Long-Term Prognosis of Patients with Myocardial Infarction Type 1 and Type 2 with and without Involvement of Coronary Vasospasm
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics Statement
2.2. Study Population
2.3. Clinical Outcomes and Definitions
2.4. Statistical Analysis
3. Results
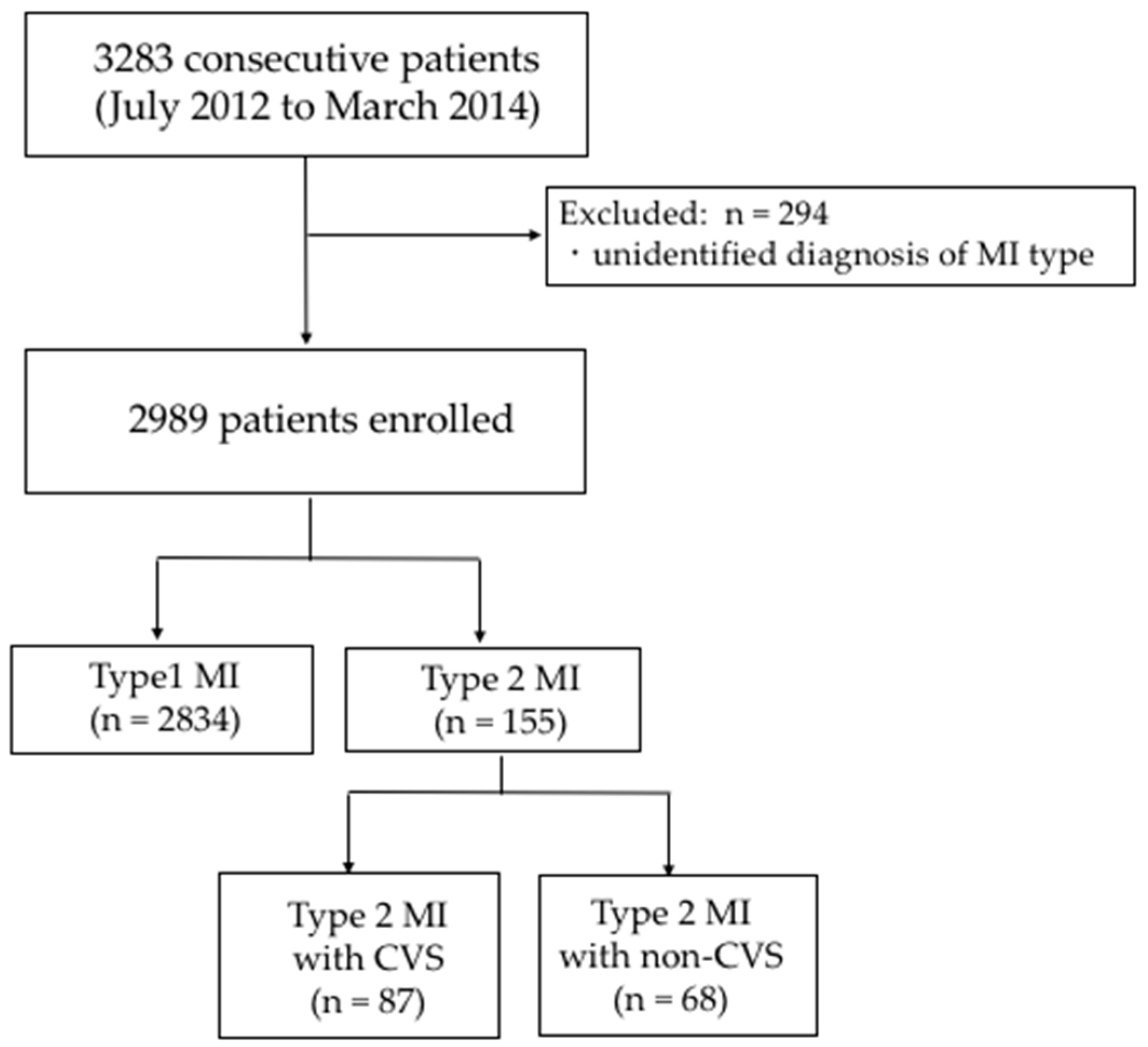
3.1. Patient Background
3.1.1. Type 1 vs. Type 2
3.1.2. Type 2 with CVS vs. with Non-CVS
3.2. Clinical Outcomes
3.3. Sensitivity Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined—A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur. Heart J. 2000, 21, 1502–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. Eur. Heart J. 2007, 28, 2525–2538. [Google Scholar] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Writing Group on the Joint, ESC/ACCF/AHA/WHF. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial, I. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2018, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Boeddinghaus, J.; Twerenbold, R.; Nestelberger, T.; APACE Investigators. Clinical validation of a novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin. Chem. 2018, 64, 1347–1360. [Google Scholar] [CrossRef] [Green Version]
- Twerenbold, R.; Neumann, J.T.; Sorensen, N.A.; Ojeda, F.; Karakas, M.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Rubini Gimenez, M.; Puelacher, C.; et al. Prospective validation of the 0/1-h algorithm for early diagnosis of myocardial infarction. J. Am. Coll. Cardiol. 2018, 72, 620–632. [Google Scholar] [CrossRef]
- Chapman, A.R.; Adamson, P.D.; Shah, A.S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Ken Lee, K.; Berry, C.; Findlay, I.; Cruikshank, A.; et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation 2020, 141, 161–171. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Newby, L.K.; Mills, N.L. A proposal for modest revision of the definition of type 1 and type 2 myocardial infarction. Circulation 2019, 140, 1773–1775. [Google Scholar] [CrossRef]
- Stein, G.Y.; Herscovici, G.; Korenfeld, R.; Matetzky, S.; Gottlieb, S.; Alon, D.; Gevrielov-Yusim, N.; Iakobishvili, Z.; Fuchs, S. Type-II myocardial infarction--patient characteristics, management and outcomes. PLoS ONE 2014, 9, e84285. [Google Scholar] [CrossRef]
- Saaby, L.; Poulsen, T.S.; Diederichsen, A.C.; Hosbond, S.; Larsen, T.B.; Schmidt, H.; Gerke, O.; Hallas, J.; Thygesen, K.; Mickley, H. Mortality rate in Type 2 myocardial infarction: Observations from an unselected hospital cohort. Am. J. Med. 2014, 127, 295–302. [Google Scholar] [CrossRef]
