Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethical Statement
2.2. Study Design and Area
2.3. Selection of Study Villages and Study Population
2.4. Clinical and Parasitological Examination
2.5. Detection for Microfilaraemia
2.6. Data Management and Analysis
3. Results
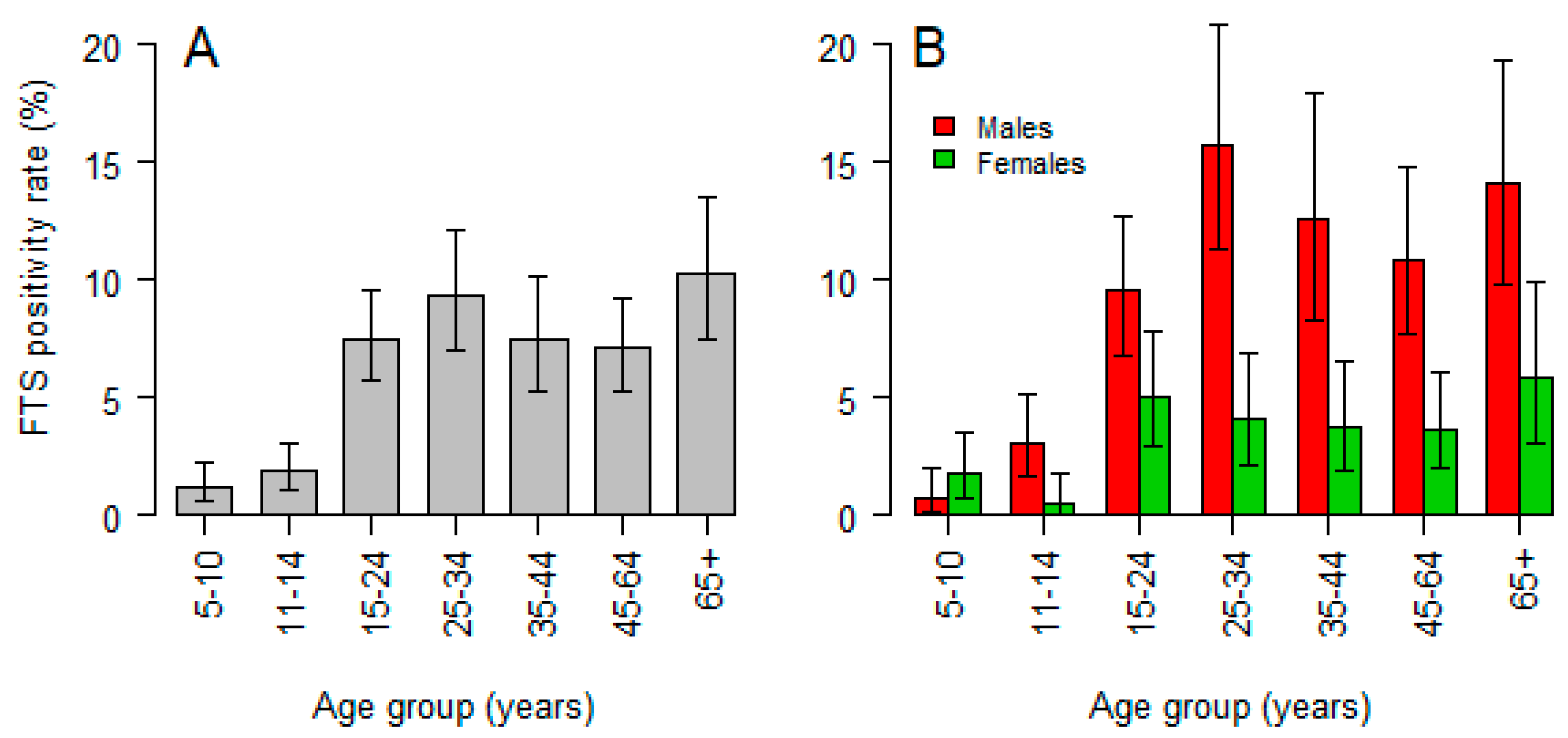
3.1. Prevalence and Correlates of Circulating Filarial Antigen
3.2. Prevalence and Correlates of Microfilaremia
3.3. Coverage of MDA from the Previous Round
3.4. Clinical Manifestation of Bancroftian Filariasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization (2019): Lymphatic Filariasis-Key Facts: 7. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 20 February 2020).
