Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies
Abstract
:1. Introduction
1.1. Medical Needs and Clinical Aspects in Colonoscopy
1.2. Ergonomics: The Problem of Musculoskeletal Injuries
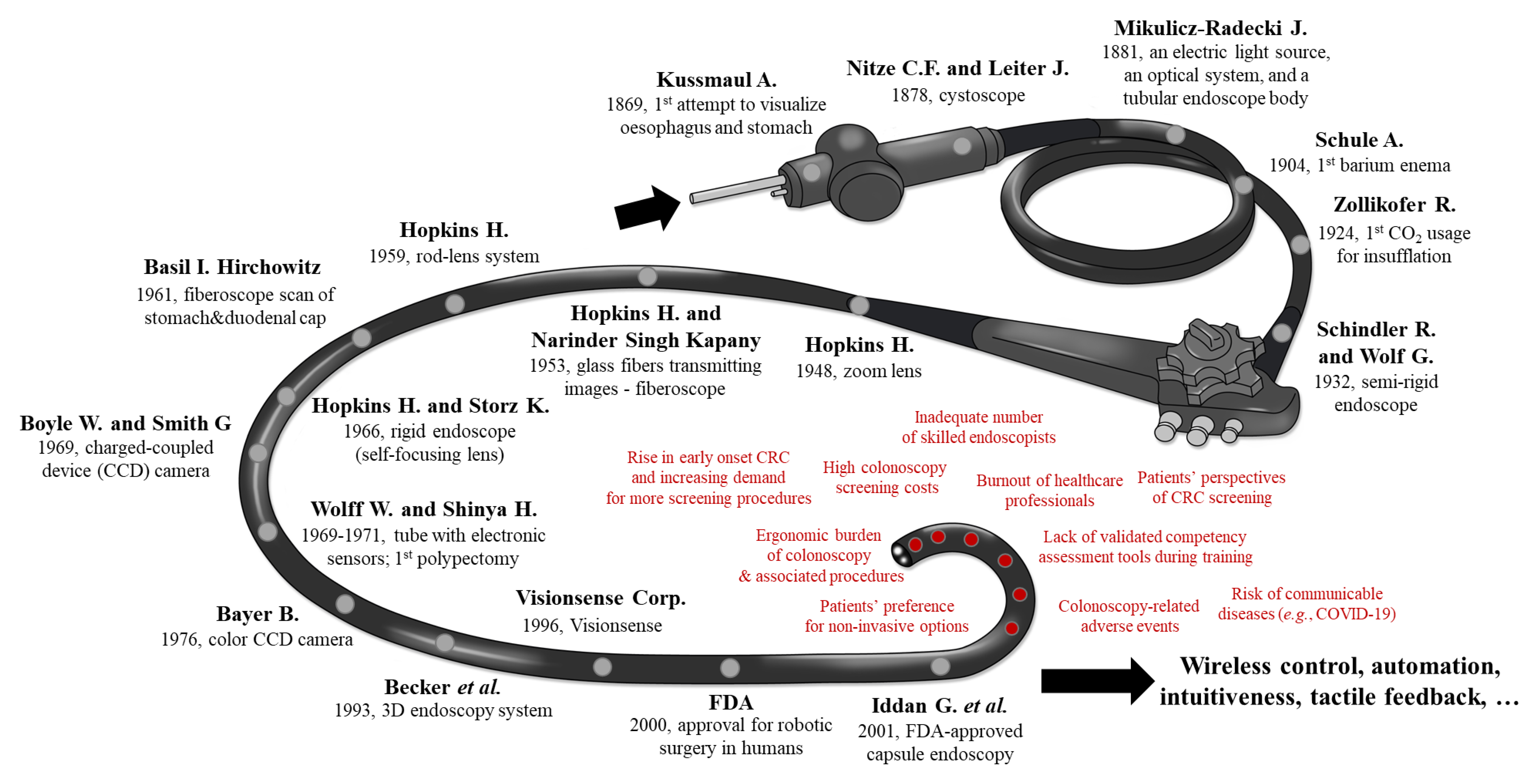
1.3. History and Key-Milestones of Colonoscopy
1.4. What Is Robotic Colonoscopy and Why Is Now the Time?
- “What is the difference between non-robotic instrumental colonoscopes (Section 2) and robotic flexible colonoscopes (Section 3 and Section 4)?” Although there is not a single definition that will cover all aspects, we hope—at least—that this contribution will continue the conversation on this debated issue. For the authors, the difference between them is nestled in its intrinsic capability to enable and perform controlled assisted actions or autonomous procedures in an unstructured deformable environment, such as in the colonic tract.
- “What are the modules needed to achieve that?” Not only embedded sensors, such as the vision camera into the PillCam™ capsule or pH/temperature sensors are needed but also a complex hardware and software architecture that enables computer-integrated modalities, i.e., the information collected by sensors, through wired or wireless communications, can be elaborated thanks to AI algorithms (Section 5) for enabling advanced and potentially-autonomous actuation and actions (i.e., navigation of the device but also activation of mechanisms for drug-delivery and tissue sampling) in a closed-loop manner.
- “Why is now the time?” Technologies are now in a mature state and thanks to the wider use of robotic systems and technologies in surgery, endoscopists are now more open in accepting and collaborating with robotic companions during their activities [29,30]. In addition, under the current circumstances, one could in parallel to the term “social distancing” coin the term “medical distancing” (not in care, emotion or relationship but more in a physical contact during medical practices) via complex personal protective equipment or very simply reducing handshakes and consultation distance. Obviously, most of us believe that the end of the SARS-CoV-2 pandemic will allow things to go back to normal, however, this global pandemic sets the scene for innovation in ways and speed that have not been seen before in the field of minimally-invasive surgery and/or in remote robotic diagnosis and therapy in medicine. So, our last question is “Is it now the time for introducing in the medical practice a new teleoperated or even autonomous robotic colonoscope?”
2. Non-Robotic Colonoscopes and Colonoscopy Adjuncts in the Clinical Practice
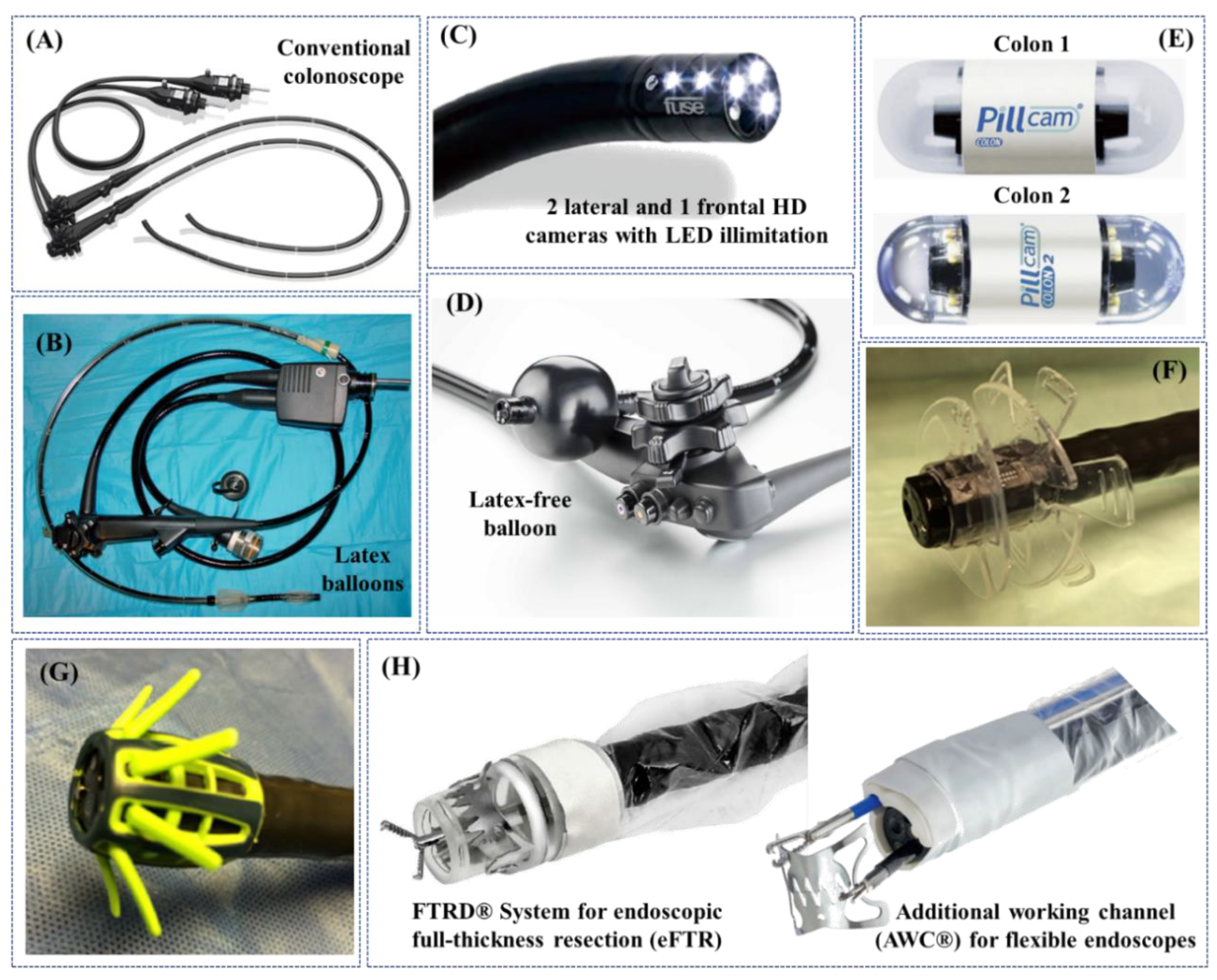
3. Robotic Flexible Colonoscopes: Commercially-Certified Instruments

4. Innovative Robotic Colonoscopes: Research Initiatives and Devices


5. Artificial Intelligence: An Enabling Factor for Enhancing Robotic Colonoscopy

6. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Keum, N.N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Robertson, D.J.; Senore, C.; Rex, D.K. Optimizing the quality of colorectal cancer screening worldwide. Gastroenterology 2019, 158, 404–417. [Google Scholar] [CrossRef]
- Ratushnyak, S.; Hoogendoorn, M.; Van Baal, P.H.M. Cost-effectiveness of cancer screening: Health and costs in life years gained. Am. J. Prev. Med. 2019, 57, 792–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaseem, A.; Crandall, C.J.; Mustafa, R.A.; Hicks, L.A.; Wilt, T.J. Screening for colorectal cancer in asymptomatic average-risk adults: A guidance statement from the american college of physicians. Ann. Intern. Med. 2019, 171, 643–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phisalprapa, P.; Supakankunti, S.; Chaiyakunapruk, N. Cost-effectiveness and budget impact analyses of colorectal cancer screenings in a low- and middle-income country: Example from Thailand. J. Med. Econ. 2019, 22, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Chan, J.L. Physician burnout: The hidden health care crisis. Clin. Gastroenterol. Hepatol. 2018, 16, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siau, K.; Anderson, J.T. Ergonomics in endoscopy: Should the endoscopist be considered and trained like an athlete? Endosc. Int. Open 2019, 7, E813–E815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krigel, A.; Chen, L.; Wright, J.D.; Lebwohl, B. Substantial increase in anesthesia assistance for outpatient colonoscopy and associated cost nationwide. Clin. Gastroenterol. Hepatol. 2019, 17, 2489–2496. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.J.; Ladabaum, U. Opportunities and challenges in moving from current guidelines to personalized colorectal cancer screening. Gastroenterology 2019, 156, 904–917. [Google Scholar] [CrossRef] [Green Version]
- Repici, A.; Maselli, R.; Colombo, M.; Gabbiadini, R.; Spadaccini, M.; Anderloni, A.; Carrara, S.; Fugazza, A.; Di Leo, M.; Galtieri, P.A.; et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest. Endosc. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Kim, H.S.; Park, H.J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef]
- Kothari, S.T.; Huang, R.J.; Shaukat, A.; Agrawal, D.; Buxbaum, J.L.; Abbas Fehmi, S.M.; Fishman, D.S.; Gurudu, S.R.; Khashab, M.A.; Jamil, L.H.; et al. ASGE review of adverse events in colonoscopy. Gastrointest. Endosc. 2019, 90, 863–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.-N.; Wang, C.-B.; Yang, C.-H.; Lai, C.-H.; Lin, H.-H. Risk of infection following colonoscopy and sigmoidoscopy in symptomatic patients. Endoscopy 2017, 49, 754–764. [Google Scholar] [CrossRef]
- Yung, D.E.; Banfi, T.; Ciuti, G.; Arezzo, A.; Dario, P.; Koulaouzidis, A. Musculoskeletal injuries in gastrointestinal endoscopists: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Attar, B.; Trick, W.; Kotwal, V. Endoscopy-related musculoskeletal injuries in gastroenterology fellows. Endosc. Int. Open 2019, 7, E808–E812. [Google Scholar] [CrossRef] [Green Version]
- Austin, K.; Schoenberger, H.; Sesto, M.; Gaumnitz, E.; Teo Broman, A.; Saha, S. Musculoskeletal injuries are commonly reported among gastroenterology trainees: Results of a national survey. Dig. Dis. Sci. 2019, 64, 1439–1447. [Google Scholar] [CrossRef]
- Shergill, A.K.; McQuaid, K.R.; Rempel, D. Ergonomics and GI endoscopy. Gastrointest. Endosc. 2009, 70, 145–153. [Google Scholar] [CrossRef]
- Eisenberg, R.L.; Margulis, A.R. Brief history of gastrointestinal radiology. Radiographics 1991, 11, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.S.; Yee, J. History, evolution, and current status of radiologic imaging tests for colorectal cancer screening. Radiology 2014, 273, S160–S180. [Google Scholar] [CrossRef]
- Modlin, I.M.; Axcan Pharma. The Evolution of Therapy in Gastroenterology: A Vintage of Digestion; Axcan Pharma: Mont St. Hilaire, QC, Canada, 2002; ISBN 2980750409. [Google Scholar]
- Wolff, W.I. Colonoscopy: History and development. Am. J. Gastroenterol. 1989, 84, 1017–1025. [Google Scholar] [PubMed]
- Wolff, W.I.; Shinya, H. A new approach to colonic polyps. Ann. Surg. 1973, 178, 367–378. [Google Scholar] [CrossRef]
- Belinsky, I.; Shinya, H.; Wolff, W.I. Colonofiberoscopy: Technique in colon examination. Am. J. Nurs. 1973, 73, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Mannalithara, A.; Singh, G.; Gerson, L.B.; Triadafilopoulos, G. Risk of perforation from a colonoscopy in adults: A large population-based study. Gastrointest. Endosc. 2009, 69, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Gelijns, A.C.; Dawkins, H. From the scalpel to the scope: Endoscopic innovations in gastroenterology, gynecology, and surgery. In Sources of Medical Technology: Universities and Industry; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Siciliano, B.; Khatib, O. Springer Handbook of Robotics; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 9783319325521. [Google Scholar]
