Severe Traumatic Brain Injury (TBI) Modulates the Kinetic Profile of the Inflammatory Response of Markers for Neuronal Damage
Abstract
1. Introduction
2. Experimental Section
3. Results
3.1. Demographics and Clinical Injury Characteristics
3.2. Systemic Profiles of Neuro Markers in Relation to Injury Pattern
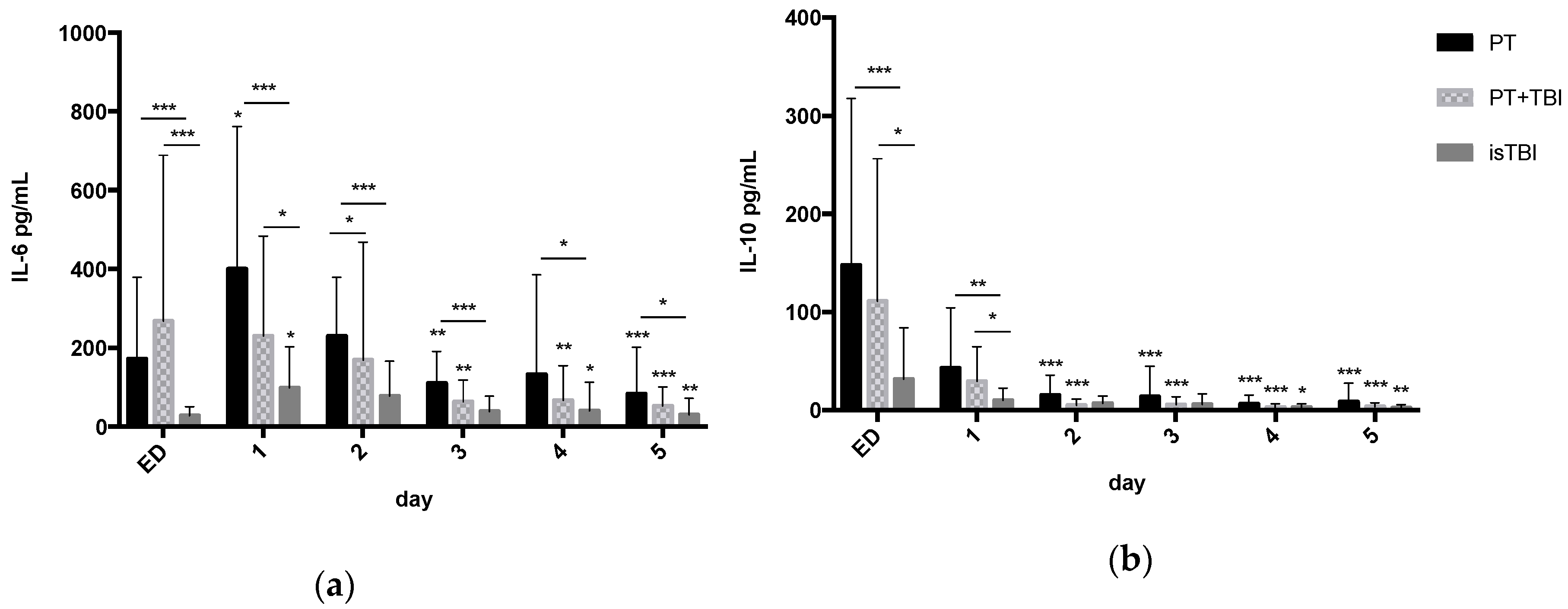
3.3. Systemic Profiles of Inflammatory Markers in Relation to Injury Pattern
3.4. Relation between Marker Expression and Injury Severity
3.5. Inflammatory Status (IL-6/IL-10 Ratio) and Kinetic Profile of Neuro Markers
3.6. Relation between Inflammatory Response and Neuronal Markers
4. Discussion
4.1. S100b Missing Diagnostic Value after Severe Trauma
4.2. Non-Specific Increase of NSE Expression by Cofounding Effect after Severe Trauma
4.3. GFAP Expression Is Induced by Multiple Injuries
4.4. Severe Traumatic Brain Injury (TBI) Modulates Kinetic Profile of Inflammatory Response
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bäckström, D.; Larsen, R.; Steinvall, I.; Fredrikson, M.; Gedeborg, R.; Sjöberg, F. Deaths caused by injury among people of working age (18–64) are decreasing, while those among older people (64+) are increasing. Eur. J. Trauma Emerg. Surg. 2018, 44, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.; Verboket, C.; Schöffski, O.; Tlatlik, J.; Marzi, I.; Nau, C. Costs and proceeds from patients admitted via the emergency room with mild craniocerebral trauma. Unfallchirurg 2018. [Google Scholar] [CrossRef]
- Störmann, P.; Wagner, N.; Köhler, K.; Auner, B.; Simon, T.-P.; Pfeifer, R.; Horst, K.; Pape, H.-C.; Hildebrand, F.; Wutzler, S.; et al. Monotrauma is associated with enhanced remote inflammatory response and organ damage, while polytrauma intensifies both in porcine trauma model. Eur. J. Trauma Emerg. Surg. 2020, 46, 31–42. [Google Scholar] [CrossRef]
- Van Wessem, K.J.P.; Leenen, L.P.H. Reduction in Mortality Rates of Postinjury Multiple Organ Dysfunction Syndrome: A Shifting Paradigm? A Prospective Population-Based Cohort Study. Shock 2018, 49, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.J.; Marzi, I. Novel concepts related to inflammatory complications in polytrauma. Eur. J. Trauma Emerg. Surg. 2018, 44, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Lenzlinger, P.M.; Morganti-Kossmann, M.C.; Laurer, H.L.; McIntosh, T.K. The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 2001, 24, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Makinde, H.M.; Just, T.B.; Cuda, C.M.; Perlman, H.; Schwulst, S.J. The Role of Microglia in the Etiology and Evolution of Chronic Traumatic Encephalopathy. Shock 2017, 48, 276–283. [Google Scholar] [CrossRef]
- Lustenberger, T.; Kern, M.; Relja, B.; Wutzler, S.; Störmann, P.; Marzi, I. The effect of brain injury on the inflammatory response following severe trauma. Immunobiology 2016, 221, 427–431. [Google Scholar] [CrossRef]
