Neural Correlates of Gender Face Perception in Transgender People
Abstract
1. Introduction
2. Methods
2.1. Participants
- Age older than 18 years;
- The use at any point in life of hormonal therapy.
- Gender affirming surgery performed.
- Illiteracy.
- Mental retardation.
- Disorder of sexual development (DSD).
- Severe and unstable psychiatric conditions (e.g., psychotic disorders, depressive disorder with suicidal ideation) assessed by mental health professionals experienced in GD during consultations and by assessing DSM 5 criteria [2]
- The use in the previous six months of any hormonal treatment and of any psychiatric medication;
- Illiteracy;
- Mental retardation;
- DSD;
- Severe and unstable psychiatric conditions (e.g., psychotic disorders, depressive disorder with suicidal ideation);
- Pregnancy or current lactation.
2.2. Sociodemographic and Psychometric Evaluations
2.3. Rationale and Description of Experimental Paradigm
2.4. MRI Data Acquisitions.
2.5. Data Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. fMRI
3.3. Psychometric Evaluations
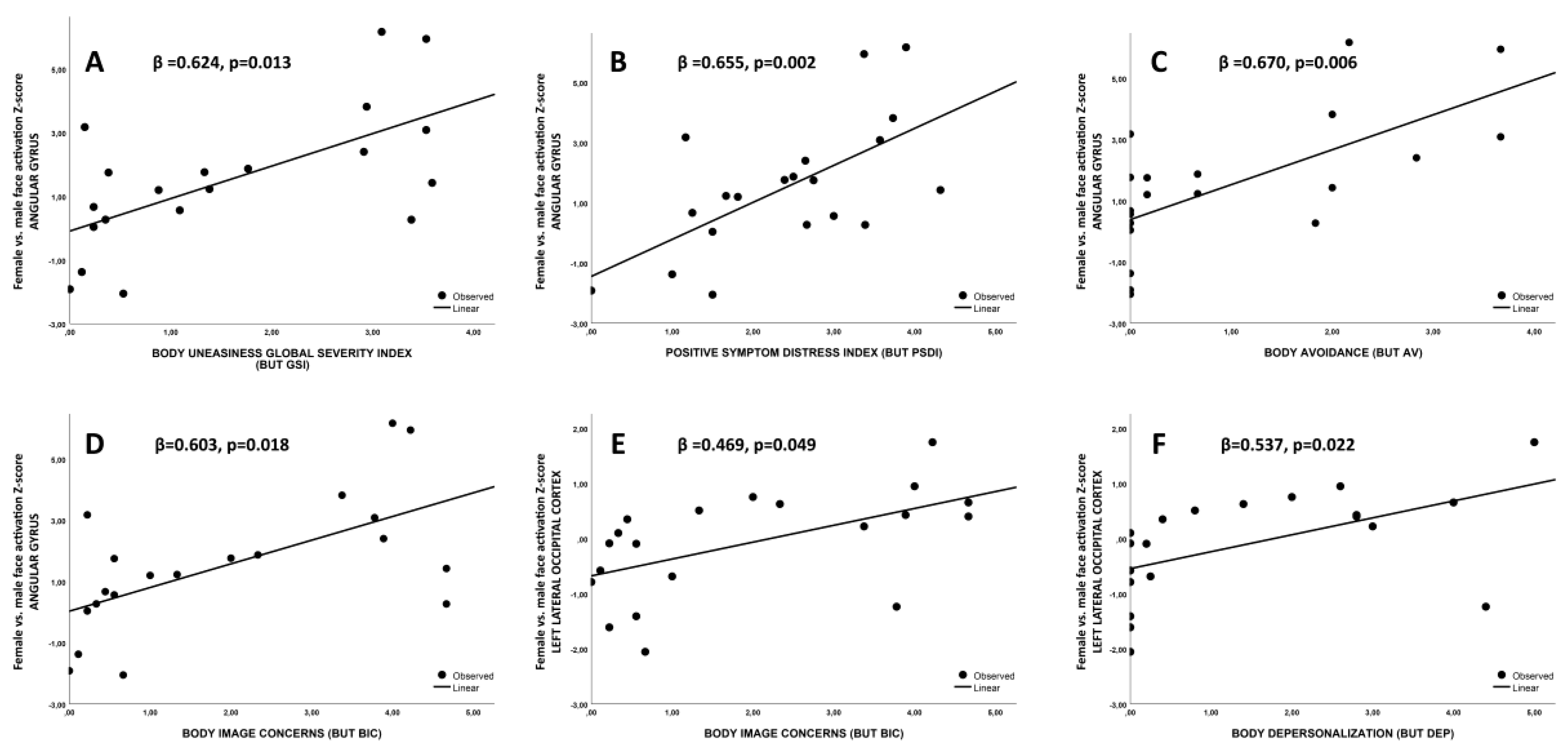
3.4. Association between fMRI Imaging Results and Body Uneasiness Levels
4. Discussion
4.1. Association between Precuneus Activation and Psychometric Evaluations
4.2. Associations between Posterior Cingulate Gyrus and Angular Gyrus Activations and Psychometric Evaluations
4.3. Associations between Lateral Occipital Cortices Activations and Psychometric Evaluations
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. International Classification of Diseases and Related Health Problems, 11th Revision (ICD-11); ICD-11 Geneva; World Health Organ: Geneva, Switzerland, 2019. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Bao, A.-M.; Swaab, D.F. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocr. 2011, 32, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ristori, J.; Cocchetti, C.; Romani, A.; Mazzoli, F.; Vignozzi, L.; Maggi, M.; Fisher, A.D. Brain Sex Differences Related to Gender Identity Development: Genes or Hormones? Int. J. Mol. Sci. 2020, 21, 2123. [Google Scholar] [CrossRef] [PubMed]
- Kreukels, B.P.; Guillamón, A. Neuroimaging studies in people with gender incongruence. Int. Rev. Psychiatry 2016, 28, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Bendahan, C.C.; Van De Beek, C.; Berenbaum, S.A. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci. Biobehav. Rev. 2005, 29, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Zubiaurre-Elorza, L.; Junque, C.; Gil, E.G.; Segovia, S.; Carrillo, B.; Rametti, G.; Guillamon, A. Cortical Thickness in Untreated Transsexuals. Cereb. Cortex 2012, 23, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.S.; Hahn, A.; Kaufmann, U.; Küblböck, M.; Hummer, A.; Ganger, S.; Seiger, R.; Winkler, D.; Swaab, D.F.; Windischberger, C.; et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J. Neurosci. 2014, 34, 15466–15475. [Google Scholar] [CrossRef]