- Boškoski, I.; Costamagna, G. Endoscopy robotics: Current and future applications. Dig. Endosc. 2019, 31, 119–124. [Google Scholar] [CrossRef] [Green Version]
- McBride, K.E.; Steffens, D.; Duncan, K.; Bannon, P.G.; Solomon, M.J. Knowledge and attitudes of theatre staff prior to the implementation of robotic-assisted surgery in the public sector. PLoS ONE 2019, 14, e0213840. [Google Scholar] [CrossRef]
- Perez, R.E.; Schwaitzberg, S.D. Robotic surgery: Finding value in 2019 and beyond. Ann. Laparosc. Endosc. Surg. 2019, 4. [Google Scholar] [CrossRef]
- Leung, W.C.; Foo, D.C.; Chan, T.; Chiang, M.; Lam, A.H.; Chan, H.H.; Cheung, C.C. Alternatives to colonoscopy for population-wide colorectal cancer screening. Hong Kong Med. J. 2016, 22, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Bynum, S.A.; Davis, J.L.; Green, B.L.; Katz, R.V. Unwillingness to participate in colorectal cancer screening: Examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am. J. Health Promot. AJHP 2012, 26, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Green, A.R.; Peters-Lewis, A.; Percac-Lima, S.; Betancourt, J.R.; Richter, J.M.; Janairo, M.-P.R.; Gamba, G.B.; Atlas, S.J. Barriers to Screening colonoscopy for low-income latino and white patients in an urban community health center. J. Gen. Intern. Med. 2008, 23, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Trevisani, L.; Zelante, A.; Sartori, S. Colonoscopy, pain and fears: Is it an indissoluble trinomial? World J. Gastrointest. Endosc. 2014, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Santhirakumar, K.; Butt, H.; Yetisen, A.K. Colonoscopy technologies for diagnostics and drug delivery. Med. Devices Sens. 2019, 2. [Google Scholar] [CrossRef]
- Van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; Van Deventer, S.J.; Dekker, E. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.; Leitão, C.; Pinto, J.; Ribeiro, H.; Pereira, F.; Caldeira, A.; Banhudo, A. Can water exchange improve patient tolerance in unsedated colonoscopy a prospective comparative study. GE Port. J. Gastroenterol. 2018, 25, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuccio, L.; Frazzoni, L.; Hassan, C.; La Marca, M.; Paci, V.; Smania, V.; De Bortoli, N.; Bazzoli, F.; Repici, A.; Rex, D.; et al. Water exchange colonoscopy increases adenoma detection rate: A systematic review with network meta-analysis of randomized controlled studies. Gastrointest. Endosc. 2018, 88, 589–597.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, S.; Zolk, K.; Radaelli, F.; Otte, J.; Rabenstein, T.; Zolk, O. Water infusion versus air insufflation for colonoscopy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Asai, S.; Fujimoto, N.; Tanoue, K.; Akamine, E.; Nakao, E.; Hashimoto, K.; Ichinona, T.; Nambara, M.; Sassa, S.; Yanagi, H.; et al. Water immersion colonoscopy facilitates straight passage of the colonoscope through the sigmoid colon without loop formation: Randomized controlled trial. Dig. Endosc. 2015, 27, 345–353. [Google Scholar] [CrossRef]
- Jia, H.; Pan, Y.; Guo, X.; Zhao, L.; Wang, X.; Zhang, L.; Dong, T.; Luo, H.; Ge, Z.; Liu, J.; et al. Water exchange method significantly improves adenoma detection rate: A multicenter, randomized controlled trial. Am. J. Gastroenterol. 2017, 112, 568–576. [Google Scholar] [CrossRef]
- Siau, K.; Beintaris, I. My approach to water-assisted colonoscopy. Frontline Gastroenterol. 2019, 10, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Ciocîrlan, M. Low-cost disposable endoscope: Pros and cons. Endosc. Int. Open 2019, 7, E1184–E1186. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Naber, S.K.; Doria-Rose, V.P.; Pabiniak, C.; Johanson, C.; Fischer, S.E.; Lansdorp-Vogelaar, I.; Kuntz, K.M. Estimation of benefits, burden, and harms of colorectal cancer screening strategies. JAMA 2016, 315, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Niederreiter, M.; Niederreiter, L.; Schmiderer, A.; Tilg, H.; Djanani, A. Colorectal cancer screening and prevention—Pros and cons. Memo Mag. Eur. Med. Oncol. 2019, 12, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Iino, S.; Ido, K.; Sugano, K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest. Endosc. 2001, 53, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Gay, G.; Delvaux, M. Double-balloon colonoscopy after failed conventional colonoscopy: A pilot series with a new instrument. Endoscopy 2007, 39, 788–792. [Google Scholar] [CrossRef]
- Neerincx, M.; Terhaar Sive Droste, J.S.; Mulder, C.J.J.; Räkers, M.; Bartelsman, J.F.W.M.; Loffeld, R.J.; Tuynman, H.A.R.E.; Brohet, R.M.; Van Der Hulst, R.W.M. Colonic work-up after incomplete colonoscopy: Significant new findings during follow-up. Endoscopy 2010, 42, 730–735. [Google Scholar] [CrossRef] [Green Version]
- Mönkemüller, K.; Knippig, C.; Rickes, S.; Fry, L.C.; Schulze, A.; Malfertheiner, P. Usefulness of the double-balloon enteroscope in colonoscopies performed in patients with previously failed colonoscopy. Scand. J. Gastroenterol. 2007, 42, 277–278. [Google Scholar] [CrossRef]
- Moreels, T.G.; MacKen, E.J.; Roth, B.; Van Outryve, M.J.; Pelckmans, P.A. Cecal intubation rate with the double-balloon endoscope after incomplete conventional colonoscopy: A study in 45 patients. J. Gastroenterol. Hepatol. (Aust.) 2010, 25, 80–83. [Google Scholar] [CrossRef]
- Pasha, S.F.; Harrison, M.E.; Das, A.; Corrado, C.M.; Arnell, K.N.; Leighton, J.A. Utility of double-balloon colonoscopy for completion of colon examination after incomplete colonoscopy with conventional colonoscope. Gastrointest. Endosc. 2007, 65, 848–853. [Google Scholar] [CrossRef]
- Kaltenbach, T.; Soetikno, R.; Friedland, S. Use of a double balloon enteroscope facilitates caecal intubation after incomplete colonoscopy with a standard colonoscope. Dig. Liver Dis. 2006, 38, 921–925. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Siersema, P.D.; Halpern, Z.; Segol, O.; Melhem, A.; Suissa, A.; Santo, E.; Sloyer, A.; Fenster, J.; Moons, L.M.G.; et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: An international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014, 15, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, A.; Szura, M.; Solecki, R.; Bogacki, P.; Bachul, P.; Walocha, J.A. The impact of full-spectrum endoscopy on pathological lesion detection in different regions of the colon: A randomised, controlled trial. Arch. Med. Sci. 2019, 15. [Google Scholar] [CrossRef]