- Ichimori, K.; King, J.D.; Engels, D.; Yajima, A.; Mikhailov, A.; Lammie, P.; Ottesen, E.A. Global programme to eliminate lymphatic filariasis: The processes underlying programme success. PLoS Negl. Trop. Dis. 2014, 8, e3328. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization (2019), Weekly Epidemiological Record, 2019, Global Programme to Eliminate Lymphatic Filariasis: Progress Report, 2018, No 41, 94, 457–472. Available online: https://apps.who.int/iris/bitstream/handle/10665/329087/WER9441-eng-fre.pdf?ua=1 (accessed on 3 May 2020).
- Babu, S.; Nutman, T.B. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockarie, M.J.; Taylor, M.J.; Gyapong, J.O. Current practices in the management of lymphatic filariasis. Expert Rev. Anti. Infect. Ther. 2009, 7, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Hoerauf, A.; Pfarr, K.; Mand, S.; Debrah, A.Y.; Specht, S. Filariasis in Africa--treatment challenges and prospects. Clin. Microbiol. Infect. 2011, 17, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, J.W. Parasitology for Medical and Clinical Laboratory Professionals; Delmar: Clifton Park, NY, USA, 2012; p. xvii. 316p. [Google Scholar]
- Shenoy, R.K. Clinical and pathological aspects of filarial lymphedema and its management. Korean J. Parasitol. 2008, 46, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfarr, K.M.; Debrah, A.Y.; Specht, S.; Hoerauf, A. Filariasis and lymphoedema. Parasite Immunol. 2009, 31, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Ottesen, E.A. The global programme to eliminate lymphatic filariasis. Trop. Med. Int. Health 2000, 5, 591–594. [Google Scholar] [CrossRef]
- WHO. Progress Report 2000–2009 and Strategic Plan 2010–2020 of the Global Programme to Eliminate Lymphatic Filariasis: Halfway towards Eliminating Lymphatic Filariasis; WHO, World Health Organization: Geneva, Switzerland, 2010; Available online: http://apps.who.int/iris/bitstream/10665/44473/1/9789241500722_eng.pdf?ua=1/ (accessed on 11 March 2020).
- WHO. World Health Organization (2011), Monitoring and Epidemiological Assessment of Mass Drug Administration in the Global Programme to Eliminate Lymphatic Filariasis: A Manual for National Elimination Programmes; WHO: Geneva, Switzerland, 2011; ISBN 978 92 4 150148 4. Available online: https://www.who.int/lymphatic_filariasis/resources/9789241501484/en/ (accessed on 3 May 2020).
- World Health Organization Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis. 2017. Available online: http://apps.who.int/iris/bitstream/handle/10665/259381/9789241550161-eng.pdf;jsessionid=DF69AA4AEC1CE3111C7F14B62463CDAC?sequence=1 (accessed on 27 March 2019).