- Luders, E.; Sánchez, F.; Gaser, C.; Toga, A.W.; Narr, K.L.; Hamilton, L.S.; Vilain, E. Regional gray matter variation in male-to-female transsexualism. NeuroImage 2009, 46, 904–907. [Google Scholar] [CrossRef]
- Rametti, G.; Carrillo, B.; Gómez-Gil, G.; Junque, C.; Segovia, S.; Gomez, A.; Guillamon, A. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J. Psychiatr. Res. 2011, 45, 199–204. [Google Scholar] [CrossRef]
- Rametti, G.; Carrillo, B.; Gómez-Gil, E.; Junque, C.; Segovia, S.; Gomez, A.; Guillamon, A. The white matter microstructure in male to female transsexuals before cross-sex hormonal treatment. A DTI study. J. Psychiatr. Res. 2011, 45, 949–954. [Google Scholar] [CrossRef]
- Hoekzema, E.; Schagen, S.E.; Kreukels, B.P.; Veltman, D.J.; Cohen-Kettenis, P.T.; De Waal, H.D.-V.; Bakker, J. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology 2015, 55, 59–71. [Google Scholar] [CrossRef]
- Simon, L.; Kozák, L.R.; Simon, V.; Czobor, P.; Unoka, Z.; Szabó, A.; Csukly, G. Regional Grey Matter Structure Differences between Transsexuals and Healthy Controls—A Voxel Based Morphometry Study. PLoS ONE 2013, 8, e83947. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Manzouri, A.H.; Dhejne, C.; Bergström, K.; Arver, S.; Feusner, J.D.; Savic, I. Testosterone Effects on the Brain in Transgender Men. Cereb. Cortex 2017, 28, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Manzouri, A.; Kosidou, K.; Savić, I. Anatomical and Functional Findings in Female-to-Male Transsexuals: Testing a New Hypothesis. Cereb. Cortex 2015, 27, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, G.; Souza-Duran, F.L.; Chaim-Avancini, T.M.; Serpa, M.H.; Cavallet, M.; Pereira, C.M.A.; Santos, P.P.; Squarzoni, P.; Da Costa, N.A.; Busatto, G.F.; et al. Grey and white matter volumes either in treatment-naïve or hormone-treated transgender women: A voxel-based morphometry study. Sci. Rep. 2018, 8, 736. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Landré, L.; T’Sjoen, G.; Wierckx, K. A Structural Magnetic Resonance Imaging Study in Transgender Persons on Cross-Sex Hormone Therapy. Neuroendocrinology 2016, 105, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chöning, S.; Engelien, A.; Bauer, C.; Kugel, H.; Kersting, A.; Roestel, C.; Zwitserlood, P.; Pyka, M.; Dannlowski, U.; Lehmann, W.; et al. Neuroimaging differences in spatial cognition between men and male-to-female transsexuals before and during hormone therapy. J. Sex. Med. 2010, 7, 1858–1867. [Google Scholar] [CrossRef]
- Soleman, R.S.; Schagen, S.E.; Veltman, D.J.; Kreukels, B.P.C.; Cohen-Kettenis, P.; Lambalk, C.B.; Wouters, F.; De Waal, H.A.D.-V. Sex Differences in Verbal Fluency during Adolescence: A Functional Magnetic Resonance Imaging Study in Gender Dysphoric and Control Boys and Girls. J. Sex. Med. 2013, 10, 1969–1977. [Google Scholar] [CrossRef]