- Kudo, T.; Saito, Y.; Ikematsu, H.; Hotta, K.; Takeuchi, Y.; Shimatani, M.; Kawakami, K.; Tamai, N.; Mori, Y.; Maeda, Y.; et al. New-generation full-spectrum endoscopy versus standard forward-viewing colonoscopy: A multicenter, randomized, tandem colonoscopy trial (J-FUSE Study). Gastrointest. Endosc. 2018, 88, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Rodríguez, H.; Diez-Redondo, P.; Pérez-Miranda, M.; Gonzalez Sagrado, M.; Conde, R.; De La Serna, C. Role of full-spectrum endoscopy in colorectal cancer screening: Randomized trial. J. Clin. Gastroenterol. 2019, 53, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Shirin, H.; Shpak, B.; Epshtein, J.; Karstensen, J.G.; Hoffman, A.; De Ridder, R.; Testoni, P.A.; Ishaq, S.; Reddy, D.N.; Gross, S.A.; et al. G-EYE colonoscopy is superior to standard colonoscopy for increasing adenoma detection rate: An international randomized controlled trial (with videos). Gastrointest. Endosc. 2019, 89, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, Z.; Gross, S.A.; Gralnek, I.M.; Shpak, B.; Pochapin, M.; Hoffman, A.; Mizrahi, M.; Rochberger, Y.S.; Moshkowitz, M.; Santo, E.; et al. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: A randomized tandem study. Endoscopy 2015, 47, 238–244. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, K.E.; Soons, E.; Siersema, P.D. The role of behind folds visualizing techniques and technologies in improving adenoma detection rate. Curr. Treat. Options Gastroenterol. 2019, 17, 394–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417–418. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Iakovidis, D.K.; Karargyris, A.; Rondonotti, E. Wireless endoscopy in 2020: Will it still be a capsule? World J. Gastroenterol. 2015, 21, 5119–5130. [Google Scholar] [CrossRef]
- Cummins, G.; Cox, B.F.; Ciuti, G.; Anbarasan, T.; Desmulliez, M.P.Y.; Cochran, S.; Steele, R.; Plevris, J.N.; Koulaouzidis, A. Gastrointestinal diagnosis using non-white light imaging capsule endoscopy. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 429–447. [Google Scholar] [CrossRef] [Green Version]
- Voska, M.; Zavoral, M.; Grega, T.; Majek, O.; Martinek, J.; Tacheci, I.; Benes, M.; Vojtechova, G.; Drastich, P.; Bures, J.; et al. Accuracy of colon capsule endoscopy for colorectal neoplasia detection in individuals referred for a screening colonoscopy. Gastroenterol. Res. Pract. 2019. [Google Scholar] [CrossRef] [Green Version]
- Spada, C.; Pasha, S.F.; Gross, S.A.; Leighton, J.A.; Schnoll-Sussman, F.; Correale, L.; González Suárez, B.; Costamagna, G.; Hassan, C. Accuracy of first- and second-generation colon capsules in endoscopic detection of colorectal polyps: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1533–1543.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lifshitz, R.; Kimchy, Y.; Gelbard, N.; Leibushor, A.; Golan, O.; Elgali, A.; Hassoon, S.; Kaplan, M.; Smirnov, M.; Shpigelman, B.; et al. An x-ray-based capsule for colorectal cancer screening incorporating single photon counting technology. In Proceedings of the Medical Imaging 2017: Physics of Medical Imaging, Orlando, FL, USA, 11–16 February 2017; p. 1013210. [Google Scholar]
- Dik, V.K.; Gralnek, I.M.; Segol, O.; Suissa, A.; Belderbos, T.D.G.; Moons, L.M.G.; Segev, M.; Domanov, S.; Rex, D.K.; Siersema, P.D. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy—Results of the CLEVER study. Endoscopy 2015, 47, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, D.K.; Repici, A.; Gross, S.A.; Hassan, C.; Ponugoti, P.L.; Garcia, J.R.; Broadley, H.M.; Thygesen, J.C.; Sullivan, A.W.; Tippins, W.W.; et al. High-definition colonoscopy versus Endocuff versus EndoRings versus full-spectrum endoscopy for adenoma detection at colonoscopy: A multicenter randomized trial. Gastrointest. Endosc. 2018, 88, 335–344.e2. [Google Scholar] [CrossRef] [Green Version]
- Floer, M.; Biecker, E.; Fitzlaff, R.; Röming, H.; Ameis, D.; Heinecke, A.; Kunsch, S.; Ellenrieder, V.; Ströbel, P.; Schepke, M.; et al. Higher adenoma detection rates with Endocuff-assisted colonoscopy—A randomized controlled multicenter trial. PLoS ONE 2014, 9, e114267. [Google Scholar] [CrossRef]
- Nutalapati, V.; Kanakadandi, V.; Desai, M.; Olyaee, M.; Rastogi, A. Cap-assisted colonoscopy: A meta-analysis of high-quality randomized controlled trials. Endosc. Int. Open 2018, 6, E1214–E1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieling, T. Cap-assisted endoscopy: Do we have enough evidence? Endosc. Int. Open 2018, 6, E1224–E1226. [Google Scholar] [CrossRef] [Green Version]
- Pohl, H.; Bensen, S.; Toor, A.; Gordon, S.; Levy, L.; Berk, B.; Anderson, P.; Anderson, J.; Rothstein, R.; MacKenzie, T.; et al. Cap-assisted colonoscopy and detection of Adenomatous Polyps (CAP) study: A randomized trial. Endoscopy 2015, 47, 891–897. [Google Scholar] [CrossRef]
- Szold, A.; Bergamaschi, R.; Broeders, I.; Dankelman, J.; Forgione, A.; Langø, T.; Melzer, A.; Mintz, Y.; Morales-Conde, S.; Rhodes, M.; et al. Frontiers of robotic endoscopic capsules: A review. Surg. Endosc. 2015, 29, 253–288. [Google Scholar] [CrossRef]
- Kurniawan, N.; Keuchel, M. Flexible gastro-intestinal endoscopy—Clinical challenges and technical achievements. Comput. Struct. Biotechnol. J. 2017, 15, 168–179. [Google Scholar] [CrossRef]
- Tal, A.O.; Vermehren, J.; Albert, J.G. Colon capsule endoscopy: Current status and future directions. World J. Gastroenterol. 2014, 20, 16596–16602. [Google Scholar] [CrossRef]
- Yeung, C.; Cheung, J.L.; Sreedhar, B. Emerging next-generation robotic colonoscopy systems towards painless colonoscopy. J. Dig. Dis. 2019, 20, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickhoff, A.; Jakobs, R.; Kamal, A.; Mermash, S.; Riemann, J.; Van Dam, J. In vitro evaluation of forces exerted by a new computer-assisted colonoscope (the NeoGuide Endoscopy System). Endoscopy 2006, 38, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, A.; Van Dam, J.; Jakobs, R.; Kudis, V.; Hartmann, D.; Damian, U.; Weickert, U.; Schilling, D.; Riemann, J.F. Computer-assisted colonoscopy (the NeoGuide Endoscopy System): Results of the first human clinical trial (“PACE Study”). Am. J. Gastroenterol. 2007, 102, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.S.; Armijo, P.R.; Krause, C.; Choudhury, S.A.; Oleynikov, D. Review of emerging surgical robotic technology. Surg. Endosc. 2018, 32, 1636–1655. [Google Scholar] [CrossRef] [PubMed]
- Rösch, T.; Adler, A.; Pohl, H.; Wettschureck, E.; Koch, M.; Wiedenmann, B.; Hoepffner, N. A motor-driven single-use colonoscope controlled with a hand-held device: A feasibility study in volunteers. Gastrointest. Endosc. 2008, 67, 1139–1146. [Google Scholar] [CrossRef]
- Li, Z.; Chiu, P.W.Y. Robotic Endoscopy. Visc. Med. 2018, 34, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gluck, N.; Melhem, A.; Halpern, Z.; Mergener, K.; Santo, E. A novel self-propelled disposable colonoscope is effective for colonoscopy in humans (with video). Gastrointest. Endosc. 2016, 83, 998–1004.e1. [Google Scholar] [CrossRef] [Green Version]
- Shike, M.; Fireman, Z.; Eliakim, R.; Segol, O.; Sloyer, A.; Cohen, L.B.; Goldfarb-Albak, S.; Repici, A. Sightline ColonoSight system for a disposable, power-assisted, non-fiber-optic colonoscopy (with video). Gastrointest. Endosc. 2008, 68, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Trecca, A.; Catalano, F.; Bella, A.; Borghini, R. Robotic colonoscopy: Efficacy, tolerability and safety. Preliminary clinical results from a pilot study. Surg Endosc 2020, 34, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Tumino, E.; Passoni, G.R.; Morandi, E.; Capria, A. Functional evaluation of the Endotics System, a new disposable self-propelled robotic colonoscope: In vitro tests and clinical trial. Int. J. Artif. Organs 2009, 32, 517–527. [Google Scholar] [CrossRef]
- Tumino, E.; Sacco, R.; Bertini, M.; Bertoni, M.; Parisi, G.; Capria, A. Endotics system vs colonoscopy for the detection of polyps. World J. Gastroenterol. 2010, 16, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Tumino, E.; Parisi, G.; Bertoni, M.; Bertini, M.; Metrangolo, S.; Ierardi, E.; Cervelli, R.; Bresci, G.; Sacco, R. Use of robotic colonoscopy in patients with previous incomplete colonoscopy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 819–826. [Google Scholar] [PubMed]
