Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical and Laboratory Assessments
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Comparisons of Characteristics between Subjects with and without NAFLD before and after Propensity Score (PS) Matching
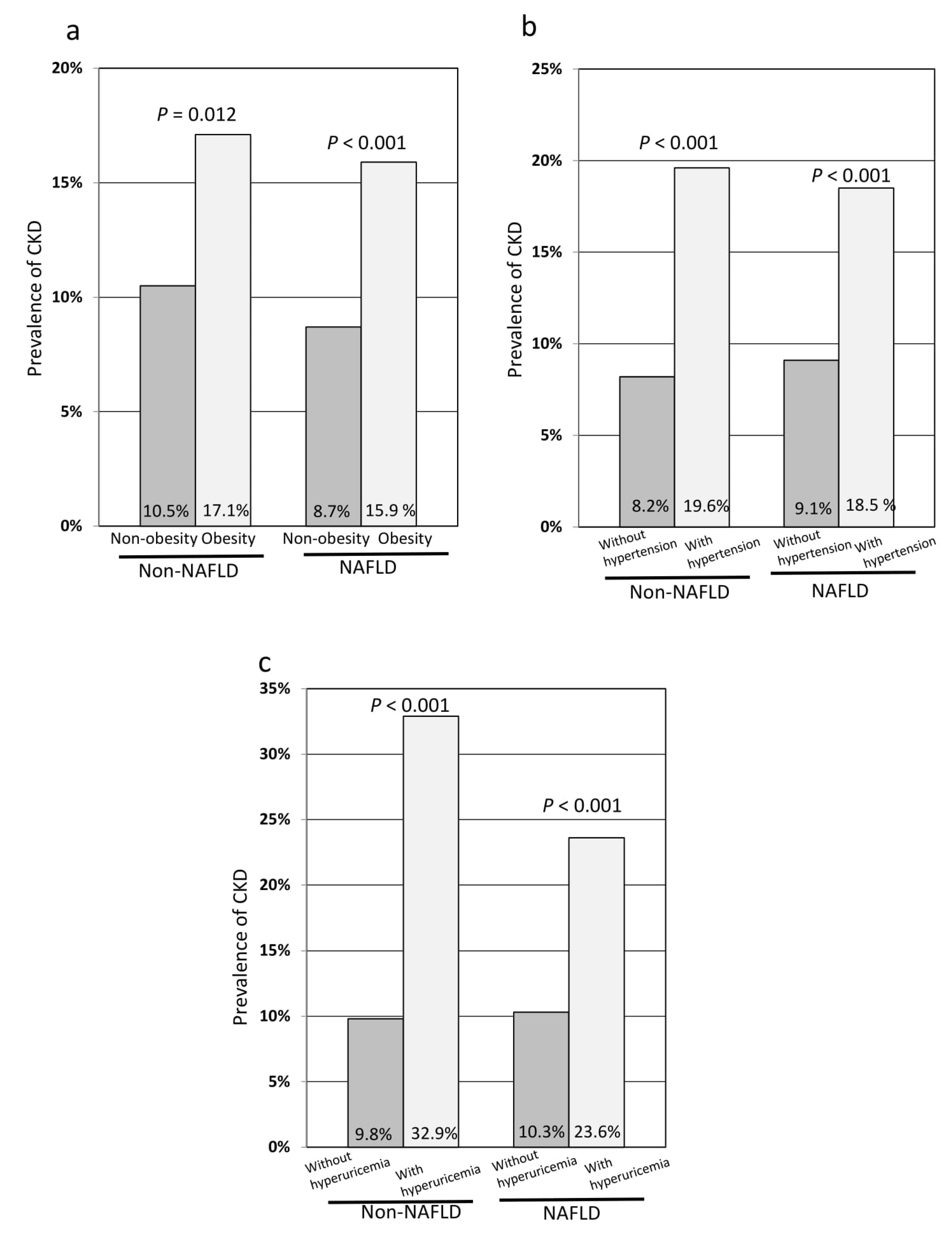
3.3. Comparisons of the Prevalence of Chronic Kidney Disease (CKD) in Subgroup Analyses after PS Matching
3.4. Risk Factors of CKD in the Logistic Regression Analysis after PS Matching
3.5. Risk Factors of CKD in Subjects with and without Non-Alcoholic Fatty Liver Disease (NAFLD)
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- James, M.T.; Hemmelgarn, B.R.; Tonelli, M. Early recognition and prevention of chronic kidney disease. Lancet 2010, 375, 1296–1309. [Google Scholar] [CrossRef]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Chien, K.L.; Lin, H.J.; Lee, B.C.; Hsu, H.C.; Lee, Y.T.; Chen, M.F. A prediction model for the risk of incident chronic kidney disease. Am. J. Med. 2010, 123, 836–846.e832. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of Chronic Kidney Disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Watanabe, T.; Iseki, K.; Yamagata, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Moriyama, T.; Ando, Y.; et al. Prevalence of chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2009, 13, 621–630. [Google Scholar] [CrossRef]
- Weng, S.C.; Wu, C.L.; Kor, C.T.; Chiu, P.F.; Wu, M.J.; Chang, C.C.; Tarng, D.C. Migraine and subsequent chronic kidney disease risk: A nationwide population-based cohort study. BMJ Open 2017, 7, e018483. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Zoppini, G.; Abaterusso, C.; Bonora, E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: Is there a link? J. Hepatol. 2011, 54, 1020–1029. [Google Scholar] [CrossRef]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, S.; Bachmayer, S.; Wernly, B.; Bakula, A.; Huber-Schonauer, U.; Semmler, G.; Schmied, C.; Aigner, E.; Datz, C. Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort. J. Clin. Med. 2020, 9, 1065. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Suzuki, F.; Kobayashi, M.; Suzuki, Y.; Kawamura, Y.; Matsumoto, N.; Akuta, N.; Kobayashi, M.; Sezaki, H.; Saito, S.; et al. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern. Med. 2011, 50, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kim, J.Y.; Choi, E.; Kim, J.S.; Chang, Y.; Sung, K.C. The fatty liver index as a predictor of incident chronic kidney disease in a 10-year prospective cohort study. PLoS ONE 2017, 12, e0180951. [Google Scholar] [CrossRef]
- Jang, H.R.; Kang, D.; Sinn, D.H.; Gu, S.; Cho, S.J.; Lee, J.E.; Huh, W.; Paik, S.W.; Ryu, S.; Chang, Y.; et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: A cohort study. Sci. Rep. 2018, 8, 4718. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Jang, H.R.; Gu, S.; Cho, S.J.; Paik, S.W.; Ryu, S.; Chang, Y.; Lazo, M.; Guallar, E.; et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J. Hepatol. 2017, 67, 1274–1280. [Google Scholar] [CrossRef]
