Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Lung Function Measurements
2.4. Hospital Measurements
2.5. Home Monitor
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
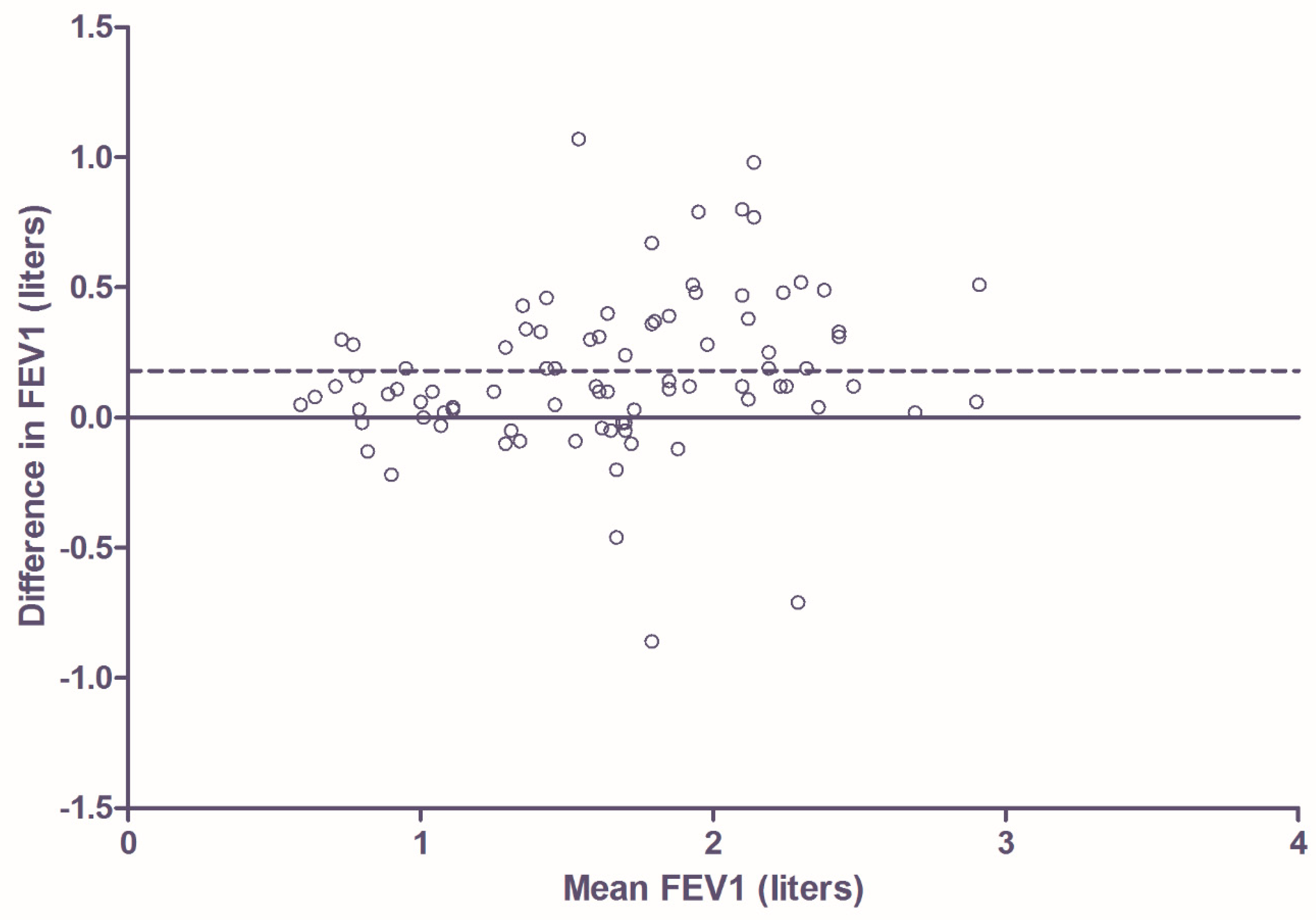
3.2. Comparison of Home Monitor FEV1 Measurement with FEV1 Measurement Using Pneumotachometer in Hospital Setting in Patients with CF
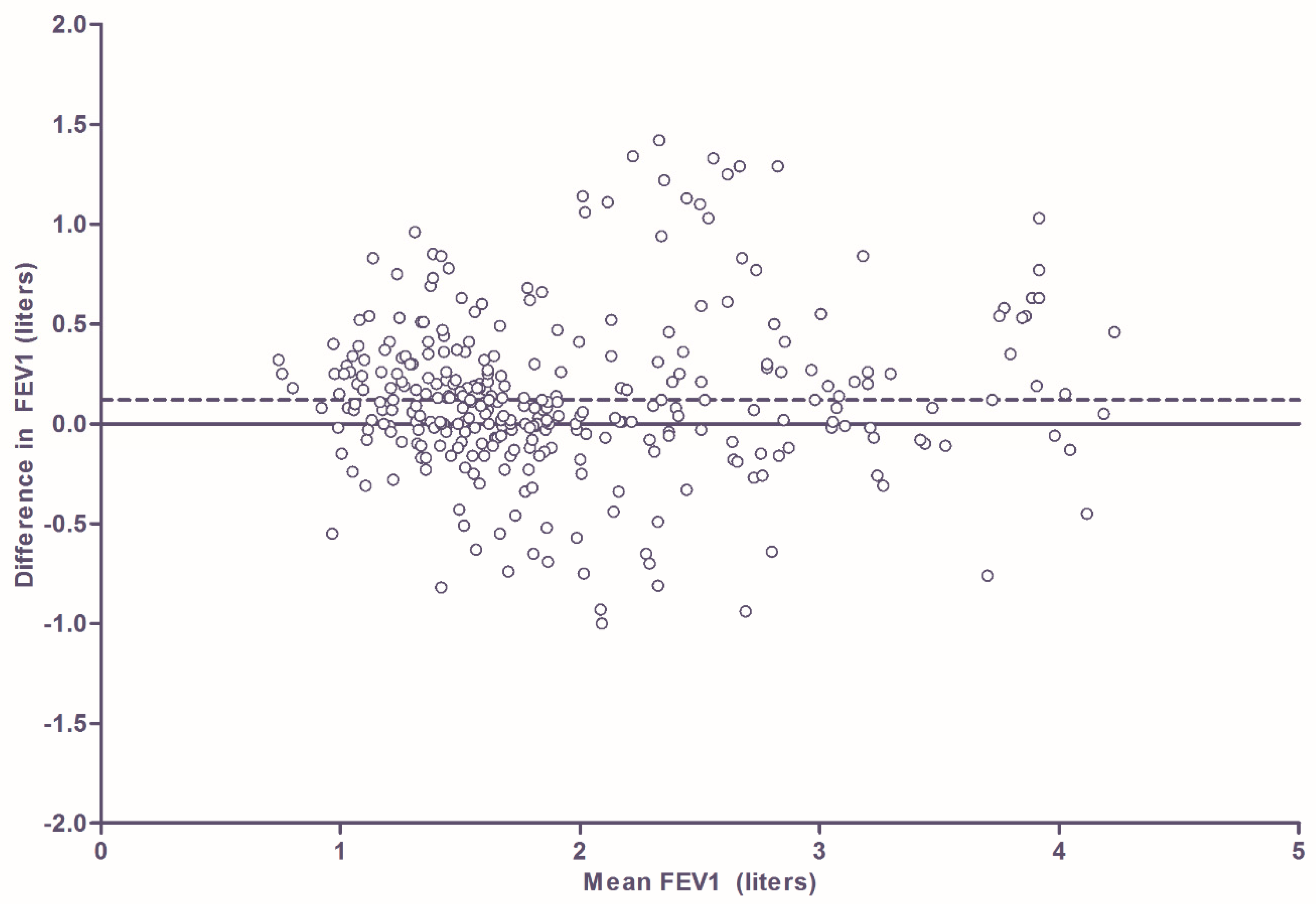
3.3. Comparison of Home Monitor FEV1 Measurement with FEV1 Measurement Using Pneumotachometer in Hospital Setting in Patients with Asthma
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papadopoulos, N.G.; Arakawa, H.; Carlsen, K.-H.; Custovic, A.; Gern, J.; Lemanske, R.; Le Souef, P.; Makela, M.; Roberts, G.; Wong, G.; et al. International consensus on (icon) pediatric asthma. Allergy 2012, 67, 976–997. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (UK). Cystic Fibrosis, Diagnosis and Management; NICE Guideline, No. 78. Available online: https://www.ncbi.nlm.nih.gov/books/NBK464183 (accessed on 30 April 2020).
- Smyth, A.R.; Bell, S.C.; Bojcin, S.; Bryon, M.; Duff, A.; Flume, P.; Kashirskaya, N.; Munck, A.; Ratjen, F.; Schwarzenberg, S.J.; et al. European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J. Cyst. Fibros. 2014, 13 (Suppl. 1), 23–42. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (updated 2020). Available online: http://www.ginasthma.org (accessed on 30 April 2020).
