Poor Time in Therapeutic Range Control is Associated with Adverse Clinical Outcomes in Patients with Non-Valvular Atrial Fibrillation: A Report from the Nationwide COOL-AF Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Data Collection
2.4. Assessment of Clinical Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lip, G.Y.H.; Brechin, C.M.; Lane, D.A. The Global Burden of Atrial Fibrillation and Stroke: A Systematic Review of the Epidemiology of Atrial Fibrillation in Regions outside North America and Europe. Chester 2012, 142, 1489–1498. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chester 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y. Stroke Prevention in Atrial Fibrillation. Eur. Heart J. 2017, 38, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Haas, S.; Ten Cate, H.; Accetta, G.; Angchaisuksiri, P.; Bassand, J.P.; Camm, A.J.; Corbalan, R.; Darius, H.; Fitzmaurice, D.A.; Goldhaber, S.Z.; et al. Quality of Vitamin K Antagonist Control and 1-Year Outcomes in Patients with Atrial Fibrillation: A Global Perspective from the GARFIELD-AF Registry. PLoS ONE 2016, 11, e0164076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.E.; Okumura, K.; Zhang, S.; Chao, T.F.; Siu, C.W.; Wei Lim, T.; Saxena, A.; Takahashi, Y.; Siong Teo, W. 2017 Consensus of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation. J. Arrhythm. 2017, 33, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A. Time in the Therapeutic Range for Patients Taking Warfarin in Clinical Trials: Useful, but Also Misleading, Misused, and Overinterpreted. Circulation 2017, 135, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.E.; Wang, K.L.; Lip, G.Y. Stroke Prevention in Atrial Fibrillation: An Asian Perspective. Thromb. Haemost. 2014, 111, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, A.Y.; Yao, J.F.; Brar, S.S.; Jorgensen, M.B.; Chen, W. Racial/Ethnic Differences in the Risk of Intracranial Hemorrhage among Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2007, 50, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Goto, S.; Accetta, G.; Angchaisuksiri, P.; Camm, A.J.; Cools, F.; Haas, S.; Kayani, G.; Koretsune, Y.; Lim, T.W.; et al. Vitamin K Antagonist Control in Patients with Atrial Fibrillation in Asia Compared with Other Regions of the World: Real-world Data from the GARFIELD-AF Registry. Int. J. Cardiol. 