- Thomsen, E.K.; Sanuku, N.; Baea, M.; Satofan, S.; Maki, E.; Lombore, B.; Schmidt, M.S.; Siba, P.M.; Weil, G.J.; Kazura, J.W.; et al. Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis. Clin. Infect. Dis. 2016, 62, 334–341. [Google Scholar] [CrossRef]
- Ottesen, E.A. Major progress toward eliminating lymphatic filariasis. N. Engl. J. Med. 2002, 347, 1885–1886. [Google Scholar] [CrossRef]
- Tisch, D.J.; Michael, E.; Kazura, J.W. Mass chemotherapy options to control lymphatic filariasis: A systematic review. Lancet Infect. Dis. 2005, 5, 514–523. [Google Scholar] [CrossRef]
- Fischer, P.U.; King, C.L.; Jacobson, J.A.; Weil, G.J. Potential Value of Triple Drug Therapy with Ivermectin, Diethylcarbamazine, and Albendazole (IDA) to Accelerate Elimination of Lymphatic Filariasis and Onchocerciasis in Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005163. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, P.E.; Pedersen, E.M.; Rwegoshora, R.T.; Malecela, M.N.; Derua, Y.A.; Magesa, S.M. Lymphatic filariasis control in Tanzania: Effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS Negl. Trop. Dis. 2010, 4, e696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisoka, W.J.; Tersbol, B.P.; Meyrowitsch, D.W.; Simonsen, P.E.; Mushi, D.L. Community Members’ Perceptions of Mass Drug Administration for Control of Lymphatic Filariasis in Rural and Urban Tanzania. J. Biosoc. Sci. 2016, 48, 94–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsen, P.E.; Meyrowitsch, D.W.; Jaoko, W.G.; Malecela, M.N.; Mukoko, D.; Pedersen, E.M.; Ouma, J.H.; Rwegoshora, R.T.; Masese, N.; Magnussen, P.; et al. Bancroftian filariasis infection, disease, and specific antibody response patterns in a high and a low endemicity community in East Africa. Am. J. Trop. Med. Hyg. 2002, 66, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rwegoshora, R.T.; Pedersen, E.M.; Mukoko, D.A.; Meyrowitsch, D.W.; Masese, N.; Malecela-Lazaro, M.N.; Ouma, J.H.; Michael, E.; Simonsen, P.E. Bancroftian filariasis: Patterns of vector abundance and transmission in two East African communities with different levels of endemicity. Ann. Trop. Med. Parasitol. 2005, 99, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, P.E.; Derua, Y.A.; Kisinza, W.N.; Magesa, S.M.; Malecela, M.N.; Pedersen, E.M. Lymphatic filariasis control in Tanzania: Effect of six rounds of mass drug administration with ivermectin and albendazole on infection and transmission. BMC Infect Dis 2013, 13, 335. [Google Scholar] [CrossRef] [Green Version]
- Mshana, H.J.; Baraka, V.; Misinzo, G.; Makunde, W.H. Current Epidemiological Assessment of Bancroftian Filariasis in Tanga Region, Northeastern Tanzania. J. Trop. Med. 2016, 2016, 7408187. [Google Scholar] [CrossRef]
- Simonsen, P.E.; Derua, Y.A.; Magesa, S.M.; Pedersen, E.M.; Stensgaard, A.S.; Malecela, M.N.; Kisinza, W.N. Lymphatic filariasis control in Tanga Region, Tanzania: Status after eight rounds of mass drug administration. Parasites Vectors 2014, 7, 507. [Google Scholar] [CrossRef]
- Hotez, P.J. The neglected tropical diseases and the neglected infections of poverty: Overview of their common features, global disease burden and distribution, new control tools, and prospects for disease elimination. Causes Impacts Negl. Trop. Zoonotic Dis. 2011. [Google Scholar] [CrossRef]
- Tanzania Forest Service Agency (TFS). Available online: http://www.tfs.go.tz/index.php/en/forests/nilo (accessed on 28 April 2020).
- Esri ArcGIS Software, California, USA. Available online: https://www.esri.uconn.edu/software/arcgis-student/ (accessed on 20 May 2020).
- Meyrowitsch, D.W.; Simonsen, P.E.; Makunde, W.H. Bancroftian filariasis: Analysis of infection and disease in five endemic communities of north-eastern Tanzania. Ann. Trop. Med. Parasitol. 1995, 89, 653–663. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 17 February 2019).
- NTDCP. Annual Report 2017–2018, Neglected Tropical Diseases Control Programme; Ministry of Health: Dar-es-Salaam, Tanzania, 2018.
- NTDCP. Survey Report 2007, Neglected Tropical Diseases Control Programme; Ministry of Health: Dar-es-Salaam, Tanzania, 2007.