- Chapman, A.R.; Shah, A.S.V.; Lee, K.K.; Anand, A.; Francis, O.; Adamson, P.; McAllister, D.A.; Strachan, F.E.; Newby, D.E.; Mills, N.L. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation 2018, 137, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.; Hambraeus, K.; Sundstrom, J.; Erlinge, D.; Jernberg, T.; Lindahl, B.; TOTAL-AMI study group. Type 2 myocardial infarction in clinical practice. Heart 2015, 101, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kishida, H.; Hata, N.; Kusama, Y.; Suzuki, T.; Saito, T.; Nejima, J.; Otsu, F.; Yasutake, M.; Koumi, S.; Nakagomi, A. Factors influencing the clinical course and the long-term prognosis of patients with variant angina. Jpn. Heart J. 1987, 28, 293–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braunwald, E. Coronary spasm and acute myocardial infarction—New possibility for treatment and prevention. N. Engl. J. Med. 1978, 299, 1301–1303. [Google Scholar] [CrossRef]
- Pristipino, C.; Beltrame, J.F.; Finocchiaro, M.L.; Hattori, R.; Fujita, M.; Mongiardo, R.; Cianflone, D.; Sanna, T.; Sasayama, S.; Maseri, A. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation 2000, 101, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Fujino, M.; Ogawa, H.; Yasuda, S.; Noguchi, T.; Nakao, K.; Ozaki, Y.; Kimura, K.; Suwa, S.; Fujimoto, K.; et al. Clinical presentation, management and outcome of Japanese patients with acute myocardial infarction in the troponin era—Japanese registry of acute myocardial infarction diagnosed by universal definition (J-MINUET). Circ. J. 2015, 79, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Group JCSJW. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef] [Green Version]
- Nestelberger, T.; Boeddinghaus, J.; Badertscher, P.; Twerenbold, R.; Wildi, K.; Breitenbucher, D.; Sabti, Z.; Puelacher, C.; Rubini Gimenez, M.; Kozhuharov, N.; et al. Effect of definition on incidence and prognosis of type 2 myocardial infarction. J. Am. Coll. Cardiol. 2017, 70, 1558–1568. [Google Scholar] [CrossRef]
- Raphael, C.E.; Roger, V.L.; Sandoval, Y.; Singh, M.; Bell, M.; Lerman, A.; Rihal, C.S.; Gersh, B.J.; Lewis, B.; Lennon, R.J.; et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation 2020, 141, 454–463. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, A.; DeFilippis, E.M.; Qamar, A.; Biery, D.W.; Almarzooq, Z.; Collins, B.; Fatima, A.; Jackson, C.; Galazka, P.; et al. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J. Am. Coll. Cardiol. 2020, 75, 1003–1013. [Google Scholar] [CrossRef]
- Roger, V.L.; Weston, S.A.; Gerber, Y.; Killian, J.M.; Dunlay, S.M.; Jaffe, A.S.; Bell, M.R.; Kors, J.; Yawn, B.P.; Jacobsen, S.J. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation 2010, 121, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kojima, S. Modern state of acute myocardial infarction in the interventional era: Observational case-control study—Japanese acute coronary syndrome study (JACSS). J. Cardiol. 2009, 54, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daida, H.; Miyauchi, K.; Ogawa, H.; Yokoi, H.; Matsumoto, M.; Kitakaze, M.; Kimura, T.; Matsubara, T.; Ikari, Y.; Kimura, K.; et al. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: Prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ. J. 2013, 77, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatani, D.; Sakata, Y.; Suna, S.; Usami, M.; Matsumoto, S.; Shimizu, M.; Sumitsuji, S.; Kawano, S.; Ueda, Y.; Hamasaki, T.; et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ. J. 2013, 77, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Neumann, J.T.; Sorensen, N.A.; Rubsamen, N.; Ojeda, F.; Renne, T.; Qaderi, V.; Teltrop, E.; Kramer, S.; Quantius, L.; Zeller, T.; et al. Discrimination of patients with type 2 myocardial infarction. Eur. Heart J. 2017, 38, 3514–3520. [Google Scholar] [CrossRef]
- Bonnefoy, E.; Lapostolle, F.; Leizorovicz, A.; Steg, G.; McFadden, E.P.; Dubien, P.Y.; Cattan, S.; Boullenger, E.; Machecourt, J.; Lacroute, J.M.; et al. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: A randomised study. Lancet 2002, 360, 825–829. [Google Scholar] [CrossRef]
- Widimsky, P.; Budesinsky, T.; Vorac, D.; Groch, L.; Zelizko, M.; Aschermann, M.; Branny, M.; St’asek, J.; Formanek, P.; Investigators, P.S.G. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur. Heart J. 2003, 24, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Yasue, H.; Ogawa, H.; Tanaka, H.; Miyazaki, S.; Hattori, R.; Saito, M.; Ishikawa, K.; Masuda, Y.; Yamaguchi, T.; Motomiya, T.; et al. Effects of aspirin and trapidil on cardiovascular events after acute myocardial infarction. Japanese Antiplatelets Myocardial Infarction Study (JAMIS) Investigators. Am. J. Cardiol. 1999, 83, 1308–1313. [Google Scholar] [CrossRef]
- Baigent, C.; Collins, R.; Appleby, P.; Parish, S.; Sleight, P.; Peto, R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998, 316, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Intervention with Pravastatin in Ischaemic Disease Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Cheitlin, M.D.; McAllister, H.A.; De Castro, C.M. Myocardial infarction without atherosclerosis. JAMA 1975, 231, 951–959. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjold, A.; Gard, A.; Jernberg, T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef]
- Lambrecht, S.; Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Hosbond, S.; Diederichsen, A.C.P.; Thygesen, K.; Mickley, H. Different causes of death in patients with myocardial infarction type 1, type 2, and myocardial injury. Am. J. Med. 2018, 131, 548–554. [Google Scholar] [CrossRef]
- Bertrand, M.E.; LaBlanche, J.M.; Tilmant, P.Y.; Thieuleux, F.A.; Delforge, M.R.; Carre, A.G.; Asseman, P.; Berzin, B.; Libersa, C.; Laurent, J.M. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation 1982, 65, 1299–1306. [Google Scholar] [CrossRef] [Green Version]