- Vos, P.E.; Lamers, K.J.B.; Hendriks, J.C.M.; Van Haaren, M.; Beems, T.; Zimmerman, C.; Van Geel, W.; De Reus, H.; Biert, J.; Verbeek, M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 2004, 62, 1303–1310. [Google Scholar] [CrossRef]
- Thelin, E.P.; Nelson, D.W.; Bellander, B.M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2017, 159, 209–225. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Yada, K.; Morii, S.; Kitahara, T.; Ohwada, T. Diagnostic significance of serum neuron-specific enolase and myelin basic protein assay in patients with acute head injury. Surg. Neurol. 1995, 43, 267–271. [Google Scholar] [CrossRef]
- Park, D.W.; Park, S.H.; Hwang, S.K. Serial measurement of S100B and NSE in pediatric traumatic brain injury. Child’s Nerv. Syst. 2019, 35, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit. Care 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kapural, M.; Krizanac-Bengez, L.; Barnett, G.; Perl, J.; Masaryk, T.; Apollo, D.; Rasmussen, P.; Mayberg, M.R.; Janigro, D. Serum S-100β as a possible marker of blood-brain barrier disruption. Brain Res. 2002, 940, 102–104. [Google Scholar] [CrossRef]
- Ercole, A.; Thelin, E.P.; Holst, A.; Bellander, B.M.; Nelson, D.W. Kinetic modelling of serum S100b after traumatic brain injury. BMC Neurol. 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dadas, A.; Washington, J.; Diaz-Arrastia, R.; Janigro, D. Biomarkers in traumatic brain injury (TBI): A review. Neuropsychiatr. Dis. Treat. 2018, 14, 2989–3000. [Google Scholar] [CrossRef]
- Nomellini, V.; Kaplan, L.J.; Sims, C.A.; Caldwell, C.C. Chronic Critical Illness and Persistent Inflammation: What can we Learn from the Elderly, Injured, Septic, and Malnourished? Shock 2018, 49, 4–14. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Maier, B.; Lefering, R.; Lehnert, M.; Laurer, H.L.; Steudel, W.I.; Neugebauer, E.A.; Marzi, I. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock 2007, 28, 668–674. [Google Scholar] [CrossRef]
- Swartz, K.R.; Liu, F.; Sewell, D.; Schochet, T.; Campbell, I.; Sandor, M.; Fabry, Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001, 896, 86–95. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Marriott, I. The interleukin-10 family of cytokines and their role in the CNS. Front. Cell. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Frink, M.; Van Griensven, M.; Kobbe, P.; Brin, T.; Zeckey, C.; Vaske, B.; Krettek, C.; Hildebrand, F. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand. J. Trauma. Resusc. Emerg. Med. 2009, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, J.; Christiansen, M.; TØnnesen, E.; Espersen, K.; Lippert, F.K.; Rasmussen, L.S. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol. Scand. 2009, 53, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altmann, D.G.; Egger, M.; Pocock, S.C.; Gøtzsche, P.C.; Vandenbroucke, J.P. Das Strengthening the Reporting of Observational Studies in Epidemiology (STROBE-) Statement. Internist (Berl) 2008, 49, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, B.; Kanz, K.G.; Lackner, C.K.; Mutschler, W.; Sturm, J. Die bedeutung des Advanced Trauma Life Support® (ATLS®) im schockraum. Unfallchirurg 2004, 107, 844–850. [Google Scholar] [CrossRef]