- Liao, Z.; Hou, X.; Lin-Hu, E.Q.; Sheng, J.Q.; Ge, Z.Z.; Jiang, B.; Hou, X.H.; Liu, J.Y.; Li, Z.; Huang, Q.Y.; et al. Accuracy of magnetically controlled capsule endoscopy, compared with conventional gastroscopy, in detection of gastric diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 1266–1273.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, F.; Tumino, E.; Passoni, G.R.; Rigante, A.; Barbera, R.; Tauro, A.; Cosentino, P.E. Robotic colonoscopy. In Colonoscopy; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Ciuti, G.; Menciassi, A.; Dario, P. Capsule endoscopy: From current achievements to open challenges. IEEE Rev. Biomed. Eng. 2011, 4, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ciuti, G.; Caliò, R.; Camboni, D.; Neri, L.; Bianchi, F.; Arezzo, A.; Koulaouzidis, A.; Schostek, S.; Stoyanov, D.; Oddo, C.M.; et al. Frontiers of robotic endoscopic capsules: A review. J. Micro Bio Robot. 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lee, D.; Joe, S.; Lee, B.I.; Kim, B. The flexible caterpillar based robotic colonoscope actuated by an external motor through a flexible shaft. J. Mech. Sci. Technol. 2014, 28, 4415–4420. [Google Scholar] [CrossRef]
- Lee, D.; Joe, S.; Choi, J.; Lee, B.I.; Kim, B. An elastic caterpillar-based self-propelled robotic colonoscope with high safety and mobility. Mechatronics 2016, 39, 54–62. [Google Scholar] [CrossRef]
- Lee, D.; Joe, S.; Jung, J.-H.; Kim, J.-U.; Kim, B. A simple and reliable reel mechanism-based robotic colonoscope for high mobility. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2018, 232, 2753–2763. [Google Scholar] [CrossRef]
- Lee, D.; Joe, S.; Kang, H.; An, T.; Kim, B. A reel mechanism-based robotic colonoscope with high safety and maneuverability. Surg. Endosc. 2019, 33, 322–332. [Google Scholar] [CrossRef]
- Sliker, L.J.; Kern, M.D.; Schoen, J.A.; Rentschler, M.E. Surgical evaluation of a novel tethered robotic capsule endoscope using micro-patterned treads. Surg. Endosc. 2012, 26, 2862–2869. [Google Scholar] [CrossRef]
- Kern, M.D.; Ortega Alcaide, J.; Rentschler, M.E. Soft material adhesion characterization for in vivo locomotion of robotic capsule endoscopes: Experimental and modeling results. J. Mech. Behav. Biomed. Mater. 2014, 39, 257–269. [Google Scholar] [CrossRef]
- Formosa, G.A.; Prendergast, J.M.; Edmundowicz, S.A.; Rentschler, M.E. Novel optimization-based design and surgical evaluation of a treaded robotic capsule colonoscope. IEEE Trans. Robot. 2019, 36, 545–552. [Google Scholar] [CrossRef]
- Alcaide, J.O.; Pearson, L.; Rentschler, M.E. Design, modeling and control of a SMA-actuated biomimetic robot with novel functional skin. In Proceedings of the IEEE International Conference on Robotics and Automation, Singapore, 29 May–3 June 2017; pp. 4338–4345. [Google Scholar]
- Wang, K.; Ma, J.; Wang, F.; Wang, Z.; Yan, G.; Zhou, Y. Full-driving soft robotic colonoscope in compliant colon tissue. J. Med. Eng. Technol. 2017, 41, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Bernth, J.E.; Arezzo, A.; Liu, H. A novel robotic meshworm with segment-bending anchoring for colonoscopy. IEEE Robot. Autom. Lett. 2017, 2, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, H.; Welch, C.R.; Pourghodrat, A.; Nelson, C.A.; Oleynikov, D.; Dasgupta, P.; Terry, B.S. Design and preliminary evaluation of a self-steering, pneumatically driven colonoscopy robot. J. Med. Eng. Technol. 2017, 41, 223–236. [Google Scholar] [CrossRef]
- Manfredi, L.; Capoccia, E.; Ciuti, G.; Cuschieri, A. A soft pneumatic inchworm double balloon (SPID) for colonoscopy. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Consis Medical (Beer-Sheva, Israel). Available online: http://consis-medical.com/ (accessed on 26 May 2020).
- Sliker, L.; Ciuti, G.; Rentschler, M.; Menciassi, A. Magnetically driven medical devices: A review. Expert Rev. Med. Devices 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- VECTOR EU Project. Available online: www.vector-project.com (accessed on 26 May 2020).
- Ciuti, G.; Valdastri, P.; Menciassi, A.; Dario, P. Robotic magnetic steering and locomotion of capsule endoscope for diagnostic and surgical endoluminal procedures. Robotica 2010, 28. [Google Scholar] [CrossRef] [Green Version]
- Ciuti, G.; Donlin, R.; Valdastri, P.; Arezzo, A.; Menciassi, A.; Morino, M.; Dario, P. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy 2010, 42. [Google Scholar] [CrossRef] [Green Version]
- Arezzo, A.; Menciassi, A.; Valdastri, P.; Ciuti, G.; Lucarini, G.; Salerno, M.; Di Natali, C.; Verra, M.; Dario, P.; Morino, M. Experimental assessment of a novel robotically-driven endoscopic capsule compared to traditional colonoscopy. Dig. Liver Dis. 2013, 45. [Google Scholar] [CrossRef]
- Valdastri, P.; Ciuti, G.; Verbeni, A.; Menciassi, A.; Dario, P.; Arezzo, A.; Morino, M. Magnetic air capsule robotic system: Proof of concept of a novel approach for painless colonoscopy. Surg. Endosc. Other Interv. Tech. 2012, 26, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Sliker, L.J.; Ciuti, G.; Rentschler, M.E.; Menciassi, A. Frictional resistance model for tissue-capsule endoscope sliding contact in the gastrointestinal tract. Tribol. Int. 2016, 102, 472–484. [Google Scholar] [CrossRef]
- Li, J.; Barjuei, E.S.; Ciuti, G.; Hao, Y.; Zhang, P.; Menciassi, A.; Huang, Q.; Dario, P. Magnetically-driven medical robots: An analytical magnetic model for endoscopic capsules design. J. Magn. Magn. Mater. 2018, 452, 278–287. [Google Scholar] [CrossRef]
- Salerno, M.; Ciuti, G.; Lucarini, G.; Rizzo, R.; Valdastri, P.; Menciassi, A.; Landi, A.; Dario, P. A discrete-time localization method for capsule endoscopy based on on-board magnetic sensing. Meas. Sci. Technol. 2012, 23, 015701. [Google Scholar] [CrossRef]
- Taddese, A.Z.; Slawinski, P.R.; Pirotta, M.; De Momi, E.; Obstein, K.L.; Valdastri, P. Enhanced real-time pose estimation for closed-loop robotic manipulation of magnetically actuated capsule endoscopes. Int. J. Robot. Res. 2018, 37, 890–911. [Google Scholar] [CrossRef]
- Ciuti, G.; Salerno, M.; Lucarini, G.; Valdastri, P.; Arezzo, A.; Menciassi, A.; Morino, M.; Dario, P. A comparative evaluation of control interfaces for a robotic-aided endoscopic capsule platform. IEEE Trans. Robot. 2012, 28. [Google Scholar] [CrossRef] [Green Version]
- Slawinski, P.R.; Simaan, N.; Taddese, A.Z.; Obstein, K.L.; Valdastri, P. Sensitivity ellipsoids for force control of magnetic robots with localization uncertainty. IEEE Trans. Robot. 2019, 35, 1123–1135. [Google Scholar] [CrossRef]
- Barducci, L.; Pittiglio, G.; Norton, J.C.; Obstein, K.L.; Valdastri, P. Adaptive dynamic control for magnetically actuated medical robots. IEEE Robot. Autom. Lett. 2019, 4, 3633–3640. [Google Scholar] [CrossRef] [Green Version]
- Slawinski, P.R.; Taddese, A.Z.; Musto, K.B.; Sarker, S.; Valdastri, P.; Obstein, K.L. Autonomously controlled magnetic flexible endoscope for colon exploration. Gastroenterology 2018, 154, 1577–1579.e1. [Google Scholar] [CrossRef] [Green Version]
- Norton, J.C.; Slawinski, P.R.; Lay, H.S.; Martin, J.W.; Cox, B.F.; Cummins, G.; Desmulliez, M.P.Y.; Clutton, R.E.; Obstein, K.L.; Cochran, S.; et al. Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci. Robot. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Endoo EU Project. Available online: www.endoo-project.eu (accessed on 26 May 2020).