- Burke, S.M.; Kreukels, B.P.C.; Cohen-Kettenis, P.T.; Veltman, D.J.; Klink, D.T.; Bakker, J. Male-typical visuospatial functioning in gynephilic girls with gender dysphoria—organizational and activational effects of testosterone. J. Psychiatry Neurosci. 2016, 41, 395–404. [Google Scholar] [CrossRef]
- Clemens, B.; Junger, J.; Pauly, K.; Neulen, J.; Neuschaefer-Rube, C.; Frölich, D.; Mingoia, G.; Derntl, B.; Habel, U. Male-to-female gender dysphoria: Gender-specific differences in resting-state networks. Brain Behav. 2017, 7, e00691. [Google Scholar] [CrossRef]
- Mueller, S.C.; Wierckx, K.; Jackson, K.; T’Sjoen, G. Circulating androgens correlate with resting-state MRI in transgender men. Psychoneuroendocrinology 2016, 73, 91–98. [Google Scholar] [CrossRef]
- Clemens, B.; Derntl, B.; Smith, E.; Junger, J.; Neulen, J.; Mingoia, G.; Schneider, F.; Abel, T.; Bzdok, D.; Habel, U. Predictive Pattern Classification Can Distinguish Gender Identity Subtypes from Behavior and Brain Imaging. Cereb. Cortex 2020, 30, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Feusner, J.D.; Dervisic, J.; Kosidou, K.; Dhejne, C.; Bookheimer, S.Y.; Savic, I. Female-to-Male Transsexual Individuals Demonstrate Different Own Body Identification. Arch. Sex. Behav. 2015, 45, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Manzouri, A.; Savic, I. Possible Neurobiological Underpinnings of Homosexuality and Gender Dysphoria. Cereb. Cortex 2018, 29, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Majid, D.S.A.; Burke, S.M.; Manzouri, A.; Moody, T.D.; Dhejne, C.; Feusner, J.D.; Savic, I. Neural Systems for Own-body Processing Align with Gender Identity Rather Than Birth-assigned Sex. Cereb. Cortex 2019, 30, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Junque, C.; Gómez-Gil, E.; Abos, A.; Mueller, S.C.; Guillamon, A. Brain network interactions in transgender individuals with gender incongruence. NeuroImage 2020, 211, 116613. [Google Scholar] [CrossRef]
- Feusner, J.; Lidström, A.; Moody, T.D.; Dhejne, C.; Bookheimer, S.Y.; Savic, I. Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging Behav. 2016, 11, 964–976. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Craig, A.D. Interception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Uddin, L.Q. Idiosyncratic connectivity in autism: Developmental and anatomical considerations. Trends Neurosci. 2015, 38, 261–263. [Google Scholar] [CrossRef]
- T’Sjoen, G.; Arcelus, J.; De Vries, A.L.; Fisher, A.D.; Nieder, T.O.; Özer, M.; Motmans, J. European Society for Sexual Medicine Position Statement “Assessment and Hormonal Management in Adolescent and Adult Trans People, With Attention for Sexual Function and Satisfaction”. J. Sex. Med. 2020, 17, 570–584. [Google Scholar] [CrossRef]