- Swaminathan, S.; Perumal, V.; Adinarayanan, S.; Kaliannagounder, K.; Rengachari, R.; Purushothaman, J. Epidemiological assessment of eight rounds of mass drug administration for lymphatic filariasis in India: Implications for monitoring and evaluation. PLoS Negl. Trop. Dis. 2012, 6, e1926. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Ngasalla, B.; Derua, Y.A.; Tarimo, D.; Malecela, M.N. Lymphatic filariasis elimination efforts in Rufiji, southeastern Tanzania: Decline in circulating filarial antigen prevalence in young school children after twelve rounds of mass drug administration and utilization of long-lasting insecticide-treated nets. Int. J. Infect. Dis. 2017, 61, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manhenje, I.; Galan-Puchades, M.T.; Fuentes, M.V. Socio-environmental variables and transmission risk of lymphatic filariasis in central and northern Mozambique. Geospat. Health 2013, 7, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunyo, S.K.; Appawu, M.; Nkrumah, F.K.; Baffoe-Wilmot, A.; Pedersen, E.M.; Simonsen, P.E. Lymphatic filariasis on the coast of Ghana. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 634–638. [Google Scholar] [CrossRef]
- Emidi, B.; Kisinza, W.N.; Mmbando, B.P.; Malima, R.; Mosha, F.W. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites Vectors 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Derua, Y.A.; Kisinza, W.N.; Simonsen, P.E. Differential effect of human ivermectin treatment on blood feeding Anopheles gambiae and Culex quinquefasciatus. Parasites Vectors 2015, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Michael, E.; Singh, B.K.; Mayala, B.K.; Smith, M.E.; Hampton, S.; Nabrzyski, J. Continental-scale, data-driven predictive assessment of eliminating the vector-borne disease, lymphatic filariasis, in sub-Saharan Africa by 2020. BMC Med. 2017, 15, 176. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.L.; Won, K.Y.; Becker, L.; Soares Magalhaes, R.J.; Fuimaono, S.; Melrose, W.; Lammie, P.J.; Graves, P.M. Seroprevalence and spatial epidemiology of Lymphatic Filariasis in American Samoa after successful mass drug administration. PLoS Negl. Trop. Dis. 2014, 8, e3297. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Vlaminck, J.; Kunyu-Shako, B.; Pion, S.D.; Awaca-Uvon, N.P.; Weil, G.J.; Mumba, D.; Boussinesq, M. Measurement of Circulating Filarial Antigen Levels in Human Blood with a Point-of-Care Test Strip and a Portable Spectrodensitometer. Am. J. Trop. Med. Hyg. 2016, 94, 1324–1329. [Google Scholar] [CrossRef] [Green Version]
- Njomo, D.W.; Amuyunzu-Nyamongo, M.; Magambo, J.K.; Njenga, S.M. The role of personal opinions and experiences in compliance with mass drug administration for lymphatic filariasis elimination in Kenya. PLoS ONE 2012, 7, e48395. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.V.; Kar, S.K. Coverage, compliance and some operational issues of mass drug administration during the programme to eliminate lymphatic filariasis in Orissa, India. Trop. Med. Int. Health 2004, 9, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Lakwo, T.L.; Gasarasi, D.B. Non-adherence to community directed treatment with ivermectin for onchocerciasis control in Rungwe district, southwest Tanzania. East. Afr. Med. J. 2006, 83, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ochieng-Ooko, V.; Ochieng, D.; Sidle, J.E.; Holdsworth, M.; Wools-Kaloustian, K.; Siika, A.M.; Yiannoutsos, C.T.; Owiti, M.; Kimaiyo, S.; Braitstein, P. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull. World Health Organ. 2010, 88, 681–688. [Google Scholar] [CrossRef]
- WHO. World Health Organization (2018): Global Programme to Eliminate Lymphatic Filariasis: Progress Report. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/275719/WER9344.pdf?ua=1 (accessed on 20 February 2020).