2016, 223, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R.; Cannegieter, S.C.; van der Meer, F.J.; Briet, E. A Method to Determine the Optimal Intensity of Oral Anticoagulant Therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, S.; Kearon, C.; SCAS; SCIST; Haemostasis. Definition of Major Bleeding in Clinical Investigations of Antihemostatic Medicinal Products in Non-surgical Patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Krittayaphong, R.; Winijkul, A.; Methavigul, K.; Wongtheptien, W.; Wongvipaporn, C.; Wisaratapong, T.; Kunjara-Na-Ayudhya, R.; Boonyaratvej, S.; Komoltri, C.; Kaewcomdee, P.; et al. Risk Profiles and Pattern of Antithrombotic Use in Patients with Non-valvular Atrial Fibrillation in Thailand: A Multicenter Study. BMC Cardiovasc. Disord. 2018, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Hayden, D.T.; Hannon, N.; Callaly, E.; Ni Chroinin, D.; Horgan, G.; Kyne, L.; Duggan, J.; Dolan, E.; O’Rourke, K.; Williams, D.; et al. Rates and Determinants of 5-Year Outcomes After Atrial Fibrillation-Related Stroke: A Population Study. Stroke 2015, 46, 3488–3493. [Google Scholar] [CrossRef]
- Hannon, N.; Daly, L.; Murphy, S.; Smith, S.; Hayden, D.; Ni Chroinin, D.; Callaly, E.; Horgan, G.; Sheehan, O.; Honari, B.; et al. Acute Hospital, Community, and Indirect Costs of Stroke Associated with Atrial Fibrillation: Population-based Study. Stroke 2014, 45, 3670–3674. [Google Scholar] [CrossRef] [Green Version]
- Dilokthornsakul, P.; Nathisuwan, S.; Krittayaphong, R.; Chutinet, A.; Permsuwan, U. Cost-Effectiveness Analysis of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Thai Patients with Non-Valvular Atrial Fibrillation. Heart Lung Circ. 2019. [Google Scholar] [CrossRef]
- Morgan, C.L.; McEwan, P.; Tukiendorf, A.; Robinson, P.A.; Clemens, A.; Plumb, J.M. Warfarin Treatment in Patients with Atrial Fibrillation: Observing Outcomes Associated with Varying Levels of INR Control. Thromb. Res. 2009, 124, 37–41. [Google Scholar] [CrossRef]
- Ho, C.W.; Ho, M.H.; Chan, P.H.; Hai, J.J.; Cheung, E.; Yeung, C.Y.; Lau, K.K.; Chan, K.H.; Lau, C.P.; Lip, G.Y.; et al. Ischemic Stroke and Intracranial Hemorrhage with Aspirin, Dabigatran, and Warfarin: Impact of Quality of Anticoagulation Control. Stroke 2015, 46, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Oldgren, J.; Healey, J.S.; Ezekowitz, M.; Commerford, P.; Avezum, A.; Pais, P.; Zhu, J.; Jansky, P.; Sigamani, A.; Morillo, C.A.; et al. Variations in Cause and Management of Atrial Fibrillation in a Prospective Registry of 15,400 Emergency Department Patients in 46 Countries: The RE-LY Atrial Fibrillation Registry. Circulation 2014, 129, 1568–1576. [Google Scholar] [CrossRef] [Green Version]