- Cuzzolaro, M.; Vetrone, G.; Marano, G.; Garfinkel, P. The Body Uneasiness Test (BUT): Development and validation of a new body image assessment scale. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2006, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Hartmann, D.; Mognetti, M.; Prunas, A. La valutazione della disforia di genere: La versione italiana del gender identity/gender dysphoria questionnaire. Riv. DI Sessuol. Clin. 2013, 35–51. [Google Scholar] [CrossRef]
- Prunas, A.; Sarno, I.; Preti, E.; Madeddu, F.; Perugini, M. Psychometric properties of the Italian version of the SCL-90-R: A study on a large community sample. Eur. Psychiatry 2011, 27, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.D.; Castellini, G.; Casale, H.; Fanni, E.; Bandini, E.; Campone, B.; Ferruccio, N.; Maseroli, E.; Boddi, V.; Dèttore, D.; et al. Hypersexuality, Paraphilic Behaviors, and Gender Dysphoria in Individuals with Klinefelter’s Syndrome. J. Sex. Med. 2015, 12, 2413–2424. [Google Scholar] [CrossRef]
- Bandini, E.; Fisher, A.D.; Castellini, G.; Sauro, C.L.; Lelli, L.; Meriggiola, M.C.; Casale, H.; Benni, L.; Ferruccio, N.; Faravelli, C.; et al. Gender Identity Disorder and Eating Disorders: Similarities and Differences in Terms of Body Uneasiness. J. Sex. Med. 2013, 10, 1012–1023. [Google Scholar] [CrossRef]
- Fisher, A.D.; Castellini, G.; Bandini, E.; Casale, H.; Fanni, E.; Benni, L.; Ferruccio, N.; Meriggiola, M.C.; Manieri, C.; Gualerzi, A.; et al. Cross-Sex Hormonal Treatment and Body Uneasiness in Individuals with Gender Dysphoria. J. Sex. Med. 2014, 11, 709–719. [Google Scholar] [CrossRef]
- Fisher, A.D.; Castellini, G.; Ristori, J.; Casale, H.; Cassioli, E.; Sensi, C.; Fanni, E.; Amato, A.M.L.; Bettini, E.; Mosconi, M.; et al. Cross-Sex Hormone Treatment and Psychobiological Changes in Transsexual Persons: Two-Year Follow-Up Data. J. Clin. Endocrinol. Metab. 2016, 101, 4260–4269. [Google Scholar] [CrossRef]
- Lundqvist, D.; Flykt, A.; Ohman, A. Karolinska directed emotional faces database of standardized facial images. Psychol. Sect. Dep. Clin. Neurosci. Karolinska Hosp. 1998, 76, S-171. [Google Scholar]
- Yang, H.; Huang, D.; Wang, Y.; Jain, A.K. Learning Face Age Progression: A Pyramid Architecture of GANs. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 31–39. [Google Scholar]
- Esfahani, S.N.; Latifi, S. Image Generation with Gans-based Techniques: A Survey. Int. J. Comput. Sci. Inf. Technol. 2019, 11, 33–50. [Google Scholar] [CrossRef]
- Armann, R.G.M.; Bülthoff, I. Male and female faces are only perceived categorically when linked to familiar identities–And when in doubt, he is a male. Vis. Res. 2012, 63, 69–80. [Google Scholar] [CrossRef]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.; Paus, T.; Caspers, S.; Grosbras, M.-H.; Evans, A.C.; Zilles, K.; Amunts, K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 2007, 36, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pinsk, M.A.; Arcaro, M.; Weiner, K.S.; Kalkus, J.F.; Inati, S.J.; Gross, C.G.; Kastner, S. Neural representations of faces and body parts in macaque and human cortex: A comparative FMRI study. J. Neurophysiol. 2009, 101, 2581–2600. [Google Scholar] [CrossRef]
