeaphorn, N.; Gonzalez-Suarez, M.L.; Cheungpasitporn, W. Promises of Big Data and Artificial Intelligence in Nephrology and Transplantation. J. Clin. Med. 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinstock, C.A.; Gandhi, M.; Cheungpasitporn, W.; Mitema, D.; Prieto, M.; Dean, P.; Cornell, L.; Cosio, F.; Stegall, M. Kidney Transplant With Low Levels of DSA or Low Positive B-Flow Crossmatch: An Underappreciated Option for Highly Sensitized Transplant Candidates. Transplantation 2017, 101, 2429–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheungpasitporn, W.; Kremers, W.K.; Lorenz, E.; Amer, H.; Cosio, F.G.; Stegall, M.D.; Gandhi, M.J.; Schinstock, C.A. De novo donor-specific antibody following BK nephropathy: The incidence and association with antibody-mediated rejection. Clin. Transplant. 2018, 32, e13194. [Google Scholar] [CrossRef] [PubMed]
- Leeaphorn, N.; Thongprayoon, C.; Chon, W.J.; Cummings, L.S.; Mao, M.A.; Cheungpasitporn, W. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am. J. Transplant. 2020, 20, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Cherikh, W.; Sood, P.; Hariharan, S. Kidney allograft surveillance biopsy practices across US transplant centers: A UNOS survey. Clin. Transplant. 2017, 31, e12945. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Vernerey, D.; Tinel, C.; Aubert, O.; Duong van Huyen, J.P.; Rabant, M.; Verine, J.; Nochy, D.; Empana, J.P.; Martinez, F.; et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J. Am. Soc. Nephrol. Jasn 2015, 26, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Schinstock, C.A.; Cosio, F.; Cheungpasitporn, W.; Dadhania, D.M.; Everly, M.J.; Samaniego-Picota, M.D.; Cornell, L.; Stegall, M.D. The Value of Protocol Biopsies to Identify Patients With De Novo Donor-Specific Antibody at High Risk for Allograft Loss. Am. J. Transplant. 2017, 17, 1574–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, P. Post-transplant monitoring of renal allografts: Are we there yet? Curr. Opin. Immunol. 2009, 21, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Josephson, M.A. Monitoring and managing graft health in the kidney transplant recipient. Clin. J. Am. Soc. Nephrol. Cjasn 2011, 6, 1774–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eikmans, M.; Gielis, E.M.; Ledeganck, K.J.; Yang, J.; Abramowicz, D.; Claas, F.F.J. Non-invasive Biomarkers of Acute Rejection in Kidney Transplantation: Novel Targets and Strategies. Front. Med. (Lausanne) 2018, 5, 358. [Google Scholar] [CrossRef]
- Nasr, M.; Sigdel, T.; Sarwal, M. Advances in diagnostics for transplant rejection. Expert Rev. Mol. Diagn. 2016, 16, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Koyawala, N.; Reese, P.P.; Hall, I.E.; Jia, Y.; Thiessen-Philbrook, H.R.; Mansour, S.G.; Doshi, M.D.; Akalin, E.; Bromberg, J.S.; Harhay, M.N.; et al. Urine injury biomarkers are not associated with kidney transplant failure. Transplantation 2019. [Google Scholar] [CrossRef]
- Naesens, M.; Sarwal, M.M. Molecular diagnostics in transplantation. Nat. Rev. Nephrol. 2010, 6, 614–628. [Google Scholar] [CrossRef]
- Halloran, P.F.; Famulski, K.S.; Reeve, J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat. Rev. Nephrol. 2016, 12, 534–548. [Google Scholar] [CrossRef]
- Strom, T.B.; Suthanthiran, M. Transcriptional profiling to assess the clinical status of kidney transplants. Nat. Clin. Pract. Nephrol. 2006, 2, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.M.; Volkmann, I.; Fiedler, J.; Schmidt, M.; Scheffner, I.; Haller, H.; Gwinner, W.; Thum, T. Urinary miR-210 as a Mediator of Acute T-Cell Mediated Rejection in Renal Allograft Recipients. Am. J. Transplant. 2011, 11, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Lewis, R.A.; Wang, D.Z. MicroRNAs in heart development. Curr. Top. Dev. Biol. 2012, 100, 279–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erpicum, P.; Hanssen, O.; Weekers, L.; Lovinfosse, P.; Meunier, P.; Tshibanda, L.; Krzesinski, J.M.; Hustinx, R.; Jouret, F. Non-invasive approaches in the diagnosis of acute rejection in kidney transplant recipients, part II: Omics analyses of urine and blood samples. Clin. Kidney J. 2017, 10, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- First, M.R.; Peddi, V.R.; Mannon, R.; Knight, R.; Marsh, C.L.; Kurian, S.M.; Rice, J.C.; Maluf, D.; Mandelbrot, D.; Patel, A.; et al. Investigator Assessment of the Utility of the TruGraf Molecular Diagnostic Test in Clinical Practice. Transplant. Proc. 2019, 51, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Pereira, A.B.; Chang, J.; Matas, A.; Picton, M.; De Freitas, D.; Bromberg, J.; Seron, D.; Sellares, J.; Einecke, G.; et al. Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: The INTERCOM study. Am. J. Transplant. 2013, 13, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Pereira, A.B.; Chang, J.; Matas, A.; Picton, M.; De Freitas, D.; Bromberg, J.; Seron, D.; Sellares, J.; Einecke, G.; et al. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: An international prospective study (INTERCOM). Am. J. Transplant. 2013, 13, 2865–2874. [Google Scholar] [CrossRef] [Green Version]
- Anglicheau, D.; Suthanthiran, M. Noninvasive prediction of organ graft rejection and outcome using gene expression patterns. Transplantation 2008, 86, 192–199. [Google Scholar] [CrossRef]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Ding, R.C.; Sharma, V.K.; Schwartz, J.E.; Suthanthiran, M. Urinary cell mRNA profiles predictive of human kidney allograft status. Immunol. Rev. 2014, 258, 218–240. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Hartono, C.; Ding, R.; Sharma, V.K.; Ramaswamy, R.; Qian, B.; Serur, D.; Mouradian, J.; Schwartz, J.E.; Suthanthiran, M. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N. Engl. J. Med. 2001, 344, 947–954. [Google Scholar] [CrossRef]
- Ding, R.; Li, B.; Muthukumar, T.; Dadhania, D.; Medeiros, M.; Hartono, C.; Serur, D.; Seshan, S.V.; Sharma, V.K.; Kapur, S.; et al. CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation 2003, 75, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Suthanthiran, M.; Schwartz, J.E.; Ding, R.; Abecassis, M.; Dadhania, D.; Samstein, B.; Knechtle, S.J.; Friedewald, J.; Becker, Y.T.; Sharma, V.K.; et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N. Engl. J. Med. 2013, 369, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt-Minkowski, P.; De Serres, S.A.; Ho, J. Developing renal allograft surveillance strategies-urinary biomarkers of cellular rejection. Can. J. Kidney Health Dis. 2015, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostom, A.G.; Steubl, D.; Friedman, A.N. Hypothesis: Potential Utility of Serum and Urine Uromodulin Measurement in Kidney Transplant Recipients? Transplant. Direct 2017, 3, e219. [Google Scholar] [CrossRef] [PubMed]
- Oetting, W.S.; Rogers, T.B.; Krick, T.P.; Matas, A.J.; Ibrahim, H.N. Urinary β2-microglobulin is associated with acute renal allograft rejection. Am. J. Kidney Dis. 2006, 47, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.E.; Chen, T.; LeBlanc, J.F.; Wei, X.; Gjertson, D.W.; Li, K.C.; Khalighi, M.A.; Lassman, C.R.; Veale, J.L.; Gritsch, H.A.; et al. Apolipoprotein A1 and C-terminal fragment of alpha-1 antichymotrypsin are candidate plasma biomarkers associated with acute renal allograft rejection. Transplantation 2011, 92, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Hricik, D.E.; Rodriguez, V.; Riley, J.; Bryan, K.; Tary-Lehmann, M.; Greenspan, N.; Dejelo, C.; Schulak, J.A.; Heeger, P.S. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am. J. Transplant. 2003, 3, 878–884. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, E.J.; Kim, M.J.; Park, Y.J.; Han, K.; Yang, H.J.; Kim, J.Y.; Choi, B.S.; Yang, C.W.; Kim, Y.S.; et al. Pretransplant donor-specific interferon-gamma ELISPOT assay predicts acute rejection episodes in renal transplant recipients. Transplant. Proc. 2007, 39, 3057–3060. [Google Scholar] [CrossRef]
- Koscielska-Kasprzak, K.; Drulis-Fajdasz, D.; Kaminska, D.; Mazanowska, O.; Krajewska, M.; Gdowska, W.; Bieniecki, W.; Chudoba, P.; Polak, W.; Janczak, D. Pretransplantation cellular alloreactivity is predictive of acute graft rejection and 1-year graft function in kidney transplant recipients. Transplant. Proc. 2009, 41, 3006–3008. [Google Scholar] [CrossRef]