- Reiffel, J.A. Time to Revisit the Time in the Therapeutic Range. J. Atr. Fibrillation 2017, 9, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aribou, Z.M.; Mondry, A. Anticoagulation Needs in Asians with Atrial Fibrillation: A Mythbuster. Ann. Acad. Med. Singapore 2014, 43, 275–278. [Google Scholar] [PubMed]
- Rao, M.P.; Vinereanu, D.; Wojdyla, D.M.; Alexander, J.H.; Atar, D.; Hylek, E.M.; Hanna, M.; Wallentin, L.; Lopes, R.D.; Gersh, B.J.; et al. Clinical Outcomes and History of Fall in Patients with Atrial Fibrillation Treated with Oral Anticoagulation: Insights From the ARISTOTLE Trial. Am. J. Med. 2018, 131, 269–275.e2. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Cheng, G.; Chan, T.Y. Use of Herbal Medicines by Patients Receiving Warfarin. Drug. Saf. 2003, 26, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.; Gueorguieva, I.; Wise, S.; Soon, D.; Aarons, L. Ethnic Differences in the Population Pharmacokinetics and Pharmacodynamics of Warfarin. J. Pharm. Pharm. 2010, 37, 3–24. [Google Scholar] [CrossRef]
- Chen, K.P.; Huang, C.X.; Huang, D.J.; Cao, K.J.; Ma, C.S.; Wang, F.Z.; Zhang, S. Anticoagulation Therapy in Chinese Patients with Non-valvular Atrial Fibrillation: A Prospective, Multi-center, Randomized, Controlled Study. Chin. Med. J. (Engl.) 2012, 125, 4355–4360. [Google Scholar]
- Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). Circ. J. 2014, 78, 1997–2021. [CrossRef] [Green Version]
- Pandey, A.K.; Xu, K.; Zhang, L.; Gupta, S.; Eikelboom, J.; Cook, O.; McIntyre, W.F.; Lopes, R.D.; Crowther, M.; Belley-Cote, E.P.; et al. Lower Versus Standard INR Targets in Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Thromb. Haemost. 2020, 120, 484–494. [Google Scholar] [CrossRef]
- Gateman, D.; Trojnar, M.E.; Agarwal, G. Time in therapeutic range: Warfarin Anticoagulation for Atrial Fibrillation in a Community-based Practice. Can. Fam. Physician 2017, 63, e425–e431. [Google Scholar]