- Haasper, C.; Junge, M.; Ernstberger, A.; Brehme, H.; Hannawald, L.; Langer, C.; Nehmzow, J.; Otte, D.; Sander, U.; Krettek, C.; et al. Die Abbreviated Injury Scale (AIS). Unfallchirurg 2010, 113, 366–372. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W. The Injury Severity Score. J. Trauma-Inj. Infect. Crit. Care 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Stevenson, M.; Segui-Gomez, M.; Lescohier, I.; Di Scala, C.; McDonald-Smith, G. An overview of the injury severity score and the new injury severity score. Inj. Prev. 2001, 7, 10–13. [Google Scholar] [CrossRef]
- Fröhlich, M.; Caspers, M.; Lefering, R.; Driessen, A.; Bouillon, B.; Maegele, M.; Wafaisade, A. Do elderly trauma patients receive the required treatment? Epidemiology and outcome of geriatric trauma patients treated at different levels of trauma care. Eur. J. Trauma Emerg. Surg. 2019, 1–7. [Google Scholar] [CrossRef]
- Bechtel, K.; Frasure, S.; Marshall, C.; Dziura, J.; Simpson, C. Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatrics 2009, 124. [Google Scholar] [CrossRef]
- Ohrt-Nissen, S.; Friis-Hansen, L.; Dahl, B.; Stensballe, J.; Romner, B.; Rasmussen, L.S. How does extracerebral trauma affect the clinical value of S100B measurements? Emerg. Med. J. 2011, 28, 941–944. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.F.; Wartchow, K.; Hansen, F.; Lunardi, P.; Guerra, M.C.; Nardin, P.; Gonçalves, C.A. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 43, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Hertz, H.; Mauritz, W.; Harada, N.; Jafarmadar, M.; Albrecht, M.; Redl, H.; Bahrami, S. Nonspecific increase of systemic neuron-specific enolase after trauma: Clinical and experimental findings. Shock 2005, 24, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.; Herden, C.; Roeb, E.; Roderfeld, M.; Eschbach, D.; Steinfeldt, T.; Wulf, H.; Ruchholtz, S.; Uhl, E.; Schöller, K. Cerebral Alterations Following Experimental Multiple Trauma and Hemorrhagic Shock. Shock 2018, 49, 164–173. [Google Scholar] [CrossRef]
- Xu, C.M.; Luo, Y.L.; Li, S.; Li, Z.X.; Jiang, L.; Zhang, G.X.; Owusu, L.; Chen, H.L. Multifunctional neuron-specific enolase: ITS role in lung diseases. Biosci. Rep. 2019, 39, 1–16. [Google Scholar] [CrossRef]
- Crawford, A.M.; Yang, S.; Hu, P.; Li, Y.; Lozanova, P.; Scalea, T.M.; Stein, D.M. Concomitant chest trauma and traumatic brain injury, biomarkers correlate with worse outcomes. J. Trauma Acute Care Surg. 2019, 87, S146–S151. [Google Scholar] [CrossRef]