- Reinsmoen, N.L.; Cornett, K.M.; Kloehn, R.; Burnette, A.D.; McHugh, L.; Flewellen, B.K.; Matas, A.; Savik, K. Pretransplant donor-specific and non-specific immune parameters associated with early acute rejection. Transplantation 2008, 85, 462–470. [Google Scholar] [CrossRef]
- Zitzner, J.R.; Tambur, A.R. Role of ELISPOT Assays in Risk Assessment Pre-and Post-Kidney Transplantation. Cells 2012, 1, 100–110. [Google Scholar] [CrossRef]
- Christakoudi, S.; Runglall, M.; Mobillo, P.; Tsui, T.L.; Duff, C.; Domingo-Vila, C.; Kamra, Y.; Delaney, F.; Montero, R.; Spiridou, A.; et al. Development of a multivariable gene-expression signature targeting T-cell-mediated rejection in peripheral blood of kidney transplant recipients validated in cross-sectional and longitudinal samples. EBioMedicine 2019, 41, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigdel, T.; Nguyen, M.; Liberto, J.; Dobi, D.; Junger, H.; Vincenti, F.; Laszik, Z.; Sarwal, M.M. Assessment of 19 Genes and Validation of CRM Gene Panel for Quantitative Transcriptional Analysis of Molecular Rejection and Inflammation in Archival Kidney Transplant Biopsies. Front. Med. (Lausanne) 2019, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herath, S.; Erlich, J.; Au, A.Y.M.; Endre, Z.H. Advances in Detection of Kidney Transplant Injury. Mol. Diagn. 2019, 23, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Dengu, F. Next-generation sequencing methods to detect donor-derived cell-free DNA after transplantation. Transplant. Rev. (Orlando) 2020, 100542. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. Jasn 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Gielis, E.M.; Ledeganck, K.J.; De Winter, B.Y.; Del Favero, J.; Bosmans, J.L.; Claas, F.H.; Abramowicz, D.; Eikmans, M. Cell-Free DNA: An Upcoming Biomarker in Transplantation. Am. J. Transplant. 2015, 15, 2541–2551. [Google Scholar] [CrossRef] [Green Version]
- Pattar, S.K.; Greenway, S.C. Circulating nucleic acids as biomarkers for allograft injury after solid organ transplantation: Current state-of-the-art. Transpl. Res. Risk Manag. 2019, 11, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Moreira, V.G.; Garcia, B.P.; Martin, J.M.B.; Suarez, F.O.; Alvarez, F.V. Cell-Free DNA as a Noninvasive Acute Rejection Marker in Renal Transplantation. Clin. Chem. 2009, 55, 1958–1966. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, A.V.; Melkonyan, H.S.; Tomei, L.D.; Umansky, S.R. Circulating nucleic acids and apoptosis. Ann. N. Y. Acad. Sci. 2001, 945, 239–249. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA—Apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Oellerich, M.; Schulz, U.; Schauerte, V.; Reinhard, L.; Fuchs, U.; Knabbe, C.; Zittermann, A.; Olbricht, C.; Gummert, J.F.; et al. Donor-Derived Cell-Free DNA Is a Novel Universal Biomarker for Allograft Rejection in Solid Organ Transplantation. Transplant. Proc. 2015, 47, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Vitalone, M.J.; Tran, T.Q.; Dai, H.; Hsieh, S.C.; Salvatierra, O.; Sarwal, M.M. A Rapid Noninvasive Assay for the Detection of Renal Transplant Injury. Transplantation 2013, 96, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromberg, J.S.; Brennan, D.C.; Poggio, E.; Bunnapradist, S.; Langone, A.; Sood, P.; Matas, A.J.; Mannon, R.B.; Mehta, S.; Sharfuddin, A. Biological variation of donor-derived cell-free DNA in renal transplant recipients: Clinical implications. J. Appl. Lab. Med. 2017, 2, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Altug, Y.; Liang, N.; Ram, R.; Ravi, H.; Ahmed, E.; Brevnov, M.; Swenerton, R.K.; Zimmermann, B.; Malhotra, M.; Demko, Z.P.; et al. Analytical Validation of a Single-nucleotide Polymorphism-based Donor-derived Cell-free DNA Assay for Detecting Rejection in Kidney Transplant Patients. Transplantation 2019, 103, 2657–2665. [Google Scholar] [CrossRef] [Green Version]
- Mandel, P.; Metais, P. Les acides nucleiques du plasma sanguine chez l’homme. C. R. Acad. Sci. Paris 1948, 142, 241–243. [Google Scholar]
- Lo, Y.M.; Tein, M.S.; Pang, C.C.; Yeung, C.K.; Tong, K.L.; Hjelm, N.M. Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet 1998, 351, 1329–1330. [Google Scholar] [CrossRef]