- Ogawa, H.; Hamatani, Y.; Doi, K.; Tezuka, Y.; An, Y.; Ishii, M.; Iguchi, M.; Masunaga, N.; Esato, M.; Chun, Y.H.; et al. Sex-Related Differences in the Clinical Events of Patients With Atrial Fibrillation- The Fushimi AF Registry. Circ. J. 2017, 81, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Hizbullah; Ahmed, S.; Noor Mumtaz, M.; Zulfiqar, Z.; Amir Hamza, S.; Siraj, S.; Jelani, M.; Imran, I.; Khan, A. Genetic Variations in Drug-metabolizing Enzyme CYP2C9 among Major Ethnic Groups of Pakistani Population. Gene 2020, 746, 144659. [Google Scholar] [CrossRef]
- Tzourio, C.; Arima, H.; Harrap, S.; Anderson, C.; Godin, O.; Woodward, M.; Neal, B.; Bousser, M.G.; Chalmers, J.; Cambien, F.; et al. APOE Genotype, Ethnicity, and the Risk of Cerebral Hemorrhage. Neurology 2008, 70, 1322–1328. [Google Scholar] [CrossRef]
- Camm, A.J.; Accetta, G.; Ambrosio, G.; Atar, D.; Bassand, J.P.; Berge, E.; Cools, F.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; et al. Evolving Antithrombotic Treatment Patterns for Patients with Newly Diagnosed Atrial Fibrillation. Heart 2017, 103, 307–314. [Google Scholar] [CrossRef]
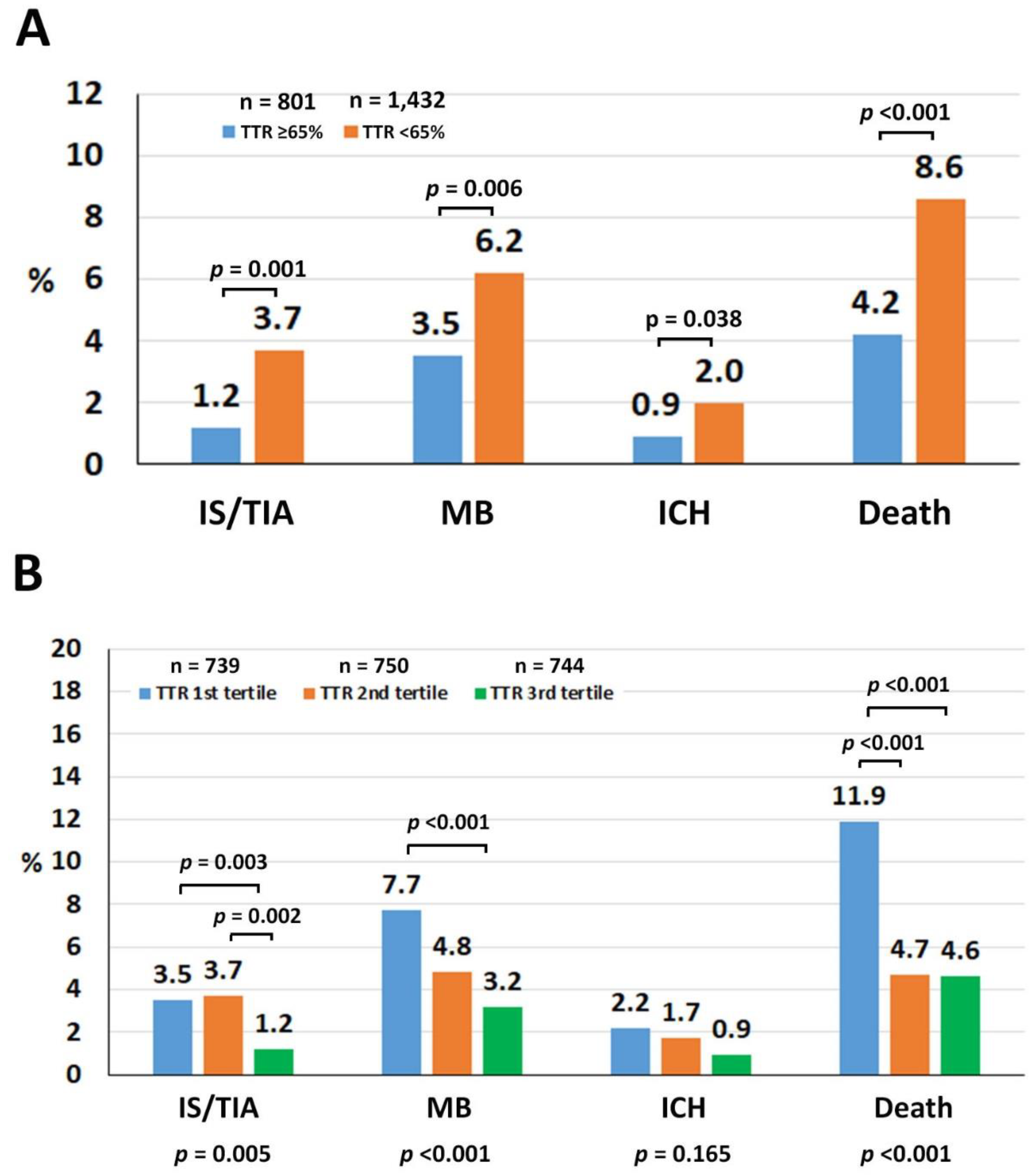



| Variables | All (n = 2233) | TTR ≥ 65% (n = 801) | TTR < 65% (n = 1432) | p |
|---|---|---|---|---|
| Age (years) | 68.44 ± 10.579 | 68.22 ± 10.626 | 68.56 ± 10.554 | 0.469 |
| Female gender | 980 (43.9%) | 351 (43.8%) | 629 (43.9%) | 0.962 |
| Time after AF diagnosis (years) | 3.56 ± 4.412 | 3.82 ± 4.700 | 3.42 ± 4.238 | 0.039 |
| Atrial fibrillation | 0.085 | |||
| - Paroxysmal | 631 (28.3%) | 249 (31.1%) | 382 (26.7%) | |
| - Persistent | 421 (18.9%) | 145 (18.1%) | 276 (19.3%) | |
| - Permanent | 1181 (52.9%) | 407 (50.8%) | 774 (54.1%) | |
| Symptomatic atrial fibrillation | 1720 (77.0%) | 592 (73.9%) | 1128 (78.8%) | 0.009 |
| History of heart failure | 628 (28.1%) | 192 (24.0%) | 436 (30.4%) | 0.001 |
| History of coronary artery disease | 356 (15.9%) | 124 (15.5%) | 232 (16.2%) | 0.656 |
| Having CIED | 216 (9.7%) | 102 (12.7%) | 114 (8.0%) | <0.001 |
| History of ischemic stroke/TIA | 485 (21.7%) | 193 (24.1%) | 292 (20.4%) | 0.042 |
| Hypertension | 1641 (73.5%) | 574 (71.7%) | 1067 (74.5%) | 0.143 |
| Diabetes mellitus | 610 (27.3%) | 184 (23.0%) | 426 (29.7%) | 0.001 |
| Smoking | 414 (18.5%) | 152 (19.0%) | 262 (18.3%) | 0.692 |
| Dyslipidemia | 1320 (59.1%) | 485 (60.5%) | 835 (58.3%) | 0.302 |
| Renal replacement therapy | 20 (0.9%) | 5 (0.6%) | 15 (1.0%) | 0.309 |
| Dementia | 20 (0.9%) | 8 (1.0%) | 12 (0.8%) | 0.699 |
| History of bleeding | 241 (10.8%) | 73 (9.1%) | 168 (11.7%) | 0.056 |
| CHA2DS2-VASc score | 3.35 ± 1.563 | 3.27 ± 1.612 | 3.39 ± 1.533 | 0.075 |