- Fabien-Dupuis, C.; Niver, B.; Shillingford, N.; Wang, L.; Kokorowski, P.J.; Zhou, S. Melanotic Neuroectodermal Tumor of Infancy Presenting With Fast-Growing Scrotal Swelling: A Case Report and Literature Review. Pediatr Dev. Pathol. 2017, 20, 411–415. [Google Scholar] [CrossRef]




| Ward Name | Village Name | All Participants | Children (5–14 Years) | |||
|---|---|---|---|---|---|---|
| N | Mean Age (Years) | Median Age (IQR) | N | Median Age (IQR) | ||
| Boma | Kichakamiba | 151 | 32.1 | 17.8 (14.0–46.3) | 43 | 12.8 (11.3–13.7) |
| Subutuni | 257 | 35.2 | 31.4 (16.5–50.0) | 54 | 9.9 (7.4–12.0) | |
| Doda | B’Mwarongo | 216 | 29.8 | 16.3 (11.2–47.0) | 105 | 11 (10.0–13.0) |
| Kwale | Kichalikani | 262 | 33.3 | 29.0 (15.4–47.4) | 63 | 10 (6.8–11.8) |
| Kizingani | 403 | 29.4 | 20.0 (11.0–44.0) | 149 | 9.5 (7.0–12.0) | |
| Kwale | 225 | 30.4 | 27.5 (13.0–41.6) | 70 | 9.9 (7.0–13.0) | |
| Mongavyeru | 600 | 26.8 | 22.0 (12.0–40.0) | 203 | 9.4 (7.0–12.0) | |
| Manza | Manza | 291 | 26.1 | 16.4 (13.3–36.4) | 108 | 13.2 (12.2–13.7) |
| Mwandusi | 332 | 27.1 | 23.0 (11.2–38.7) | 128 | 9.9 (7.8–12.2) | |
| Tawalani | 181 | 39.1 | 37.0 (20.0–55.0) | 29 | 9.2 (7.0–12.0) | |
| Maramba | Maramba A | 238 | 31.3 | 26.8 (12.0–46.2) | 81 | 10 (7.0–12.0) |
| Mayomboni | Mayomboni | 74 | 23.3 | 13.7 (13.0–29.0) | 51 | 13 (12.4–13.9) |
| Ndumbani | 223 | 34.7 | 31.6 (15.4–50.0) | 53 | 9.4 (7.4–11.4) | |
| Moa | Moa | 212 | 33.3 | 29.0 (13.0–49.0) | 68 | 10.3 (7.9–12.8) |
| Zingibari | 450 | 25.5 | 14.0 (11.0–36.1) | 242 | 11 (9.0–13.0) | |
| Total | 4115 | 29.9 | 22.7 (12.5–44.5) | 1447 | 11 (8.0–13.0) | |
| Ward Name | Village Name | General Population | Children (5–14 years) | ||||
|---|---|---|---|---|---|---|---|
| N | % Positive | 95% CI | N | % Positive | 95% CI | ||
| Boma | Kichakamiba | 151 | 1.3 | 0.1–4.7 | 56 | 0 | 0–6.4 |
| Subutuni | 257 | 4.2 | 2.1–7.5 | 57 | 1.8 | 0–9.4 | |
| Doda | B’mwarongo | 216 | 2.8 | 1.02–5.9 | 107 | 1.9 | 0.2–6.5 |
| Kwale | Kichalikani | 262 | 5.3 | 2.9–8.8 | 68 | 1.5 | 0–7.9 |
| Kizingani | 403 | 8.9 | 6.3–12.0 | 165 | 3.6 | 1.3–7.7 | |
| Kwale | 225 | 5.3 | 2.7–9.1 | 78 | 7.7 | 2.9–16.0 | |
| Mongavyeru | 600 | 13.5 | 10–16.4 | 221 | 3.6 | 1.6–7.0 | |
| Manza | Manza | 291 | 2.1 | 0.7–4.4 | 134 | 0.7 | 0–4.1 |
| Mwandusi | 332 | 1.2 | 0.3–3.1 | 139 | 0.7 | 0.01–3.9 | |
| Tawalani | 181 | 3.9 | 1.5–7.8 | 34 | 0 | 0–10.0 | |
| Maramba | Maramba A | 238 | 8.4 | 5.2–12.6 | 91 | 2.2 | 0.3–7.7 |
| Mayomboni | Mayomboni | 74 | 2.7 | 0.3–9.4 | 54 | 0 | 0–6.6 |
| Ndumbani | 223 | 7.6 | 4.5–11.0 | 58 | 0 | 0–6.2 | |
| Moa | Moa | 212 | 4.2 | 1.9–7.9 | 70 | 0 | 0–5.1 |