- Sugiishi, M.; Takatsu, F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 1993, 87, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Kasanuki, H.; Honda, T.; Haze, K.; Sumiyoshi, T.; Horie, T.; Yagi, M.; Yamaguchi, J.; Ishii, Y.; Fujii, S.Y.; Nagashima, M.; et al. A large-scale prospective cohort study on the current status of therapeutic modalities for acute myocardial infarction in Japan: Rationale and initial results of the HIJAMI Registry. Am. Heart J. 2005, 150, 411–418. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yasue, H.; Nakayama, M.; Shimasaki, Y.; Sumida, H.; Sugiyama, S.; Kugiyama, K.; Ogawa, H.; Ogawa, Y.; Saito, Y.; et al. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum. Genet. 1998, 103, 65–69. [Google Scholar] [CrossRef]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Kugiyama, K.; Ogawa, H.; Motoyama, T.; Saito, Y.; Ogawa, Y.; Miyamoto, Y.; et al. T−786→C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation 1999, 99, 2864–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Ogawa, H.; Kugiyama, K.; Mizuno, Y.; Harada, E.; Nakamura, S.; Ito, T.; et al. T−786→C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with myocardial infarction, especially without coronary organic stenosis. Am. J. Cardiol. 2000, 86, 628–634. [Google Scholar] [CrossRef]
- Kugiyama, K.; Yasue, H.; Okumura, K.; Ogawa, H.; Fujimoto, K.; Nakao, K.; Yoshimura, M.; Motoyama, T.; Inobe, Y.; Kawano, H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation 1996, 94, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kugiyama, K.; Ohgushi, M.; Sugiyama, S.; Motoyama, T.; Kawano, H.; Hirashima, O.; Yasue, H. Supersensitive dilator response to nitroglycerin but not to atrial natriuretic peptide in spastic coronary arteries in coronary spastic angina. Am. J. Cardiol. 1997, 79, 606–610. [Google Scholar] [CrossRef]
- Oshima, S.; Yasue, H.; Ogawa, H.; Okumura, K.; Matsuyama, K. Fibrinopeptide A is released into the coronary circulation after coronary spasm. Circulation 1990, 82, 2222–2225. [Google Scholar] [CrossRef] [Green Version]
- Misumi, I.; Ogawa, H.; Masuda, T.; Sakamoto, T.; Okumura, K.; Yasue, H. Increased plasma plasminogen activator inhibitor activity after coronary spasm. Int. J. Cardiol. 1993, 41, 21–29. [Google Scholar] [CrossRef]
- Kaikita, K.; Ogawa, H.; Yasue, H.; Sakamoto, T.; Suefuji, H.; Sumida, H.; Okumura, K. Soluble P-selectin is released into the coronary circulation after coronary spasm. Circulation 1995, 92, 1726–1730. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Yamanaga, K.; Miyazaki, T.; Akasaka, T.; Tabata, N.; Arima, Y.; Sueta, D.; Sakamoto, K.; et al. Impact of aspirin on the prognosis in patients with coronary spasm without significant atherosclerotic stenosis. Int. J. Cardiol. 2016, 220, 328–332. [Google Scholar] [CrossRef]


| Type 1 MI (n = 2834) | Type 2 MI (n = 155) | p | Type 2 MI | |||
|---|---|---|---|---|---|---|
| Non-CVS (n = 68) | With CVS (n = 87) | p | ||||
| Age (years) | 68.7 ± 12.5 | 65.8 ± 14.4 | 0.003 | 72.4 ± 11.9 | 60.7 ± 14.5 | 0.029 |
| Female (%) | 699 (24.7) | 53 (34.2) | 0.008 | 23 (33.8) | 30 (34.5) | 0.930 |
| BMI (kg/m2) | 23.6 ± 3.9 | 23.1 ± 3.2 | 0.156 | 22.7 ± 3.6 | 23.2 ± 3.9 | 0.112 |
| Onset to admission (min) | 345.7 ± 479.5 | 382.8 ± 553.3 | 0.062 | 337.6 ± 506.0 | 418.2 ± 588.2 | 0.381 |
| HR at admission (bpm) | 79.0 ± 21.7 | 78.9 ± 19.8 | 0.340 | 83.3 ± 22.6 | 75.1 ± 16.8 | 0.033 |