- Hainfellner, J.A.; Voigtländer, T.; Ströbel, T.; Mazal, P.R.; Maddalena, A.S.; Aguzzi, A.; Budka, H. Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. J. Neuropathol. Exp. Neurol. 2001, 60, 449–461. [Google Scholar] [CrossRef]
- Foerch, C.; Pfeilschifter, W.; Zeiner, P.; Brunkhorst, R. Saures gliafaserprotein beim patienten mit akuten schlaganfallsymptomen: Diagnostischer marker einer hirnblutung. Nervenarzt 2014, 85, 982–989. [Google Scholar] [CrossRef]
- Lumpkins, K.M.; Bochicchio, G.V.; Keledjian, K.; Simard, J.M.; McCunn, M.; Scalea, T. Glial fibrillary acidic protein is highly correlated with brain injury. J. Trauma 2008, 65, 778–782. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; McCartney, K.; Moore, T.A.; Thundyil, J.; Gelderblom, M.; Manzanero, S.; Arumugam, T.V. Intestinal ischemia-reperfusion injury leads to inflammatory changes in the brain. Shock 2011, 36, 424–430. [Google Scholar] [CrossRef]
- Shultz, S.R.; Sun, M.; Wright, D.K.; Brady, R.D.; Liu, S.; Beynon, S.; Schmidt, S.F.; Kaye, A.H.; Hamilton, J.A.; O’Brien, T.J.; et al. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 2015, 35, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Morganti-Kossmann, M.C.; Satgunaseelan, L.; Bye, N.; Kossmann, T. Modulation of immune response by head injury. Injury 2007, 38, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Schwerdtfeger, K.; Mautes, A.; Holanda, M.; Müller, M.; Steudel, W.I. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock 2001, 15, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowsky, C.; Schöning, B.; Cobanov, J.; Lanksch, W.R.; Volk, H.D.; Döcke, W.D. Early il-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J. Trauma 2002, 52, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Doré, S. Role of interleukin-10 in acute brain injuries. Front. Neurol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Sapan, H.B.; Paturusi, I.; Islam, A.A.; Yusuf, I.; Patellongi, I.; Massi, M.N.; Pusponegoro, A.D.; Arief, S.K.; Labeda, I.; Rendy, L.; et al. Interleukin-6 and interleukin-10 plasma levels and mRNA expression in polytrauma patients. Chin. J. Traumatol. 2017, 20, 318–322. [Google Scholar] [CrossRef]
- Kalabalikis, P.; Papazoglou, K.; Gouriotis, D.; Papadopoulos, N.; Kardara, M.; Papageorgiou, F.; Papadatos, J. Correlation between serum IL-6 and CRP levels and severity of head injury in children. Intensive Care Med. 1999, 25, 288–292. [Google Scholar] [CrossRef]
- Wrba, L.; Ohmann, J.J.; Eisele, P.; Chakraborty, S.; Braumüller, S.; Braun, C.K.; Klohs, B.; Schultze, A.; Von Baum, H.; Palmer, A.; et al. Remote intestinal injury early after experimental polytrauma and hemorrhagic shock. Shock 2019, 52, e45–e51. [Google Scholar] [CrossRef]