- Weiner, K.S.; Grill-Spector, K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. NeuroImage 2010, 52, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.; Reddish, M.; Bellgowan, P.S.F.; Martin, A. The Selectivity and Functional Connectivity of the Anterior Temporal Lobes. Cereb. Cortex 2009, 20, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Knierim, K.; Ludlow, D.H.; Hanelin, J.; Ramachandran, T.; Glover, G.; Mackey, S.C. Reflecting upon Feelings: An fMRI Study of Neural Systems Supporting the Attribution of Emotion to Self and Other. J. Cogn. Neurosci. 2004, 16, 1746–1772. [Google Scholar] [CrossRef]
- Vocks, S.; Stahn, C.; Loenser, K.; Legenbauer, T. Eating and Body Image Disturbances in Male-to-Female and Female-to-Male Transsexuals. Arch. Sex. Behav. 2008, 38, 364–377. [Google Scholar] [CrossRef]
- Kruse, B.; Bogler, C.; Haynes, J.-D.; Schütz-Bosbach, S. Am I seeing myself, my friend or a stranger? The role of personal familiarity in visual distinction of body identities in the human brain. Cortex 2016, 83, 86–100. [Google Scholar] [CrossRef]
- Joassin, F.; Pesenti, M.; Maurage, P.; Verreckt, E.; Bruyer, R.; Campanella, S. Cross-modal interactions between human faces and voices involved in person recognition. Cortex 2011, 47, 367–376. [Google Scholar] [CrossRef]
- Hölig, C.; Föcker, J.; Best, A.; Röder, B.; Büchel, C. Activation in the angular gyrus and in the pSTS is modulated by face primes during voice recognition. Hum. Brain Mapp. 2017, 38, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kuhl, B.A. Reconstructing Perceived and Retrieved Faces from Activity Patterns in Lateral Parietal Cortex. J. Neurosci. 2016, 36, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Allison, T.; Puce, A.; McCarthy, G. Social perception from visual cues: Role of the STS region. Trends Cogn. Sci. 2000, 4, 267–278. [Google Scholar] [CrossRef]
- Brothers, L. The social brain: A project for integrating primate behaviour and neurophysiology in a new domain. Concepts Neurosci. 1990, 1, 27–51. [Google Scholar]
- Binder, J.R.; Desai, R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011, 15, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.D.; Castellini, G.; Ristori, J.; Casale, H.; Giovanardi, G.; Carone, N.; Fanni, E.; Mosconi, M.; Ciocca, G.; Jannini, E.A.; et al. Who has the worst attitudes toward sexual minorities? Comparison of transphobia and homophobia levels in gender dysphoric individuals, the general population and health care providers. J. Endocrinol. Investig. 2016, 40, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J.; Press, D.Z.; Keenan, J.P.; O’Connor, M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology 2002, 58, 71–78. [Google Scholar] [CrossRef]