| Killip Class (III/IV) | 433 (15.3) | 13 (8.4) | 0.018 | 11 (16.2) | 2 (2.3) | 0.007 |
| Hypertension (%) | 1885 (67.0) | 103 (66.5) | 0.886 | 44 (64.7) | 59 (67.8) | 0.910 |
| Diabetes mellitus (%) | 1015 (36.6) | 40 (25.8) | 0.006 | 24 (35.3) | 16 (18.4) | 0.002 |
| Dyslipidemia (%) | 1435 (51.2) | 95 (61.3) | 0.015 | 43 (63.2) | 52 (59.8) | 0.047 |
| Chronic kidney disease (%) | 1261 (44.5) | 68 (43.9) | 0.876 | 43 (63.2) | 25 (28.7) | <0.001 |
| Current smoking (%) | 921(33.6) | 51 (33.8) | 0.972 | 19 (27.9) | 32 (38.6) | 0.388 |
| Prior MI (%) | 350 (12.4) | 18 (11.6) | 0.769 | 11 (16.2) | 7 (8.0) | 0.299 |
| Prior PCI (%) | 424 (15.1) | 32 (20.6) | 0.062 | 24 (35.3) | 8(9.2) | <0.001 |
| Atrial fibrillation (%) | 166 (5.6) | 12 (7.8) | 0.347 | 9 (13.4) | 3 (3.4) | 0.023 |
| Prior stroke (%) | 276 (9.9) | 17 (10.9) | 0.651 | 12 (17.6) | 5 (5.8) | 0.041 |
| Composite of PAD (%) | 121 (4.5) | 14 (9.4) | 0.006 | 11 (17.2) | 3 (3.5) | <0.001 |
| Urgent CAG (%) | 2655 (93.8) | 130 (83.9) | <0.001 | 59 (86.8) | 71 (81.6) | <0.001 |
| Max CPK (IU/L) | 2444.1 ± 2952.8 | 1065.4 ± 1828.3 | <0.001 | 1712.5 ± 2347.7 | 559.7 ± 1049.9 | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 3.3 | 12.9 ± 2.5 | 0.009 | 11.7 ± 2.9 | 13.9 ± 1.7 | <0.001 |
| eGFR (mL/min/1.73 m2) | 65.8 ± 45.7 | 63.6 ± 27.8 | 0.006 | 50.7 ± 27.3 | 73.7 ± 24.0 | 0.006 |
| LDL-cholesterol (mg/dL) | 117.5 ± 37.8 | 100.8 ± 37.4 | <0.001 | 95.5 ± 37.1 | 104.5 ± 37.4 | <0.001 |
| HDL-cholesterol (mg/dL) | 46.7 ± 13.4 | 49.8 ± 15.2 | 0.006 | 46.2 ± 14.8 | 52.6 ± 15.1 | 0.003 |
| HbA1c (%) | 6.3 ± 1.4 | 5.9 ± 0.8 | <0.001 | 6.1 ± 0.8 | 5.9 ± 0.7 | <0.001 |
| NSTEMI (%) | 837 (29.5) | 93 (60.0) | <0.001 | 34 (50.0) | 59 (67.8) | <0.001 |
| Urgent revascularization (%) | 2,485 (87.8) | 63 (40.6) | <0.001 | 42 (61.8) | 21 (24.4) | <0.001 |
| Type 1 MI (n = 2834) | Type 2 MI (n = 155) | p | Type 2 MI | |||
|---|---|---|---|---|---|---|
| Non-CVS (n = 68) | With CVS (n = 87) | p | ||||
| Aspirin (%) | 2562 (97.3) | 70 (45.1) | <0.001 | 39 (70.9) | 54 (65.9) | 0.530 |
| DAPT (%) | 2058 (78.2) | 52 (33.5) | <0.001 | 27 (49.1) | 25 (30.5) | <0.001 |
| Anticoagulant Therapy (%) | 327 (12.4) | 24 (15.5) | 0.131 | 21 (36.2) | 3 (3.5) | <0.001 |
| CCBs (%) | 551 (20.8) | 94 (60.6) | <0.001 | 16 (27.6) | 78 (90.7) | <0.001 |
| β-blockades (%) | 1838 (70.2) | 53 (34.2) | <0.001 | 37 (64.9) | 16 (21.6) | <0.001 |
| Nitrates (%) | 246 (9.5) | 37 (23.9) | <0.001 | 7 (12.7) | 30 (38.5) | <0.001 |
| Nicorandil (%) | 541 (21.0) | 41 (26.4) | <0.014 | 12 (21.8) | 29 (36.3) | 0.005 |
| ACE-Is (%) | 1364 (52.8) | 52 (33.5) | 0.003 | 28 (51.9) | 24 (30.8) | 0.001 |
| ARBs (%) | 739 (28.5) | 41 (26.4) | 0.679 | 18 (31.6) | 23 (29.1) | 0.874 |
| Diuretics (%) | 23 (0.9) | 0 (0) | NA | 0 (0) | 0 (0) | >0.990 |
| Statins (%) | 2303 (87.6) | 112 (72.3) | 0.005 | 37 (67.3) | 75 (87.2) | <0.001 |