- Park, H.; Dawwas, G.K.; Liu, X.; Nguyen, M.H. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: A propensity-matched cohort study. J. Intern. Med. 2019, 286, 711–722. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Gamma-glutamyltransferase and risk of chronic kidney disease: A prospective cohort study. Clin. Chim. Acta 2017, 473, 39–44. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Duan, J.Y.; Duan, G.C.; Wang, C.J.; Liu, D.W.; Qiao, Y.J.; Pan, S.K.; Jiang, D.K.; Liu, Y.; Zhao, Z.H.; Liang, L.L.; et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in a central Chinese urban population: A cross-sectional survey. BMC Nephrol. 2020, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Sung, S.A.; Kim, Y.S.; Oh, K.H.; Ahn, C.; Kim, S.W. Hyperuricemia has increased the risk of progression of chronic kidney disease: Propensity score matching analysis from the KNOW-CKD study. Sci. Rep. 2019, 9, 6681. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kaze, A.D.; McMullan, C.J.; Isakova, T.; Waikar, S.S. Uric Acid and the Risks of Kidney Failure and Death in Individuals With CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 71, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kaewput, W.; Thongprayoon, C.; Rangsin, R.; Ruangkanchanasetr, P.; Bathini, T.; Mao, M.A.; Cheungpasitporn, W. Association between serum uric acid and chronic kidney disease in patients with hypertension: A multicenter nationwide cross-sectional study. J. Evid. -Based Med. 2019, 12, 235–242. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.; Zhang, X.; Liu, S.; Ge, Y. Association of the serum uric acid level with liver histology in biopsy-proven non-alcoholic fatty liver disease. Biomed. Rep. 2016, 5, 188–192. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Larson, T.S.; Santanello, N.; Shahinfar, S.; O’Brien, P.C.; Palumbo, P.J.; Melton, L.J., 3rd; Leibson, C.L. Trends in persistent proteinuria in adult-onset diabetes: A population-based study. Diabetes Care 2000, 23, 51–56. [Google Scholar] [CrossRef][Green Version]
- Sirota, J.C.; McFann, K.; Targher, G.; Chonchol, M.; Jalal, D.I. Association between nonalcoholic liver disease and chronic kidney disease: An ultrasound analysis from NHANES 1988-1994. Am. J. Nephrol. 2012, 36, 466–471. [Google Scholar] [CrossRef]
- Liu, H.W.; Liu, J.S.; Kuo, K.L. Association of nonalcoholic fatty liver and chronic kidney disease: An analysis of 37,825 cases from health checkup center in Taiwan. Ci Ji Yi Xue Za Zhi 2020, 32, 65–69. [Google Scholar] [CrossRef]