| CHA2DS2-VASc score ≥2 | 1993 (89.3%) | 704 (87.9%) | 1289 (90.0%) | 0.120 |
| HAS-BLED score | 1.60 ± 1.011 | 1.50 ± 0.962 | 1.65 ± 1.034 | 0.001 |
| HAS-BLED score ≥3 | 385 (17.2%) | 113 (14.1%) | 272 (19.0%) | 0.003 |
| Antiplatelet | 277 (12.4%) | 86 (10.7%) | 191 (13.3%) | 0.074 |
| HR (for TTR < 65% Alone) or Adjusted HR (for Model 1–3) (95% CI) | p-Value | |
|---|---|---|
| Ischemic stroke/TIA | ||
| TTR < 65% alone | 3.081 (1.567–6.055) | 0.001 |
| TTR < 65%, model 1 | 3.073 (1.563–6.040) | 0.001 |
| TTR < 65%, model 2 | 3.073 (1.563–6.040) | 0.001 |
| TTR < 65%, model 3 | 3.073 (1.563–6.040) | 0.001 |
| Major bleeding | ||
| TTR < 65% alone | 1.913 (1.244–2.944) | 0.003 |
| TTR < 65%, model 1 | 1.921 (1.248–2.955) | 0.003 |
| TTR < 65%, model 2 | 1.897 (1.232–2.919) | 0.004 |
| TTR < 65%, model 3 | 1.897 (1.232–2.919) | 0.004 |
| ICH | ||
| TTR < 65% alone | 2.380 (1.043–5.434) | 0.039 |
| TTR < 65%, model 1 | 2.377 (1.041–5.426) | 0.040 |
| TTR < 65%, model 2 | 2.335 (1.022–5.338) | 0.044 |
| TTR < 65%, model 3 | 2.335 (1.022–5.338) | 0.044 |
| Death | ||
| TTR < 65% alone | 2.150 (1.464–3.157) | <0.001 |
| TTR < 65%, model 1 | 2.144 (1.460–3.148) | <0.001 |
| TTR < 65%, model 2 | 2.105 (1.430–3.098) | <0.001 |
| TTR < 65%, model 3 | 2.105 (1.430–3.098) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krittayaphong, R.; Chantrarat, T.; Rojjarekampai, R.; Jittham, P.; Sairat, P.; Lip, G.Y.H. Poor Time in Therapeutic Range Control is Associated with Adverse Clinical Outcomes in Patients with Non-Valvular Atrial Fibrillation: A Report from the Nationwide COOL-AF Registry. J. Clin. Med. 2020, 9, 1698. https://doi.org/10.3390/jcm9061698
Krittayaphong R, Chantrarat T, Rojjarekampai R, Jittham P, Sairat P, Lip GYH. Poor Time in Therapeutic Range Control is Associated with Adverse Clinical Outcomes in Patients with Non-Valvular Atrial Fibrillation: A Report from the Nationwide COOL-AF Registry. Journal of Clinical Medicine. 2020; 9(6):1698. https://doi.org/10.3390/jcm9061698
Chicago/Turabian StyleKrittayaphong, Rungroj, Thoranis Chantrarat, Roj Rojjarekampai, Pongpun Jittham, Poom Sairat, and Gregory Y.H. Lip. 2020. "Poor Time in Therapeutic Range Control is Associated with Adverse Clinical Outcomes in Patients with Non-Valvular Atrial Fibrillation: A Report from the Nationwide COOL-AF Registry" Journal of Clinical Medicine 9, no. 6: 1698. https://doi.org/10.3390/jcm9061698