- Bianchi, F.; Ciuti, G.; Koulaouzidis, A.; Arezzo, A.; Stoyanov, D.; Schostek, S.; Oddo, C.M.; Menciassi, A.; Dario, P. An innovative robotic platform for magnetically-driven painless colonoscopy. Ann. Transl. Med. 2017, 5, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandao, P.; Mazomenos, E.; Ciuti, G.; Caliò, R.; Bianchi, F.; Menciassi, A.; Dario, P.; Koulaouzidis, A.; Arezzo, A.; Stoyanov, D. Fully convolutional neural networks for polyp segmentation in colonoscopy. In Proceedings of the SPIE 10134, Medical Imaging 2017: Computer-Aided Diagnosis, Orlando, FL, USA, 11–16 February 2017; p. 101340F. [Google Scholar]
- Brandao, P.; Zisimopoulos, O.; Mazomenos, E.; Ciuti, G.; Bernal, J.; Visentini-Scarzanella, M.; Menciassi, A.; Dario, P.; Koulaouzidis, A.; Arezzo, A.; et al. Towards a computed-aided diagnosis system in colonoscopy: Automatic polyp segmentation using convolution neural networks. J. Med. Robot. Res. 2018, 3, 1840002. [Google Scholar] [CrossRef] [Green Version]
- Iakovidis, D.K.; DImas, G.; Karargyris, A.; Bianchi, F.; Ciuti, G.; Koulaouzidis, A. Deep endoscopic visual measurements. IEEE J. Biomed. Health Inform. 2019, 23, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Visentini-Scarzanella, M.; Kawasaki, H.; Furukawa, R.; Bonino, M.; Arolfo, S.; Lo Secco, G.; Arezzo, A.; Menciassi, A.; Dario, P.; Ciuti, G. A structured light laser probe for gastrointestinal polyp size measurement: A preliminary comparative study. Endosc. Int. Open 2018, 6, E602–E609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarini, G.; Ciuti, G.; Mura, M.; Rizzo, R.; Menciassi, A. A new concept for magnetic capsule colonoscopy based on an electromagnetic system regular paper. Int. J. Adv. Robot. Syst. 2015, 12. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, G.; Mura, M.; Ciuti, G.; Rizzo, R.; Menciassi, A. Electromagnetic control system for capsule navigation: Novel concept for magnetic capsule maneuvering and preliminary study. J. Med. Biol. Eng. 2015, 35. [Google Scholar] [CrossRef] [Green Version]
- SUPCAM EU Project. Available online: www.supcam.eu (accessed on 26 May 2020).
- Nouda, S.; Ota, K.; Higuchi, K. Retrograde colon capsule endoscopy with the self-propelling capsule endoscope: The first human trial (with videos). Dig. Endosc. 2018, 30, 117–118. [Google Scholar] [CrossRef]
- Zarrouk, D.; Mann, M.; Degani, N.; Yehuda, T.; Jarbi, N.; Hess, A. Single actuator wave-like robot (SAW): Design, modeling, and experiments. Bioinspir. Biomim. 2016, 11. [Google Scholar] [CrossRef]
- Hawkes, E.W.; Blumenschein, L.H.; Greer, J.D.; Okamura, A.M. A soft robot that navigates its environment through growth. Sci. Robot. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Slade, P.; Gruebele, A.; Hammond, Z.; Raitor, M.; Okamura, A.M.; Hawkes, E.W. Design of a soft catheter for low-force and constrained surgery. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Vancouver, BC, Canada, 24–28 September 2017; Volume 2017, pp. 174–180. [Google Scholar]
- Chautems, C.; Tonazzini, A.; Boehler, Q.; Jeong, S.H.; Floreano, D.; Nelson, B.J. Magnetic continuum device with variable stiffness for minimally invasive surgery. Adv. Intell. Syst. 2019, 1900086. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Yang, Y.; Wu, Z.; Gao, W. Robotics in the gut. Adv. Ther. 2019, 3, 1900125. [Google Scholar] [CrossRef]
- Son, D.; Dogan, M.D.; Sitti, M. Magnetically actuated soft capsule endoscope for fine-needle aspiration biopsy. In Proceedings of the IEEE International Conference on Robotics and Automation, Singapore, 29 May–3 June 2017; pp. 1132–1139. [Google Scholar]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubálek, F.; Water, J.J.; et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, S.-E.; Misawa, M.; Mori, Y.; Hotta, K.; Ohtsuka, K.; Ikematsu, H.; Saito, Y.; Takeda, K.; Nakamura, H.; Ichimasa, K.; et al. Artificial intelligence-assisted system improves endoscopic identification of colorectal neoplasms. Clin. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S.; Misawa, M.; Takeda, K.; Kudo, T.; Itoh, H.; Oda, M.; Mori, K. Artificial intelligence for colorectal polyp detection and characterization. Curr. Treat. Options Gastroenterol. 2020. [Google Scholar] [CrossRef]
- De Groof, A.J.; Struyvenberg, M.R.; Van der Putten, J.; Van der Sommen, F.; Fockens, K.N.; Curvers, W.L.; Zinger, S.; Pouw, R.E.; Coron, E.; Baldaque-Silva, F.; et al. Deep-learning system detects neoplasia in patients with barrett’s esophagus with higher accuracy than endoscopists in a multistep training and validation study with benchmarking. Gastroenterology 2020, 158, 915–929.e4. [Google Scholar] [CrossRef]
- García-Peraza-Herrera, L.C.; Everson, M.; Lovat, L.; Wang, H.-P.; Wang, W.L.; Haidry, R.; Stoyanov, D.; Ourselin, S.; Vercauteren, T. Intrapapillary capillary loop classification in magnification endoscopy: Open dataset and baseline methodology. Int. J. Cars 2020, 15, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J.; Tajkbaksh, N.; Sanchez, F.J.; Matuszewski, B.J.; Chen, H.; Yu, L.; Angermann, Q.; Romain, O.; Rustad, B.; Balasingham, I.; et al. Comparative validation of polyp detection methods in video colonoscopy: Results from the MICCAI 2015 endoscopic vision challenge. IEEE Trans. Med. Imaging 2017, 36, 1231–1249. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.; Berzin, T.; Misawa, M.; Takeda, K. Computer-aided diagnosis for colonoscopy. Endoscopy 2017, 49, 813–819. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, O.F.; Soares, A.S.; Mazomenos, E.; Brandao, P.; Vega, R.; Seward, E.; Stoyanov, D.; Chand, M.; Lovat, L.B. Artificial intelligence and computer-aided diagnosis in colonoscopy: Current evidence and future directions. Lancet Gastroenterol. Hepatol. 2019, 4, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, O.F.; Stoyanov, D.; Lovat, L.B. Human-machine collaboration: Bringing artificial intelligence into colonoscopy. Frontline Gastroenterol. 2019, 10, 198–199. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy. Ann. Intern. Med. 2018, 169, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S. Detecting colorectal polyps via machine learning. Nat. Biomed. Eng. 2018, 2, 713–714. [Google Scholar] [CrossRef] [PubMed]
- ImageNet. Available online: http://www.image-net.org/ (accessed on 8 April 2020).
- Gastrointestinal Image ANAlysis challenge—Grand Challenge. Available online: https://giana.grand-challenge.org/ (accessed on 24 February 2020).
- EAD2019—Grand Challenge. Available online: https://ead2019.grand-challenge.org/ (accessed on 24 February 2020).
- Metz, C. AI is learning from humans. Many humans. The New York Times, 16 August 2019. [Google Scholar]
- Van der Sommen, F. Gastroenterology needs its own ImageNet. J. Med. Artif. Intell. 2019, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Robu, M.R.; Koo, B.; Schneider, C.; Van Laarhoven, S.; Stoyanov, D.; Davidson, B.; Clarkson, M.J.; Hu, Y. More unlabelled data or label more data? A study on semi-supervised laparoscopic image segmentation. In Domain Adaptation and Representation Transfer and Medical Image Learning with Less Labels and Imperfect Data; Wang, Q., Milletari, F., Nguyen, H.V., Albarqouni, S., Cardoso, M.J., Rieke, N., Xu, Z., Kamnitsas, K., Patel, V., Roysam, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 173–180. [Google Scholar]
- ai4gi|AI Solutions for Colon Polyp Detection and Differentiation. Available online: https://ai4gi.com/ (accessed on 24 February 2020).
- Odin Vision|Artificial Intelligence Enhanced Colonoscopy Procedures. Available online: https://odin-vision.com/ (accessed on 24 February 2020).
- Wision A.I. Available online: https://www.wision.com/ (accessed on 24 February 2020).
- GI Genius™ Intelligent Endoscopy Module|Medtronic (UK). Available online: https://www.medtronic.com/covidien/en-gb/products/gastrointestinal-artificial-intelligence/gi-genius-intelligent-endoscopy.html# (accessed on 24 February 2020).
- CADEYE. Available online: https://www.fujifilm.eu/eu/cadeye (accessed on 8 April 2020).
- Press Releases|PENTAX Medical (EMEA). Available online: https://www.pentaxmedical.com/pentax/en/95/1/HOYA-Group-PENTAX-Medical-Cleared-CE-Mark-for-DISCOVERYTM-an-AI-Assisted-Polyp-Detector- (accessed on 8 April 2020).