- Cohen, S.S.; Min, M.; Cummings, E.E.; Chen, X.; Sadowska, G.B.; Sharma, S.; Stonestreet, B.S. Effects of Interleukin-6 on the Expression of Tight Junction Proteins in Isolated Cerebral Microvessels from Yearling and Adult Sheep. Neuroimmunomodulation 2013, 20, 264–273. [Google Scholar] [CrossRef]
- Halbgebauer, R.; Braun, C.K.; Denk, S.; Mayer, B.; Cinelli, P.; Radermacher, P.; Wanner, G.A.; Simmen, H.P.; Gebhard, F.; Rittirsch, D.; et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J. Crit. Care 2018, 44, 229–237. [Google Scholar] [CrossRef]
- Pan, P.; Song, Y.; Du, X.; Bai, L.; Hua, X.; Xiao, Y.; Yu, X. Intestinal barrier dysfunction following traumatic brain injury. Neurol. Sci. 2019, 40, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Vanzant, E.L.; Hilton, R.E.; Lopez, C.M.; Zhang, J.; Ungaro, R.F.; Gentile, L.F.; Szpila, B.E.; Maier, R.V.; Cuschieri, J.; Bihorac, A.; et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit. Care 2015, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]


| PT (n = 43) | PT + TBI (n = 35) | isTBI (n = 26) | p-Value | |
|---|---|---|---|---|
| Male | 69.8% | 80.0% | 80.8% | |
| Age (y, ± SEM) | 46 (±2) | 43 (±3) | 61 (±3) | <0.001 |
| Age ≥ 55 years | 32.6% | 25.7% | 69.2% | <0.001 |
| ISS (±SEM) | 30 (±2) | 37 (±2) | 24 (±1) | <0.01 |
| ISS ≥ 25 | 74.4% | 77.1% | 53.9% | <0.01 |
| NISS (±SEM) | 37 (±2) | 44 (±2) | 38 (±2) | 0.135 |
| AIShead (±SEM) | 0.3 (±0.1) | 4.1 (±0.2) | 4.5 (±0.1) | <0.001 |
| AISchest ≥ 3 | 81.4% | 68.6% | 0.0% | <0.001 |
| AISabdomen ≥ 3 | 46.5% | 11.1% | 0.0% | <0.001 |
| AISextremity ≥ 3 | 51.2% | 37.1% | 0.0% | <0.001 |
| ICH (%) | 57.1% | 61.5% | 0.730 | |
| SDH (%) | 37.1% | 61.5% | 0.059 | |
| SAH (%) | 34.3% | 57.7% | 0.069 | |
| EDH (%) | 20.0% | 11.5% | 0.377 |
| PT | PT + TBI | isTBI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | GFAP | S100ß | IL6 | IL10 | Ratio | GFAP | S100ß | IL6 | IL10 | Ratio | GFAP | S100ß | IL6 | IL10 | Ratio | |
| ED | NSE | 0.21 | 0.06 | 0.024 | 0.00 | 0.12 | 0.13 | −0.05 | 0.37 | 0.46 * | − 0.12 | 0.55 ** | 0.44 * | −0.17 | −0.00 | −0.30 |
| GFAP | 0.08 | 0.18 | 0.06 | 0.04 | 0.30 | −0.11 | −0.14 | −0.06 | 0.27 | −0.03 | 0.25 | −0.58 * | ||||
| S100ß | −0.06 | 0.01 | −0.08 | −0.14 | 0.06 | −0.31 | 0.05 | 0.09 | −0.23 | |||||||
| IL6 | 0.78 *** | 0.39 | 0.64 ** | 0.31 | 0.76 *** | −0.19 | ||||||||||
| IL10 | −0.21 | −0.50 * | −0.80 *** | |||||||||||||
| 1 | NSE | −0.14 | 0.32 * | 0.09 | 0.29 | −0.15 | 0.17 | 0.07 | 0.12 | −0.35 | −0.29 | 0.44 * | 0.12 | 0.23 | 0.39 | −0.30 |
| GFAP | 0.17 | 0.18 | −0.08 | −0.09 | 0.52 ** | 0.08 | 0.27 | −0.28 | 0.54 * | −0.03 | 0.03 | −0.31 | ||||
| S100ß | −0.12 | −0.7 | −0.06 | 0.13 | 0.27 | −0.28 | 0.18 | −0.10 | 0.00 | |||||||
| IL6 | 0.41 * | 0.34 | 0.48 * | 0.33 | 0.66 ** | 0.28 | ||||||||||
| IL10 | −0.64 *** | 0.61 ** | −0.66 ** | |||||||||||||
| 2 | NSE | −0.16 | 0.23 | 0.33 | 0.00 | 0.07 | 0.29 | 0.23 | 0.06 | 0.17 | 0.11 | 0.63 * | 0.22 | 0.18 | 0.08 | 0.06 |