- Qin, S.; Wang, S.; Wang, X.; Wang, J. Liver stiffness assessed by transient elastography as a potential indicator of chronic kidney disease in patients with nonalcoholic fatty liver disease. J. Clin. Lab. Anal. 2019, 33, e22657. [Google Scholar] [CrossRef] [PubMed]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Xu, H.W.; Hsu, Y.C.; Chang, C.H.; Wei, K.L.; Lin, C.L. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol. Int. 2016, 10, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Wu, K.T.; Chen, Y.Y.; Yang, J.F.; Lin, W.Y.; Chang, N.C.; Lin, C.Y.; Huang, C.K.; Wang, C.L.; Chuang, H.Y.; et al. Higher NAFLD fibrosis score is associated with impaired eGFR. J. Med. Assoc. 2020, 119, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Fiorentino, T.V.; Arturi, F.; Perticone, M.; Sciacqua, A.; Perticone, F. Association between noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e88569. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- de Lédinghen, V.; Vergniol, J.; Foucher, J.; Merrouche, W.; le Bail, B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012, 32, 911–918. [Google Scholar] [CrossRef]
| Before PS Matching | After PS Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-NAFLD | NAFLD | p-Value | STD | Non-NAFLD | NAFLD | p-Value | STD | |
| n | 2571 | 1154 | 1097 | 1097 | ||||
| Sex, n, male | 996 (38.7) | 755 (65.4) | <0.001 | 0.51 | 714 (65.1) | 702 (64.0) | 0.592 | 0.02 |
| Age, y | 53.2 ± 9.5 | 54.1 ± 8.4 | 0.003 | 0.10 | 54.3 ± 9.2 | 54.0 ± 8.5 | 0.443 | 0.03 |
| Use of antihypertensive medications | 289 (11.2) | 278 (24.1) | <0.001 | 0.45 | 214 (19.5) | 225(20.5) | 0.557 | 0.04 |
| Use of antidiabetic medications | 72 (2.8) | 77 (6.7) | <0.001 | 0.45 | 49 (4.5) | 58 (5.3) | 0.372 | 0.09 |
| Use of lipid-lowering medications | 235 (9.1) | 217 (18.8) | <0.001 | 0.41 | 155 (14.1) | 169 (15.4) | 0.400 | 0.05 |
| Cerebrovascular disease | 30 (1.2) | 15 (1.3) | 0.731 | 0.05 | 11 (1.0) | 15 (1.4) | 0.430 | 0.16 |
| Heart disease | 87 (3.4) | 55 (4.8) | 0.042 | 0.17 | 36 (3.3) | 51 (4.6) | 0.101 | 0.18 |
| Smoking (BI) | 113 ± 252 | 216 ± 342 | <0.001 | 0.34 | 185 ± 320 | 205 ± 328 | 0.15 | 0.06 |
| Before PS Matching | After PS Matching | |||||
|---|---|---|---|---|---|---|
| Non-NAFLD | NAFLD | p-Value | Non-NAFLD | NAFLD | p-Value | |
| n | 2571 | 1154 | 1097 | 1097 | ||
| BMI (kg/m2) | 22.0 ± 2.7 | 25.8 ± 3.3 | <0.001 | 22.4 ± 2.7 | 25.7 ± 3.2 | <0.001 |
| Obesity | 320 (12.4) | 635 (55.0) | <0.001 | 175 (16.0) | 590 (53.8) | <0.001 |
| Smoking | <0.001 | 0.008 | ||||
| Never | 1846 (71.8) | 626 (54.2) | 678 (61.8) | 613 (55.9) | ||
| Past | 476 (18.5) | 352 (30.5) | 261 (23.8) | 321 (29.3) | ||
| Current | 249 (9.7) | 176 (15.3) | 158 (14.4) | 163 (14.9) | ||
| Systolic BP (mmHg) | 121.9 ± 15.2 | 130.0 ± 14.5 | <0.001 | 124.7 ± 14.7 | 129.5 ± 14.4 | <0.001 |
| Diastolic BP (mmHg) | 72.7 ± 11.2 | 80.0 ± 10.8 | <0.001 | 75.2 ± 11.0 | 79.2 ± 10.8 | <0.001 |
| Hypertension | 520 (20.2) | 459 (39.8) | <0.001 | 321 (29.3) | 406 (37.0) | <0.001 |