- Snyder, T.M.; Khush, K.K.; Valantine, H.A.; Quake, S.R. Universal noninvasive detection of solid organ transplant rejection. Proc. Natl. Acad. Sci. USA 2011, 108, 6229–6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grskovic, M.; Hiller, D.J.; Eubank, L.A.; Sninsky, J.J.; Christopherson, C.; Collins, J.P.; Thompson, K.; Song, M.; Wang, Y.S.; Ross, D.; et al. Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J. Mol. Diagn. 2016, 18, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, S.C.; Bunnapradist, S.; Bromberg, J.S.; Langone, A.J.; Hiller, D.; Yee, J.P.; Sninsky, J.J.; Woodward, R.N.; Matas, A.J. Donor-derived Cell-free DNA Identifies Antibody-mediated Rejection in Donor Specific Antibody Positive Kidney Transplant Recipients. Transplant. Direct 2018, 4, e379. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, S.; De Vlaminck, I.; Khush, K.K. Adding Insult on Injury: Immunogenic Role for Donor-derived Cell-free DNA? Transplantation 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Gielis, E.M.; Ledeganck, K.J.; Dendooven, A.; Meysman, P.; Beirnaert, C.; Laukens, K.; De Schrijver, J.; Van Laecke, S.; Van Biesen, W.; Emonds, M.P.; et al. The use of plasma donor-derived, cell-free DNA to monitor acute rejection after kidney transplantation. Nephrol. Dial. Transplant. 2020, 35, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.P.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.G.; Chang, J.H.; Alhamad, T.; Bromberg, J.S.; Hiller, D.J.; Grskovic, M.; Yee, J.P.; Mannon, R.B. Repeat kidney transplant recipients with active rejection have elevated donor-derived cell-free DNA. Am. J. Transplant. 2019, 19, 1597–1598. [Google Scholar] [CrossRef]
- Stites, E.; Kumar, D.; Olaitan, O.; Swanson, S.J.; Leca, N.; Weir, M.; Bromberg, J.; Melancon, J.; Agha, I.; Fattah, H. High levels of dd-cfDNA identifies patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am. J. Transplant. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gielis, E.M.; Beirnaert, C.; Dendooven, A.; Meysman, P.; Laukens, K.; De Schrijver, J.; Van Laecke, S.; Van Biesen, W.; Emonds, M.P.; De Winter, B.Y.; et al. Plasma donor-derived cell-free DNA kinetics after kidney transplantation using a single tube multiplex PCR assay. PLoS ONE 2018, 13, e0208207. [Google Scholar] [CrossRef]
- Jordan, S.; Sawinski, D.; Dholakia, S. Donor Derived Cell Free DNA Initiates De-Novo Donor Specific Antibody (DSA) Responses. Am. J. Transplant. 2019, 19, 404–405. [Google Scholar]
- Kobashigawa, J.; Patel, J.; Kransdorf, E.; Chang, D.; Kittleson, M.; Dimbil, S.; Levine, R.; Sana, S.; Wolfson, A.; Czer, L. Does Cell-Free DNA Detect the Development of De Novo Donor Specific Antibodies. J. Heart Lung Transplant. 2019, 38, S288. [Google Scholar] [CrossRef]
- Alhamad, T.; Poggio, E.; Hiller, D.; Dholakia, S.; Sood, P. The Use of dd-cfDNA as a Predictive Tool for Outcome Decreased Kidney Function. Am. J. Transplant. 2019, 19, 404. [Google Scholar]
- Hinojosa, R.J.; Chaffin, K.; Gillespie, M.; Villarreal, V.H., Jr. Donor-derived Cell-free DNA May Confirm Real-time Response to Treatment of Acute Rejection in Renal Transplant Recipients. Transplantation 2019, 103, e61. [Google Scholar] [CrossRef] [PubMed]
| Non-Invasive Diagnosis and Prognostication of Acute Allograft Rejection Kidney Transplant Recipients |
|---|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprayoon, C.; Vaitla, P.; Craici, I.M.; Leeaphorn, N.; Hansrivijit, P.; Salim, S.A.; Bathini, T.; Cabeza Rivera, F.H.; Cheungpasitporn, W. The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status. J. Clin. Med. 2020, 9, 1480. https://doi.org/10.3390/jcm9051480
Thongprayoon C, Vaitla P, Craici IM, Leeaphorn N, Hansrivijit P, Salim SA, Bathini T, Cabeza Rivera FH, Cheungpasitporn W. The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status. Journal of Clinical Medicine. 2020; 9(5):1480. https://doi.org/10.3390/jcm9051480
Chicago/Turabian StyleThongprayoon, Charat, Pradeep Vaitla, Iasmina M. Craici, Napat Leeaphorn, Panupong Hansrivijit, Sohail Abdul Salim, Tarun Bathini, Franco H. Cabeza Rivera, and Wisit Cheungpasitporn. 2020. "The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status" Journal of Clinical Medicine 9, no. 5: 1480. https://doi.org/10.3390/jcm9051480