- Yamada, M.; Saito, Y.; Imaoka, H.; Saiko, M.; Yamada, S.; Kondo, H.; Takamaru, H.; Sakamoto, T.; Sese, J.; Kuchiba, A.; et al. Development of a real-time endoscopic image diagnosis support system using deep learning technology in colonoscopy. Sci. Rep. 2019, 9, 14465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybernet Systems Co., Ltd. EndoBRAIN®—Artificial Intelligence System that Supports Optical Diagnosis of Colorectal Polyps—Was Improved by PMSA (Pharmaceuticals and Medical Devices Agency), a Regulatory Body in Japan; Cybernet Systems Co., Ltd.: Tokyo, Japan, 2018. [Google Scholar]
- Vinsard, D.G.; Mori, Y.; Misawa, M.; Kudo, S.E.; Rastogi, A.; Bagci, U.; Rex, D.K.; Wallace, M.B. Quality assurance of computer-aided detection and diagnosis in colonoscopy. Gastrointest. Endosc. 2019, 90, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.F.; Chapados, N.; Soudan, F.; Oertel, C.; Pérez, M.L.; Kelly, R.; Iqbal, N.; Chandelier, F.; Rex, D.K. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 2019, 68, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Kudo, S.; East, J.E.; Rastogi, A.; Bretthauer, M.; Misawa, M.; Sekiguchi, M.; Matsuda, T.; Saito, Y.; Ikematsu, H.; et al. Cost savings in colonoscopy with artificial intelligence–aided polyp diagnosis: An add-on analysis of a clinical trial (with video). Gastrointest. Endosc. 2020. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.-E.; Misawa, M. Can artificial intelligence standardise colonoscopy quality? Lancet Gastroenterol. Hepatol. 2020, 5, 331–332. [Google Scholar] [CrossRef]
- Stoyanov, D. Surgical vision. Ann. Biomed. Eng. 2012, 40, 332–345. [Google Scholar] [CrossRef]
- Mountney, P.; Stoyanov, D.; Yang, G.-Z. Three-dimensional tissue deformation recovery and tracking. IEEE Signal Process. Mag. 2010, 27, 14–24. [Google Scholar] [CrossRef]
- Min, J.K.; Kwak, M.S.; Cha, J.M. Overview of deep learning in gastrointestinal endoscopy. Gut Liver 2019, 13, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visentini-Scarzanella, M.; Sugiura, T.; Kaneko, T.; Koto, S. Deep monocular 3D reconstruction for assisted navigation in bronchoscopy. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Durr, N.J. Deep learning and conditional random fields-based depth estimation and topographical reconstruction from conventional endoscopy. Med. Image Anal. 2018, 48, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Clancy, N.T.; Stoyanov, D.; Elson, D.S. Tissue surface reconstruction aided by local normal information using a self-calibrated endoscopic structured light system. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Cham, Switzerland, 2015; Volume 9349, pp. 405–412. [Google Scholar]
- Münzer, B.; Schoeffmann, K.; Böszörmenyi, L. Content-based processing and analysis of endoscopic images and videos: A survey. Multimed. Tools Appl. 2018, 77, 1323–1362. [Google Scholar] [CrossRef] [Green Version]
- Rau, A.; Edwards, P.J.E.; Ahmad, O.F.; Riordan, P.; Janatka, M.; Lovat, L.B.; Stoyanov, D. Implicit domain adaptation with conditional generative adversarial networks for depth prediction in endoscopy. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Roth, H.R.; Lu, L.; Oda, M.; Misawa, M.; Mori, Y.; Kudo, S.; Mori, K. Towards automated colonoscopy diagnosis: Binary polyp size estimation via unsupervised depth learning. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Granada, Spain, 16–20 September 2018; Springer: Cham, Switzerland, 2018; pp. 611–619. [Google Scholar]
| Device | Distinctive Features | Advantages | Limitations | Ref.s |
|---|---|---|---|---|
| Standard Colonoscopy (SC) | Long semirigid instrument (~13 mm in diameter and ~1400 mm in length) with a 2-DoFs cable-driven steerable tip, manually introduced through the anus and pushed forward and backward to inspect the colonic wall (+ 1-DoF axial-roll). | Current reference standard for diagnosis and treatment; diagnosis and treatment in the same session; manual fine control of the endoscope tip. | Requires unpleasant laxative preparation, sedatives, and analgesia; uncomfortable procedure due to insufflation and tissue-colonoscope interaction; highly dependent of endoscopist training and ability; looping and potential risk of perforation (0.1–0.3% for diagnostic colonoscopies). | [31,32,33,34,35,36] |
| Virtual Colonoscopy Computed Tomography (CCT) | Medical imaging diagnostic procedure using x-rays to compute 3D reconstructed endoluminal views of the colon. | Alternative to conventional colonoscopy to diagnose disease, e.g., polyps and diverticulosis, without discomfort generally caused by colonoscope-lumen interaction. | Requires unpleasant laxative preparation; only CT-based morphological tissue analysis; uncomfortable procedure due to insufflation; sedatives, and analgesia often required; no tissue treatment or surgery; PDR limited (30% of the polyps are flat and obscured). | [44,45] |
| Double-Balloon Colonoscopy (DBC) | About 2 m long system including a high-resolution endoscope and two latex balloons filled with air by using pressure pumps for easing navigation. | Relatively shorter time of colon examination, and reduced conscious sedation if compared to SC; used in the cases of technical difficulties, e.g., loop formation, long colonic segments, or suspected adhesions, resulting in the discovery of advanced neoplasia, colon polyps, stenosis and Crohn’s disease, that were not identified with SC. | Same of SC (often, with reduced discomfort, looping and risk of tissue damage); lack of fluoroscopic evaluation. | [46,47,48,49,50,51,52] |
| Full Spectrum Endoscopy (FUSE) (EndoChoice Inc., Alpharetta, GA, USA) | Flexible colonoscope with extra optics (i.e., three 4K Ultra HD cameras), allowing to view the gut with a panoramic 330° FoV (behind and into folds). | Maintaining standard features and functions of SC (e.g., 3.8mm working channel), FUSE demonstrated a higher lesions detection rate (mainly in the right and middle parts of the colon), compared with SC (missing rate 7% vs. 41%). | Equivalent to SC; trials failed to replicate higher performances if compared to forward-viewing approach colonoscopy in ascending colon; lower APC than SC (1.30 ± 1.96 vs. 1.53 ± 2.33). | [53,54,55,56,67] |
| G-Eye Endoscope (NaviAid G-EYE, SMART Medical Systems Ltd., Ra’anana, Israel) | Flexible colonoscope with an integrated inflatable balloon at its distal portion. | Balloon inflation allows, during withdrawal: (1) straightening and flattening of haustral folds, (2) inhibiting slippage of the bowel, and (3) instrument stabilization and centralized optics; higher ADR and PDR, including well-formed, flat, and sessile serrated ones, if compared to SC. | Equivalent to SC due to the same forward procedure. | [57,58,59] |
| Wireless Capsule Endoscopes (PillCamTM Colon 2) (Medtronic Inc., Minneapolis, Minnesota, USA) | Pill-size wireless screening tools (~11 mm in diameter and ~32 mm in length) with a sub-VGA, adaptive 4 to 35 fps, 172° FoV, and ~0–30mm DoF frontal/rear CMOS double cameras with synchronized activated LEDs. | Minimally-invasive and painless; high-patient tolerability; negligible risk of perforation. | Requires unpleasant and aggressive laxatives preparation; low-accuracy and reliability for diagnosis; inability to control the capsule; inability to perform therapy and treatment. | [60,61,62,63,64,65] |
| EndoRingsTM (adjunct) (EndoAid Ltd., Caesarea, Israel) | Two layers of flexible, soft circular rings - gently flattening folds during withdrawal for a clear view. | Improved visibility, scope centring and control of the endoscope during withdrawal and tissue resection; elevate the ADR in comparison with the FUSE. | Not recommended in cases of acute, severe colitis or of known colonic strictures; performance subject to training and experience. | [66,67] |
| Endocuff VISIONTM (adjunct) (Olympus Corp., Tokyo, Japan) | Disposable add-on, using arms instead of flaps, to straighten out the mucosa. | Increased ADR if compared to SC, i.e., 35.4% vs. 20.7%, with comparable overall procedure time and without major adverse events. Higher APC than EndoRingsTM (1.82 ± 2.58 vs. 1.55 ± 2.42). | Not recommended in cases of acute, severe colitis or of known colonic strictures; performance subject to training and experience. | [67,68] |
| Transparent Cap (adjunct) (Reveal® Distal Attachment Cap, Steris Corp., Mentor, Ohio, USA) | Transparent distal attachment, connected to the colonoscope’s tip and designed to elevate the ADR via mucosal folds flattening and minimizing a red-out, while preventing the mucosa to adhere to the lens. | Doubtful improvement of ADR, CIR and CIT; a study reports a higher ADR of almost 20% and improved CIR and CIT. | Performance subject to training and experience. | [69,70,71] |