| GFAP | 0.30 | −0.12 | 0.38 | −0.53 * | 0.46 * | 0.23 | 0.20 | −0.20 | 0.48 * | −0.05 | −0.14 | −0.07 | ||||
| S100ß | −0.25 | 0.10 | 0.15 | 0.06 | 0.27 | 0.42 | 0.25 | 0.24 | −0.18 | |||||||
| IL6 | 0.08 | 0.31 | 0.28 | 0.61 * | 0.33 | 0.56 * | ||||||||||
| IL10 | −0.85 *** | −0.22 | −0.69 ** | |||||||||||||
| 3 | NSE | 0.02 | 0.21 | 0.20 | −0.08 | −0.02 | 0.24 | 0.23 | 0.15 | 0.18 | 0.04 | 0.54 * | 0.48 * | 0.03 | −0.05 | −0.17 |
| GFAP | 0.26 | −0.20 | 0.24 | 0.17 | 0.43 * | 0.36 | 0.27 | −0.10 | 0.50 * | −0.24 | −0.17 | −0.25 | ||||
| S100ß | −0.05 | −0.15 | 0.44 | 0.03 | 0.42 * | −0.28 | −0.05 | −0.87 | −0.08 | |||||||
| IL6 | 0.49 * | 0.26 | 0.74 | 0.46 | 0.34 | 0.38 | ||||||||||
| IL10 | −0.7 *** | −0.78 *** | −0.44 | |||||||||||||
| 4 | NSE | −0.02 | 0.27 | 0.07 | 0.01 | −0.08 | 0.24 | 0.17 | 0.15 | −0.74 | −0.00 | 0.55 * | −0.10 | −0.04 | −0.06 | 0.48 |
| GFAP | 0.26 | −0.06 | 0.22 | −0.32 | 0.44 * | 0.36 | 0.16 | 0.21 | 0.17 | −0.10 | −0.01 | 0.25 | ||||
| S100ß | 0.4 * | 0.03 | 0.22 | 0.10 | 0.28 | 0.17 | 0.08 | 0.03 | 0.06 | |||||||
| IL6 | 0.38 | 0.76 *** | 0.01 | 0.75 ** | 0.53 * | 0.84 ** | ||||||||||
| IL10 | 0.21 | −0.23 | 0.04 | |||||||||||||
| 5 | NSE | 0.00 | 0.31 | 0.07 | 0.12 | −0.20 | 0.03 | 0.25 | −0.03 | −0.02 | 0.10 | 0.56 * | 0.22 | −0.07 | −0.16 | 0.73 ** |
| GFAP | 0.27 | −0.20 | 0.00 | −0.02 | 0.49 * | 0.04 | 0.27 | −0.36 | 0.30 | −0.17 | −0.30 | 0.54 | ||||
| S100ß | 0.42 * | 0.31 | 0.21 | −0.11 | 0.42 | −0.35 | 0.21 | −0.22 | 0.31 | |||||||
| IL6 | 0.65 *** | 0.52 * | 0.42 | 0.72 ** | 0.68 *** | 0.21 | ||||||||||
| IL10 | 0.34 | −0.71 ** | 0.24 | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, C.R.; Lustenberger, T.; Woschek, M.; Störmann, P.; Henrich, D.; Radermacher, P.; Marzi, I. Severe Traumatic Brain Injury (TBI) Modulates the Kinetic Profile of the Inflammatory Response of Markers for Neuronal Damage. J. Clin. Med. 2020, 9, 1667. https://doi.org/10.3390/jcm9061667
Schindler CR, Lustenberger T, Woschek M, Störmann P, Henrich D, Radermacher P, Marzi I. Severe Traumatic Brain Injury (TBI) Modulates the Kinetic Profile of the Inflammatory Response of Markers for Neuronal Damage. Journal of Clinical Medicine. 2020; 9(6):1667. https://doi.org/10.3390/jcm9061667
Chicago/Turabian StyleSchindler, Cora Rebecca, Thomas Lustenberger, Mathias Woschek, Philipp Störmann, Dirk Henrich, Peter Radermacher, and Ingo Marzi. 2020. "Severe Traumatic Brain Injury (TBI) Modulates the Kinetic Profile of the Inflammatory Response of Markers for Neuronal Damage" Journal of Clinical Medicine 9, no. 6: 1667. https://doi.org/10.3390/jcm9061667
APA StyleSchindler, C. R., Lustenberger, T., Woschek, M., Störmann, P., Henrich, D., Radermacher, P., & Marzi, I. (2020). Severe Traumatic Brain Injury (TBI) Modulates the Kinetic Profile of the Inflammatory Response of Markers for Neuronal Damage. Journal of Clinical Medicine, 9(6), 1667. https://doi.org/10.3390/jcm9061667