- Pijnenburg, M.W.; Baraldi, E.; Brand, P.L.; Carlsen, K.H.; Eber, E.; Frischer, T.; Hedlin, G.; Kulkarni, N.; Lex, C.; Makela, M.J.; et al. Monitoring asthma in children. Eur. Respir. J. 2015, 45, 906–925. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.; Ratjen, F. Pulmonary Exacerbations in Children with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S200–S206. [Google Scholar] [PubMed]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.B. Respiratory applications of telemedicine. Thorax 2009, 64, 189–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brouwer, A.F.; Roorda, A.J.; Brand, P.L. Comparison between peak expiratory flow and FEV (1) measurements on a home spirometer and on a pneumotachograph in children with asthma. Pediatr. Pulmonol. 2007, 42, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Bastian-Lee, Y.; Chavasse, R.; Richter, H.; Seddon, P. Assessment of a Low-Cost Home Monitoring Spirometer for Children. Pediatr. Pulmonol. 2002, 33, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.M.; Fallot, A.; Balmes, J.R.; Tager, I.B. Evaluating the use of a portable spirometer in a study of pediatric asthma. Chest 2003, 123, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, D.; van Horck, M.; van de Kant, K.; Vaassen, S.; Gulikers, S.; Winkens, B.; Rosias, P.; Heynens, J.; Muris, J.; Essers, B.; et al. Electronic monitoring of symptoms and lung function to assess asthma control in children. An. Allergy Asthma Immunol. 2014, 113, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Van Horck, M.; Winkens, B.; Wesseling, G.; van Vliet, D.; van de Kant, K.; Vaassen, S.; Groot, K.W.; de Vreede, I.; Jöbsis, Q.; Dompeling, E. Early detection of pulmonary exacerbations in children with cystic fibrosis by electronic home monitoring of symptoms and lung function. Sci. Rep. 2017, 7, 12350. [Google Scholar] [CrossRef] [PubMed]
- Lechtzin, N.; Mayer-Hamblett, N.; West, N.E.; Allgood, S.; Wilhelm, E.; Khan, U.; Aitken, M.L.; Ramsey, B.W.; Boyle, M.P.; Mogayzel, P.J., Jr.; et al. Home Monitoring of Patients with Cystic Fibrosis to Identify and Treat Acute Pulmonary Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 196, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- De Jongste, J.C.; Vrijlandt, E.J.L.E. Astma bij kinderen. In Samenvatting van de Herziene richtlijnen van de Sectie Kinderlongziekten van de NVK [Asthma in Children; Summary of the Revised Guidelines of the Paediatric Pulmonology Department of the Dutch Paediatric Society]; Hilversum: Hilversum, The Netherlands, 2007. [Google Scholar]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Wensley, D.; Silverman, M. The quality of home spirometry in schoolchildren with asthma. Thorax 2001, 56, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kanniess, F.; Mark, B.; Jörres, R.A.; Magnussen, H. Assessment of accuracyand applicability of a new electronic peak flow meter and asthma monitor. Eur. Respir. J. 1998, 12, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.M.; Jurdi, R.; Roberts, C.A.; Hernandez, M.; Horne, R.; Chan, A. A Review of Portable Electronic Spirometers: Implications for Asthma Self-Management. Curr. Allergy Asthma Rep. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | ||
|---|---|---|
| CF (n = 36) | Asthma (n = 81) | |
| Age (y), mean (SD) | 9.4 (2.8) | 9.6 (3.0) |
| Male sex, n (%) | 22 (61) | 43 (53) |
| FEV1 (L), mean (SD) | 1.70 (0.53) | 1.91 (0.76) |
| FEV1%pred, mean (SD) | 87.3 (17.0) | 97.0 (14.3) |
| FVC%pred, mean (SD) | 91.9 (16.3) | 98.5 (11.6) |
| FEV1/FVC, mean (SD) | 0.81 (0.09) | 0.84 (0.08) |
| Homozygous dF508, n (%) | 25 (69) | n.a. |
| Pseudomonas colonisation, n (%) | 12 (33) | n.a. |
| BMI, median (IQR) | 16.6 (16.0–17.6) | n.a. |
| Prophylactic antibiotics, n (%) | 22 (61) | n.a. |
| ICS use, n (%) | 11 (31) | 75 (93) |
| ICS + LABA use, n (%) | n.a. | 33 (41) |
| Atopic, n (%) | n.a. | 62 (76) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerzon, F.L.G.R.; Jöbsis, Q.; Bannier, M.A.G.E.; Winkens, B.; Dompeling, E. Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF. J. Clin. Med. 2020, 9, 1617. https://doi.org/10.3390/jcm9061617
Gerzon FLGR, Jöbsis Q, Bannier MAGE, Winkens B, Dompeling E. Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF. Journal of Clinical Medicine. 2020; 9(6):1617. https://doi.org/10.3390/jcm9061617
Chicago/Turabian StyleGerzon, Frederick L.G.R., Quirijn Jöbsis, Michiel A.G.E. Bannier, Bjorn Winkens, and Edward Dompeling. 2020. "Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF" Journal of Clinical Medicine 9, no. 6: 1617. https://doi.org/10.3390/jcm9061617
APA StyleGerzon, F. L. G. R., Jöbsis, Q., Bannier, M. A. G. E., Winkens, B., & Dompeling, E. (2020). Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF. Journal of Clinical Medicine, 9(6), 1617. https://doi.org/10.3390/jcm9061617