| Device | Actuation Principle | Technical Distinctive Features | Clinical-Oriented Features, Studies, and Clinical Outcomes | Ref.s |
|---|---|---|---|---|
| NeoGuide Endoscopy System (NeoGuide Endoscopy System Inc., Los Gatos, CA USA) | Electro-mechanical actuation with a “follow-the-leader” mechanism. | 16-segment insertion tube that controls the snake-like movement of the endoscope; each independent and electromechanically-controlled segment has 2-DoFs; position sensors at the distal tip of the endoscope and at the external base of the device to obtain live view of the position of the scope’s tip, insertion depth and computed real-time 3D mapping of the colon. | Computerized mapping enables the insertion tube to change the segments shape at different insertion depths to reduce looping and unintentional lateral forces and, consequently, patient discomfort; successful and safe (reduction in the looping rate) cecal intubation in 10 patients, with a CIT (with therapeutic invention) of 34 min (range: 24–60 min); FDA obtained in 2006, and acquisition by Intuitive Surgical Inc. in 2009; no longer available on the market and technology translated to Ion, a robotic-assisted endoluminal platform for minimally invasive peripheral lung biopsy. | [76,77,78] |
| Invendoscope™ SC40 (Invendo Medical GmbH, Weinheim, Germany), then (AMBU A/S, Copenhagen, Denmark) | Electro-mechanical actuation with an inverted sleeve mechanism. | Computer-assisted single-use colonoscope propelled, forward or backward, by an inverted-sleeve mechanism composed of eight drive wheels; robotically-driven tip with LEDs and a CMOS 114° camera, electro-hydraulically flexed through a hand-held control unit to 180° in any direction with full retroflection; diameter of 18 mm and working length of 2000 mm with standard functions including: (1) suction, (2) irrigation, and (3) insufflation with a 3.2 mm working channel, also used for conventional therapeutic procedures. | CIR of 98.4% (median time: 15 min), without any pain, in 92% of patients. 27 polypectomies successfully performed in 23 patients; Invendoscope™ SC40 replaced by a manually inserted single use device with standard flexibility and a hand-held electrical control interface, namely the Invendoscope™ SC200 (as part of the InvendoscopyTM E200 system); latter, obtained the CE mark in 2016 and in January 2018 the FDA clearance for the InvendoscopyTM system E210 and for the InvendoscopeTM SC210; no longer available on the market, acquisition by Ambu A/S in 2017. | [76,80] |
| Aer-O-Scope System (GI View Ltd., Ramat Gan, Israel) | Electro-pneumatic actuation. | Self-propelling, self-steering and disposable robotic colonoscope with navigation obtained through two sealed inflatable balloons and internal pneumatic pressure (inflation of CO2) for pushing the frontal mobile balloon forward and backward; hand-held control unit to teleoperate the colonoscope’s tip with: (1) a 360° omni-directional HD vision system with a 57° FoV camera, (2) LEDs, and (3) two working channels for conventional therapeutic procedures in the latest version; monitored pressure, through electronic sensors, ≤60 mbar. | In-vivo study with 58 patients proved a CIR of 98.2% and a PDR (including all polyps larger than 5 mm) of 87.5% compared with SC, and no mucosal damage or adverse events were reported; FDA mark obtained in 2014 (and CE mark in Europe); currently available on the market. | [81] |
| ColonoSight (Stryker GI Ltd., Haifa, Israel) | Electro-pneumatic actuation. | Self-advancing system composed of: (1) a reusable colonoscope (EndoSight), with LEDs and a camera, covered by (2) a wrapped disposable multi-lumen sheath with working channel (ColonoSleeve), to prevent infection and eliminate the need for disinfection; powered by an electro-pneumatic unit that insufflates the outer sheath to generate, by progressively unfolding it, a forward force at the distal tip enabling to pull the colonoscope. | Electro-pneumatic mechanism helps reducing the overall “pushing” force; multicentre trial with 178 participants showed a 90% CIR in a mean time of 11.2 ± 6.5 min; biopsies taken in some of the procedures and no complications, e.g., bleeding or perforation, noted, thus showing promising potential over SC; FDA achieved in 2008, no longer available on the market. | [82] |
| Endotics System (ERA Endoscopy Srl, Pisa, Italy) | Electro-pneumatic actuation. | Remotely-controlled (by a hand-held control unit) disposable colonoscope able to semi-autonomously crawl the colon by using two mucosal clamping modules, located at the proximal and distal ends of the probe, and a soft extension/retraction central mechanism, mimicking an inchworm-like locomotion; steerable head, able of a 180° bending angle and, containing: (1) LEDs, (2) a CMOS camera with a 140° FoV, (3) a water and air channel for cleaning/drying the lens and for insufflation, and (4) a 3 mm working channel for conventional therapeutic procedures. | A single-centre prospective pilot study was recently performed with 56 consecutive outpatients (two consecutive blocks of 27—group A—and 28—group B—procedures); CIR was 92.7%, reaching 100% in group B; comparing the two groups, CIT significantly decreased from 55 to 22 min, whereas procedures with CIT < 20 min increased; PDR was 40% (males 62.5%, females 14.3%) and ADR was 26.7% (males 27.5%, females 14.3%); most of patients judged it as mild or no distress, with high willingness to repeat the robotic procedure (92.7%); system available on the market with CE mark obtained in 2011. | [86] |
| Device | Actuation Principle | Technical Distinctive Features | Clinical-Oriented Features, Studies, and/or Preclinical Outcomes | Ref.s |
|---|---|---|---|---|
| Kim et al. 2014, Lee et al. 2016 Flexible caterpillar-based robotic colonoscope | Electric actuation. | Flexible caterpillar-based robotic colonoscope, actuated by an external electric motor through a flexible shaft, embedding a steering module (max. bending angle of 178° and min. curvature of the radius of 20 mm). | Reliable locomotion in ex-vivo straight excised porcine colon with forward and backward velocities of 5.0 ± 0.4 mm/s and 9.5 ± 0.9 mm/s, respectively (forward velocities of 6.1 ± 1.1 mm/s and 4.7 ± 0.7 mm/s in case of 30° and 60° inclination angles, respectively); ex-vivo tests, performed in a 1 m long excised porcine colon, arranged to mimic human anatomy, revealed a velocity of 3.0 ± 0.2 mm/s with a CIR of 50% and a CIT of 8.55 min, in case of a novice operator (#8 experiments performed); in-vivo tests, performed in a live mini pig, demonstrated the capability to reach the distal transverse colon, 600 mm from the anus, but in-vivo cecal intubation failed due to the mucosa structure and faecal materials. | [91,92] |
| Lee et al. 2018 and 2019 Reel mechanism-based tethered colonoscope | Electric actuation. | Legged robotic colonoscope based on simple and reliable reel-based mechanism, actuated by an external electric motor. | High manoeuvrability of the colonoscopic device improved, in terms of safety, by harnessing a soft material for the six legs; ex-vivo tests in excised porcine colon demonstrated a 9.552 ± 1.940 mm/s velocity on a flat path, without any scratches or perforations in the porcine tissue. | [93,94] |
| Formosa et al. 2019 Multi-DOFs sensor-enabled treaded robotic colonoscope | Electric actuation. | Two independently-controlled motors drive micro-pillared treads, above and below the device, for 2-DoFs skid-steering, even in a collapsed lumen; all the functionalities of a SC, i.e., (1) camera, (2) adjustable LEDs, (3) channels for insufflation and irrigation and (4) a tool port for conventional therapeutic procedures; in addition, it embeds (5) an inertial measurement unit, magnetometer, motor encoders, and motor current sensors for potential autonomous navigation. | In-vivo preliminary test in a live pig showed endoscopic functionalities and promising results in terms of locomotion (even if it was not able to gain consistent traction in the sigmoid area, seemingly due to excessive constriction upon the non-treaded sides of the devices); ex-vivo tests demonstrated forward/reverse locomotion up to 40 mm/s on the colon mucosa (both not insufflated and distended), 2-DoFs steering, and the ability to traverse haustral folds, and functionality of endoscopic tools. | [95,96,97] |
| Ortega et al. 2017 SMA-based three modular section soft robotic colonoscope | Electric actuation. | Each module is featured by 3-DoFs (one translation, using a peristaltic motion to translate, and two rotations); nine independently controlled SMA springs as actuators and a silicone rubber skin to passively recover force to expand the springs to the original state; three air tubes, one for each section, to provide forced convection for cooling SMA springs; orientation between −90° and +90° in both pitch and roll in less than 4 s with near zero steady state error. | In-vitro tests (rigid tube and open environment) demonstrated a peristaltic motion with a maximum and average speed of 4 mm/s and 0.36 mm/s, respectively. | [98] |