| Hypoglycemic Agents (%) | 728 (27.5) | 24 (15.5) | 0.040 | 16 (27.6) | 8 (9.3) | 0.001 |
| PPIs (%) | 2260 (85.5) | 92 (59.3) | <0.001 | 43 (74.1) | 49 (57.0) | <0.001 |
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.043 | 1.026–1.060 | <0.001 |
| Female | 1.270 | 0.899–1.794 | 0.175 |
| BMI | 0.978 | 0.939–1.019 | 0.978 |
| Killip Class | 1.986 | 1.750–2.254 | <0.001 |
| Current Smoking | 1.061 | 0.731–1.540 | 0.756 |
| Chronic Kidney Disease | 2.298 | 1.611–3.276 | <0.001 |
| Prior PCI | 1.664 | 1.158–2.393 | 0.006 |
| Composite of PAD | 2.045 | 1.203–3.475 | 0.008 |
| Urgent Revascularization | 0.737 | 0.441–1.230 | 0.243 |
| Multivessel Disease | 1.365 | 1.004–1.858 | 0.047 |
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.080 | 0.996–1.171 | 0.061 |
| Heart Rate | 1.040 | 0.999–1.081 | 0.053 |
| Killip Class | 0.814 | 0.355–1.871 | 0.629 |
| Chronic Kidney Disease | 1.144 | 0.242–5.410 | 0.865 |
| Prior CABG | 1.511 | 0.080–28.567 | 0.783 |
| Urgent Revascularization | 5.085 | 1.018–25.390 | 0.047 |
| Multivessel Disease | 5.395 | 1.186–24.545 | 0.029 |
| Hemoglobin | 0.992 | 0.712–1.384 | 0.964 |
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Type 2 MI with CVS | Reference | ||
| Type 2 MI with Non-CVS | 5.007 | 1.338–18.741 | 0.017 |
| Age | 1.020 | 0.973–1.070 | 0.407 |
| Sex | 1.527 | 0.560–4.165 | 0.408 |
| BMI | 0.993 | 0.862–1.145 | 0.925 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, R.; Sakamoto, K.; Kaikita, K.; Tsujita, K.; Nakao, K.; Ozaki, Y.; Kimura, K.; Ako, J.; Noguchi, T.; Yasuda, S.; et al. Long-Term Prognosis of Patients with Myocardial Infarction Type 1 and Type 2 with and without Involvement of Coronary Vasospasm. J. Clin. Med. 2020, 9, 1686. https://doi.org/10.3390/jcm9061686
Sato R, Sakamoto K, Kaikita K, Tsujita K, Nakao K, Ozaki Y, Kimura K, Ako J, Noguchi T, Yasuda S, et al. Long-Term Prognosis of Patients with Myocardial Infarction Type 1 and Type 2 with and without Involvement of Coronary Vasospasm. Journal of Clinical Medicine. 2020; 9(6):1686. https://doi.org/10.3390/jcm9061686
Chicago/Turabian StyleSato, Ryota, Kenji Sakamoto, Koichi Kaikita, Kenichi Tsujita, Koichi Nakao, Yukio Ozaki, Kazuo Kimura, Junya Ako, Teruo Noguchi, Satoshi Yasuda, and et al. 2020. "Long-Term Prognosis of Patients with Myocardial Infarction Type 1 and Type 2 with and without Involvement of Coronary Vasospasm" Journal of Clinical Medicine 9, no. 6: 1686. https://doi.org/10.3390/jcm9061686
APA StyleSato, R., Sakamoto, K., Kaikita, K., Tsujita, K., Nakao, K., Ozaki, Y., Kimura, K., Ako, J., Noguchi, T., Yasuda, S., Suwa, S., Fujimoto, K., Nakama, Y., Morita, T., Shimizu, W., Saito, Y., Hirohata, A., Morita, Y., Inoue, T., ... Ishihara, M., on behalf of J-MINUET Investigators. (2020). Long-Term Prognosis of Patients with Myocardial Infarction Type 1 and Type 2 with and without Involvement of Coronary Vasospasm. Journal of Clinical Medicine, 9(6), 1686. https://doi.org/10.3390/jcm9061686