| AST level (IU/L) | 21 ± 6 | 25 ± 10 | <0.001 | 21 ± 5 | 25 ± 10 | <0.001 |
| ALT level (IU/L) | 19 ±10 | 33 ± 21 | <0.001 | 21 ± 9 | 33 ± 21 | <0.001 |
| GGT level (IU/L) | 24 ± 22 | 41 ± 36 | <0.001 | 28 ± 25 | 40 ± 35 | <0.001 |
| Triglyceride level (mg/dL) | 87 ± 47 | 146 ± 92 | <0.001 | 94 ± 44 | 146 ± 93 | <0.001 |
| High triglyceride level | 192 (7.5) | 399 (34.6) | <0.001 | 107 (9.8) | 376 (34.3) | <0.001 |
| Total cholesterol level (mg/dL) | 210 ± 32 | 213 ± 34 | 0.004 | 208 ± 32 | 214 ± 93 | <0.001 |
| HDL cholesterol level (mg/dL) | 65 ± 15 | 53 ± 11 | <0.001 | 62 ± 15 | 53 ± 11 | <0.001 |
| LDL cholesterol level (mg/dL) | 124 ± 29 | 135 ± 30 | <0.001 | 125 ± 28 | 136 ± 30 | <0.001 |
| High LDL cholesterol level | 726 (28.2) | 478 (41.4) | <0.001 | 318 (29.0) | 466 (42.5) | <0.001 |
| Fasting glucose level (mg/dL) | 95 ± 15 | 105 ± 19 | <0.001 | 98 ± 16 | 105 ± 18 | <0.001 |
| Diabetes mellitus | 90 (3.5) | 134 (11.6) | <0.001 | 59 (5.4) | 113 (10.3) | <0.001 |
| Uric acid level (mg/dL) | 4.8 ± 1.2 | 5.8 ± 1.2 | <0.001 | 5.2 ± 1.2 | 5.8 ± 1.3 | <0.001 |
| Hyperuricemia | 131 (5.1) | 199 (17.2) | <0.001 | 85 (7.7) | 191 (17.4) | <0.001 |
| SCr level | 0.74 | 0.81 | <0.001 | 0.81 | 0.81 | 0.742 |
| eGFR | 74.7 | 73.1 | <0.001 | 73.5 | 73.3 | 0.719 |
| CKD | 239 (9.3) | 146 (12.7) | 0.002 | 127 (11.6) | 138 (12.6) | 0.471 |
| NAFLD Status | Number of Patients | eGFR | p-Value | CKD | p-Value | |
|---|---|---|---|---|---|---|
| Age < 60 years | Non-NAFLD | 816 | 74.9 ± 11.9 | 0.394 | 74 (9.1) | 0.315 |
| NAFLD | 825 | 74.3 ± 12.6 | 87 (10.5) | |||
| Age ≥60 years | Non-NAFLD | 281 | 69.3 ± 11.3 | 0.531 | 53 (18.9) | 0.973 |
| NAFLD | 272 | 69.9 ± 12.0 | 51 (18.8) | |||
| Male sex | Non-NAFLD | 714 | 72.6 ± 12.0 | 0.125 | 98 (13.7) | 0.838 |
| NAFLD | 702 | 71.7 ± 11.2 | 99 (14.1) | |||
| Female sex | Non-NAFLD | 383 | 75.1 ± 12.0 | 0.254 | 29 (7.6) | 0.256 |
| NAFLD | 395 | 76.2 ± 14.3 | 39 (9.9) | |||
| Non-obesity | Non-NAFLD | 922 | 73.9 ± 11.9 | 0.774 | 97 (10.5) | 0.266 |
| NAFLD | 507 | 73.7 ± 11.6 | 44 (8.7) | |||
| Obesity | Non-NAFLD | 175 | 71.2 ± 12.7 | 0.147 | 30 (17.1) | 0.703 |
| NAFLD | 590 | 72.8 ± 13.2 | 94 (15.9) | |||
| Non-hypertension | Non-NAFLD | 776 | 75.0 ± 11.6 | 0.655 | 64 (8.2) | 0.554 |
| NAFLD | 691 | 75.0 ± 12.2 | 63 (9.1) | |||
| Hypertension | Non-NAFLD | 321 | 69.9 ± 12.4 | 0.282 | 63 (19.6) | 0.694 |
| NAFLD | 406 | 70.9 ± 12.9 | 75 (18.5) | |||
| Normal triglyceride level | Non-NAFLD | 990 | 73.8 ± 12.1 | 0.373 | 110 (11.1) | 0.541 |
| NAFLD | 721 | 73.3 ± 12.3 | 87 (12.1) | |||
| High triglyceride level | Non-NAFLD | 107 | 70.7 ± 11.1 | 0.038 | 17 (15.9) | 0.542 |
| NAFLD | 376 | 73.4 ± 13.3 | 51 (13.6) | |||
| Normal LDL cholesterol level | Non-NAFLD | 779 | 73.9 ± 12.4 | 0.034 | 92 (11.8) | 0.147 |
| NAFLD | 631 | 72.5 ± 12.5 | 91 (14.4) | |||