| Wang et al. 2017 Worm-like lightweight robotic colonoscope | Electric actuation. | Lightweight robot (13 mm diameter, 105 mm in length and 22.3 g in weight) with three independent segments, each one composed of a linear locomotor with micromotor, turbine-worm and wire wrapping-sliding mechanism; covered by an external soft bellow with excellent compatibility, designed to increase the static friction and decrease the kinetic friction in the contact state. | In-vivo tests in a porcine model, demonstrating an excellent locomotion capability and safety in soft tissues, with a speed ranging between 1.62 and 2.20 mm/s and passing the entire colon with a CIT of 119s. | [99] |
| Bernth et al. 2017 Cable-driven actuated worm-like robotic colonoscope | Electric actuation. | Worm-like endoscopic robot, based on an embedded electrical cable-driven actuation system; composed of three segments: the two distal segments bend, allowing steering, while the middle segment extends and contracts along the axial direction for forward and backward locomotion. | Efficient navigation through sharp bending radius curves and proper anchoring in complicated 3D and narrow colonic deformable environments; locomotion strategy avoids high pushing forces associated with SC; fabricated with soft material thus, compliant and flexible for gently passing through irregular and curved sections (potential reduced pain for patients). | [100] |
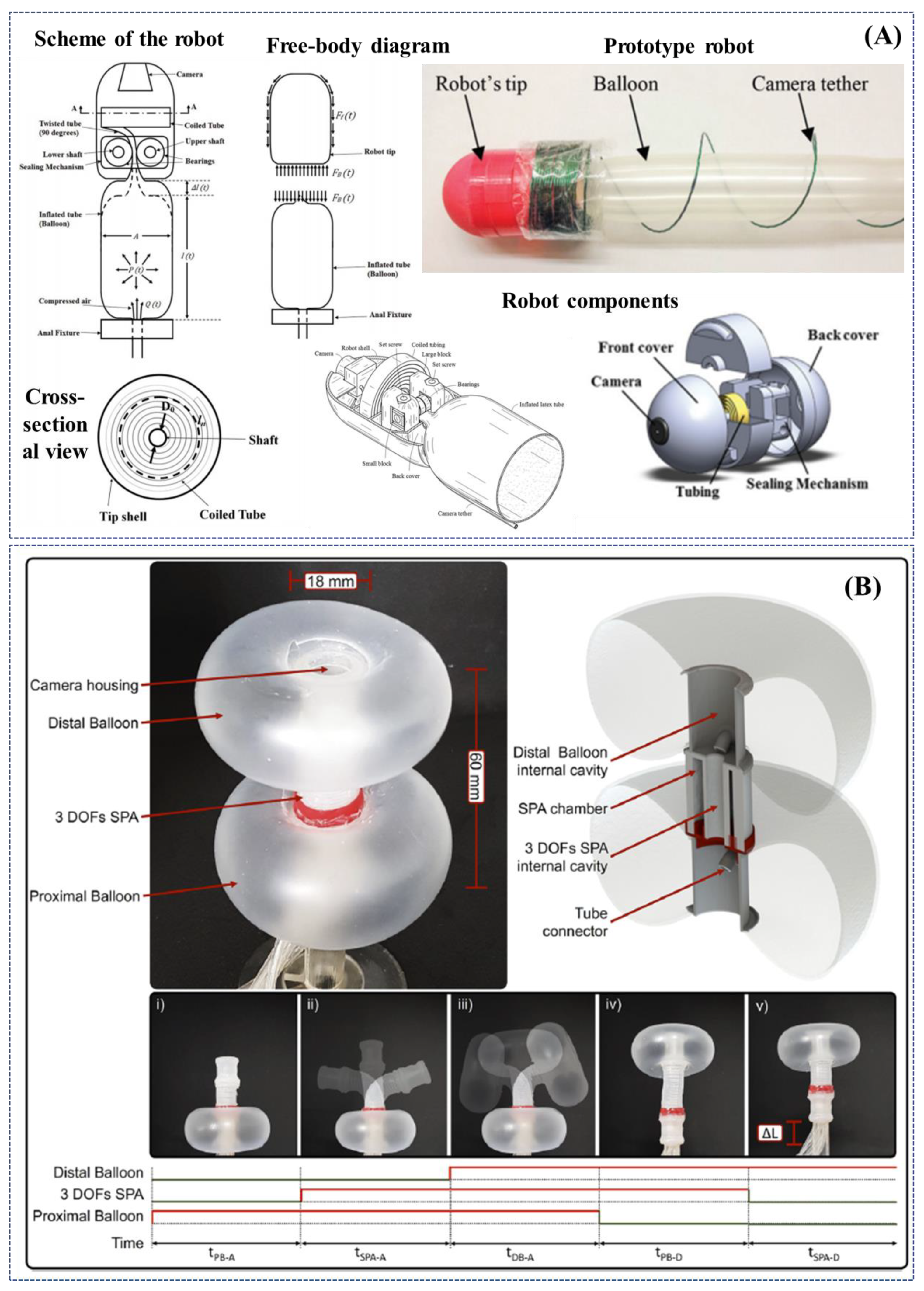
| Dehghani et al. 2017 Semiautonomous pneumatically-driven robotic colonoscope | Pneumatic actuation. | Propulsion taking advantage of a longitudinal expansion of an internal latex tube; lightweight and low inertia colonoscopic robot. | Preliminary ex-vivo tests, in excised porcine colon, demonstrated inherently prevention of loop formation (i.e., general cause of pain); successful advancement of 1500 mm, average speed of 28 mm/s and capability of traversing bends up to 150 degrees; if pressurized with 90kPa, it exerted less than 6N of normal force at the tip; a maximum force generates pressure of 44.17 mmHg at the tip (significantly lower than safe intraluminal human colonic pressure, i.e., 80 mmHg). | [101] |
| Manfredi et al. 2019 Soft pneumatic inchworm-like double balloon colonoscope (SPID) | Pneumatic actuation. | Two inflatable distal balloons for anchorage into the colonic wall, connected by a 3-DoFs central pneumatic actuator for a bio-inspired inchworm-like locomotion and bidirectional bending; external diameter of 18 mm, total length of 60 mm and weight of 10 g. | Soft and deformable structure aimed at reducing the pressure applied to the colonic wall and consequently pain and discomfort during the procedure; tested in a deformable in-vitro synthetic colonic phantom, mimicking shape and dimensions of the human anatomy; efficient navigation with an average forward speed of 2.8 mm/s (a total length of 1.4 m was covered in less than 9 min); manual withdrawal, pulling the tether with an average speed of 25 mm/s, in about 1 min. | [102] |
| Consis Medical Ltd. Semi-disposable and self-propelling robotic colonoscopes | Hydraulic actuation. | Semi-disposable and self-propelling robotic colonoscopes using hydraulic-aiding internal propulsion; composed of: (1) an inverted single-use inflatable sleeve, (2) a multiple-use electronic head, embedding a working channel, a camera, light source and air and water nozzle, and (3) an external control unit; once the electronic head is mounted and inserted into the anus, first the colon is inflated and then the device is deployed, aiding its navigation with an internal water-based hydraulic propulsion. | Hydraulic-aiding internal propulsion allows to gently approach colonic curves with a potentially-lower stress, and thus pain; examination performed withdrawing the device manually, pulling the tether and bending the camera with 2-DoFs. | [103] |
| Ciuti et al. 2010, Valdastri et al. 2012 VECTOR European project | Magnetic actuation (permanent magnets). | Magnetic-based accurate locomotion of wireless and soft-tethered capsules; use of permanent magnets embedded into the capsule and as the external source controlled by a robotic arm; continuous upgrade of the soft-tethered system in terms of modelling, localization and control towards autonomous locomotion. | Wired solution represents a trade-off between capsule and SC combining the benefits of low-invasive navigation (through “front-wheel” locomotion) with the multifunctional tether for conventional treatment; ex-vivo tests in explanted porcine colon (length of 850 mm) performed by 12 users with six to eight coloured beads, measuring 5 mm in diameter, randomly installed (number and position) along the internal surface of the colon; mean percentage of identified beads of 85 ± 11% (range 64–96%) and identified beads successfully removed; mean completion time, i.e., inspection and bead removal, of 678 ± 179 s (range 384–1082 s); preliminary in-vivo tests in pigs demonstrated an average distance travelled of 800 ± 40 mm in an average time of 900 ± 195 s, including the time devoted to inserting the tool into the dedicated channel and operating the instrument. | [105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
| ENDOO European project Soft-tethered stereoscopic robotic capsule colonoscope | Magnetic actuation (permanent magnets). | Soft-tethered magnetically-driven colonoscope with a Full-HD 170° FoV and 3–100mm DoF stereo-camera with a custom-made optics navigated by an external permanent magnet through a collaborative industrial anthropomorphic robot; advanced AI-based tools for augmented diagnosis. | Extensive experimental sessions in ex-vivo (preclinical outcomes under publication), and tests in human cadavers. | [119,120,121,122,123,124] |
| SUPCAM European project Spherical-shape magnetic capsule for colonoscopy | Magnetic actuation (hybrid). | Spherical colonoscopic capsule embedding a permanent magnet and guided by an external gravity-compensated hand-guided electromagnetic system (static electromagnetic field); omni-directional view, by a single embedded camera, through 360° rotation of an internal magnetic frame into a transparent spherical shell. | Reliable navigation in ex-vivo (explanted porcine colon) and in-vitro (synthetic plastic phantom) conditions; in-vitro tests performed by five novice users, completing the task (i.e., locomotion in a ~900 mm long simple and rigid tube with curves) with a time of 44 ± 8 s (range 26–67 s). | [125,126,127] |
| Nouda et al. 2018 PillCamTM SB2 capsule with an attached silicone magnetic fin | Magnetic actuation (hybrid). | Electromagnetic locomotion (alternating electromagnetic field through an external platform) of a 3D self-propelling capsule endoscope composed by a PillCamTM SB2 with an attached silicone fin, embedding a permanent magnet; modified capsule, 45mm in length and 11mm in diameter. | In-vivo human healthy volunteer test; the capsule, inserted in the anus and transported with endoscopic forceps in the descending colon, was able to swim in the lumen in antegrade and retrograde directions without any damage to the mucosa. | [128] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuti, G.; Skonieczna-Żydecka, K.; Marlicz, W.; Iacovacci, V.; Liu, H.; Stoyanov, D.; Arezzo, A.; Chiurazzi, M.; Toth, E.; Thorlacius, H.; et al. Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. J. Clin. Med. 2020, 9, 1648. https://doi.org/10.3390/jcm9061648
Ciuti G, Skonieczna-Żydecka K, Marlicz W, Iacovacci V, Liu H, Stoyanov D, Arezzo A, Chiurazzi M, Toth E, Thorlacius H, et al. Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. Journal of Clinical Medicine. 2020; 9(6):1648. https://doi.org/10.3390/jcm9061648
Chicago/Turabian StyleCiuti, Gastone, Karolina Skonieczna-Żydecka, Wojciech Marlicz, Veronica Iacovacci, Hongbin Liu, Danail Stoyanov, Alberto Arezzo, Marcello Chiurazzi, Ervin Toth, Henrik Thorlacius, and et al. 2020. "Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies" Journal of Clinical Medicine 9, no. 6: 1648. https://doi.org/10.3390/jcm9061648
APA StyleCiuti, G., Skonieczna-Żydecka, K., Marlicz, W., Iacovacci, V., Liu, H., Stoyanov, D., Arezzo, A., Chiurazzi, M., Toth, E., Thorlacius, H., Dario, P., & Koulaouzidis, A. (2020). Frontiers of Robotic Colonoscopy: A Comprehensive Review of Robotic Colonoscopes and Technologies. Journal of Clinical Medicine, 9(6), 1648. https://doi.org/10.3390/jcm9061648