| Zingibari | 450 | 2.7 | 1.3–4.6 | 250 | 0 | 0–1.4 | |
| Parameter | B | Std Error | Exp(B) | 95% CI for Exp(B) | p-Value | |
|---|---|---|---|---|---|---|
| Age | 0.019 | 0.003 | 1.019 | 1.014–1.025 | <0.0001 | |
| Sex (ref female) | 0.970 | 0.147 | 2.637 | 1.977–3.518 | <0.0001 | |
| Bed net use | −0.059 | 0.218 | 0.943 | 0.615–1.446 | 0.78 | |
| House windows screen | −0.216 | 0.145 | 0.805 | 0.607–1.070 | 0.14 | |
| Use of indoor spray | 0.232 | 0.245 | 1.261 | 0.780–2.039 | 0.34 | |
| Missed last MDA | 0.723 | 0.136 | 2.060 | 1.577–2.691 | <0.0001 | |
| Never used Albendazole | 0.820 | 0.144 | 2.271 | 1.712–3.013 | <0.0001 | |
| Never used Ivermectin | 0.791 | 0.137 | 2.205 | 1.686–2.885 | <0.0001 | |
| Ward | Boma | 0.005 | 0.359 | 1.005 | 0.497–2.029 | 0.99 |
| Doda | −0.137 | 0.470 | 0.872 | 0.347–2.190 | 0.77 | |
| Kwale | 1.176 | 0.239 | 3.240 | 2.030–5.172 | <0.0001 | |
| Manza | −0.417 | 0.331 | 0.659 | 0.345–1.260 | 0.21 | |
| Maramba | 1.030 | 0.322 | 2.800 | 1.489–5.265 | 0.001 | |
| Moa | 0.735 | 0.325 | 2.086 | 1.104–3.942 | 0.024 | |
| Year of MDA | Coverage in % |
|---|---|
| 2004 | 78.4 |
| 2006 | 80.6 |
| 2007 | 76.2 |
| 2009 | 44.6 |
| 2010 | 57.5 |
| 2011 | 52.6 |
| 2012 | 41.0 |
| 2013 | 53.5 |
| 2014 | 72.1 |
| 2015 | 76.0 |
| 2016 | 78.2 |
| 2017 | 78.5 |
| 2018 | 85.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fimbo, A.M.; Minzi, O.M.S.; Mmbando, B.P.; Barry, A.; Nkayamba, A.F.; Mwamwitwa, K.W.; Malishee, A.; Seth, M.D.; Makunde, W.H.; Gurumurthy, P.; et al. Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania. J. Clin. Med. 2020, 9, 1550. https://doi.org/10.3390/jcm9051550
Fimbo AM, Minzi OMS, Mmbando BP, Barry A, Nkayamba AF, Mwamwitwa KW, Malishee A, Seth MD, Makunde WH, Gurumurthy P, et al. Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania. Journal of Clinical Medicine. 2020; 9(5):1550. https://doi.org/10.3390/jcm9051550
Chicago/Turabian StyleFimbo, Adam M., Omary M.S. Minzi, Bruno P. Mmbando, Abbie Barry, Alex F. Nkayamba, Kissa W. Mwamwitwa, Alpha Malishee, Misago D. Seth, Williams H. Makunde, Parthasarathi Gurumurthy, and et al. 2020. "Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania" Journal of Clinical Medicine 9, no. 5: 1550. https://doi.org/10.3390/jcm9051550
APA StyleFimbo, A. M., Minzi, O. M. S., Mmbando, B. P., Barry, A., Nkayamba, A. F., Mwamwitwa, K. W., Malishee, A., Seth, M. D., Makunde, W. H., Gurumurthy, P., Lusingu, J. P. A., Kamuhabwa, A. A. R., & Aklillu, E. (2020). Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania. Journal of Clinical Medicine, 9(5), 1550. https://doi.org/10.3390/jcm9051550