| High LDL cholesterol level | Non-NAFLD | 318 | 72.5 ± 10.9 | 0.025 | 35 (11.0) | 0.679 |
| NAFLD | 466 | 74.4 ± 12.6 | 47 (10.1) | |||
| Non-diabetes | Non-NAFLD | 1308 | 73.4 ± 11.8 | 0.886 | 117 (11.3) | 0.519 |
| NAFLD | 984 | 73.4 ± 12.6 | 120 (12.2) | |||
| Diabetes | Non-NAFLD | 59 | 74.3 ± 15.0 | 0.473 | 10 (16.9) | 0.863 |
| NAFLD | 113 | 72.8 ± 13.1 | 18 (15.9) | |||
| Non-hyperuricemia | Non-NAFLD | 1012 | 74.1 ± 11.8 | 0.474 | 99 (9.8) | 0.725 |
| NAFLD | 906 | 74.5 ± 12.6 | 93 (10.3) | |||
| Hyperuricemia | Non-NAFLD | 85 | 65.9 ± 12.2 | 0.283 | 28 (32.9) | 0.103 |
| NAFLD | 191 | 67.5 ± 11.0 | 45 (23.6) |
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Obesity | 1.686 (1.246–2.281) | 0.001 |
| Hypertension | 1.572 (1.183–2.088) | 0.002 |
| High triglyceride level | 1.087 (0.780–1.516) | 0.622 |
| High LDL cholesterol level | 0.833 (0.621–1.118) | 0.223 |
| Diabetes | 0.922 (0.583–1.458) | 0.728 |
| Hyperuricemia | 2.877 (2.029–4.080) | <0.001 |
| NAFLD | 0.832 (0.614–1.127) | 0.220 |
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Obesity | 2.104 (1.397–3.168) | <0.001 |
| Hypertension | 1.505 (1.021–2.219) | 0.039 |
| High triglyceride level | 1.085 (0.728–1.616) | 0.688 |
| High LDL cholesterol level | 0.717 (0.484–1.063) | 0.098 |
| Diabetes | 0.950 (0.538–1.678) | 0.860 |
| Hyperuricemia | 2.413 (1.537–3.788) | <0.001 |
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Obesity | 1.229 (0.752–2.009) | 0.411 |
| Hypertension | 1.662 (1.094–2.527) | 0.017 |
| High triglyceride level | 1.227 (0.672–2.241) | 0.505 |
| High LDL cholesterol level | 0.981 (0.631–1.524) | 0.931 |
| Diabetes | 0.878 (0.406–1.898) | 0.741 |
| Hyperuricemia | 3.884 (2.228–6.772) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akahane, T.; Akahane, M.; Namisaki, T.; Kaji, K.; Moriya, K.; Kawaratani, H.; Takaya, H.; Sawada, Y.; Shimozato, N.; Fujinaga, Y.; et al. Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 1635. https://doi.org/10.3390/jcm9061635
Akahane T, Akahane M, Namisaki T, Kaji K, Moriya K, Kawaratani H, Takaya H, Sawada Y, Shimozato N, Fujinaga Y, et al. Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(6):1635. https://doi.org/10.3390/jcm9061635
Chicago/Turabian StyleAkahane, Takemi, Manabu Akahane, Tadashi Namisaki, Kosuke Kaji, Kei Moriya, Hideto Kawaratani, Hiroaki Takaya, Yasuhiko Sawada, Naotaka Shimozato, Yukihisa Fujinaga, and et al. 2020. "Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 6: 1635. https://doi.org/10.3390/jcm9061635
APA StyleAkahane, T., Akahane, M., Namisaki, T., Kaji, K., Moriya, K., Kawaratani, H., Takaya, H., Sawada, Y., Shimozato, N., Fujinaga, Y., Furukawa, M., Kitagawa, K., Ozutsumi, T., Tsuji, Y., Kaya, D., Mitoro, A., & Yoshiji, H. (2020). Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study. Journal of Clinical Medicine, 9(6), 1635. https://doi.org/10.3390/jcm9061635