- Rossion, B.; Caldara, R.; Seghier, M.; Schuller, A.-M.; Lazeyras, F.; Mayer, E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 2003, 126, 2381–2395. [Google Scholar] [CrossRef]
- Steeves, J.K.; Culham, J.C.; Duchaine, B.C.; Pratesi, C.C.; Valyear, K.; Schindler, I.; Humphrey, G.K.; Milner, A.D.; Goodale, M. The fusiform face area is not sufficient for face recognition: Evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia 2006, 44, 594–609. [Google Scholar] [CrossRef]
- Yovel, G.; Kanwisher, N. Face PerceptionDomain Specific, Not Process Specific. Neuron 2004, 44, 889–898. [Google Scholar] [CrossRef]
- Rotshtein, P.; Henson, R.N.A.; Treves, A.; Driver, J.; Dolan, R.J. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat. Neurosci. 2004, 8, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, D.; Walsh, V.; Yovel, G.; Duchaine, B. TMS Evidence for the Involvement of the Right Occipital Face Area in Early Face Processing. Curr. Boil. 2007, 17, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Safron, A.; Klimaj, V.; Sylva, D.; Rosenthal, A.M.; Li, M.; Walter, M.; Bailey, J.M. Neural Correlates of Sexual Orientation in Heterosexual, Bisexual, and Homosexual Women. Sci. Rep. 2018, 8, 673. [Google Scholar] [CrossRef]
- Savic, I.; Lindström, P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc. Natl. Acad. Sci. USA 2008, 105, 9403–9408. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Laureys, S. Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005, 150, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Lenge, M.; Bartolini, E.; Bianchi, A.; Agovi, H.; Mugnai, F.; Guerrini, R.; Giordano, F.; Viggiano, M.P.; Mascalchi, M. Left inferior frontal cortex can compensate the inhibitory functions of right inferior frontal cortex and pre-supplementary motor area. J. Neuropsychol. 2018, 13, 503–508. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Margulies, D.S.; Kelly, C.; Uddin, L.Q.; Ghaffari, M.; Kirsch, A.; Shaw, D.; Shehzad, Z.; Di Martino, A.; Biswal, B.; et al. Cingulate-Precuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry. 2008, 63, 332–337. [Google Scholar] [CrossRef]





| Cismen (n = 10) | Ciswomen (n = 10) | Transmen (n = 10) | Transwomen (n = 10) | F | p | |
|---|---|---|---|---|---|---|
| Height (cm) | 179 ± 0.8 | 163 ± 0.08 | 161 ± 0.04 ** | 1.70 ± 0.93 | 10.91 | <0.0001 |
| Weight (kg) | 72.30 ± 10.50¥ | 56.80 ± 9.04 | 56.83 ± 5.67 | 56.70 ± 10.03 | 9.96 | <0.0001 |
| Body mass index (kg/m2) | 22.48 ± 1.98 | 21.28 ± 2.06 | 21.78 ± 1.94 | 19.65 ± 2.09∞∞ | 3.21 | 0.035 |
| Educational level (years of school) | 15.40 ± 2.37 | 16.30 ± 2.63 | 11.50 ± 3.84££ | 13.70 ± 3.71 | 4.35 | 0.011 |
| VAS for Sexual orientation | 89.26 ± 31.45 | 94.94 ± 9.75 | 68.17 ± 41.92 | 71.41 ± 28.16 | 1.86 | 0.155 |
| GIDYQ-AA | 4.74 ± 0.11 | 4.80 ± 0.14 | 2.35 ± 0.24∞ | 2.20 ± 0.24∞ | 565.58 | <0.0001 |
| GD onset (years) | – | – | 11.40 ± 6.09 | 9.50 ± 4.58 | 0.62 | 0.44 |
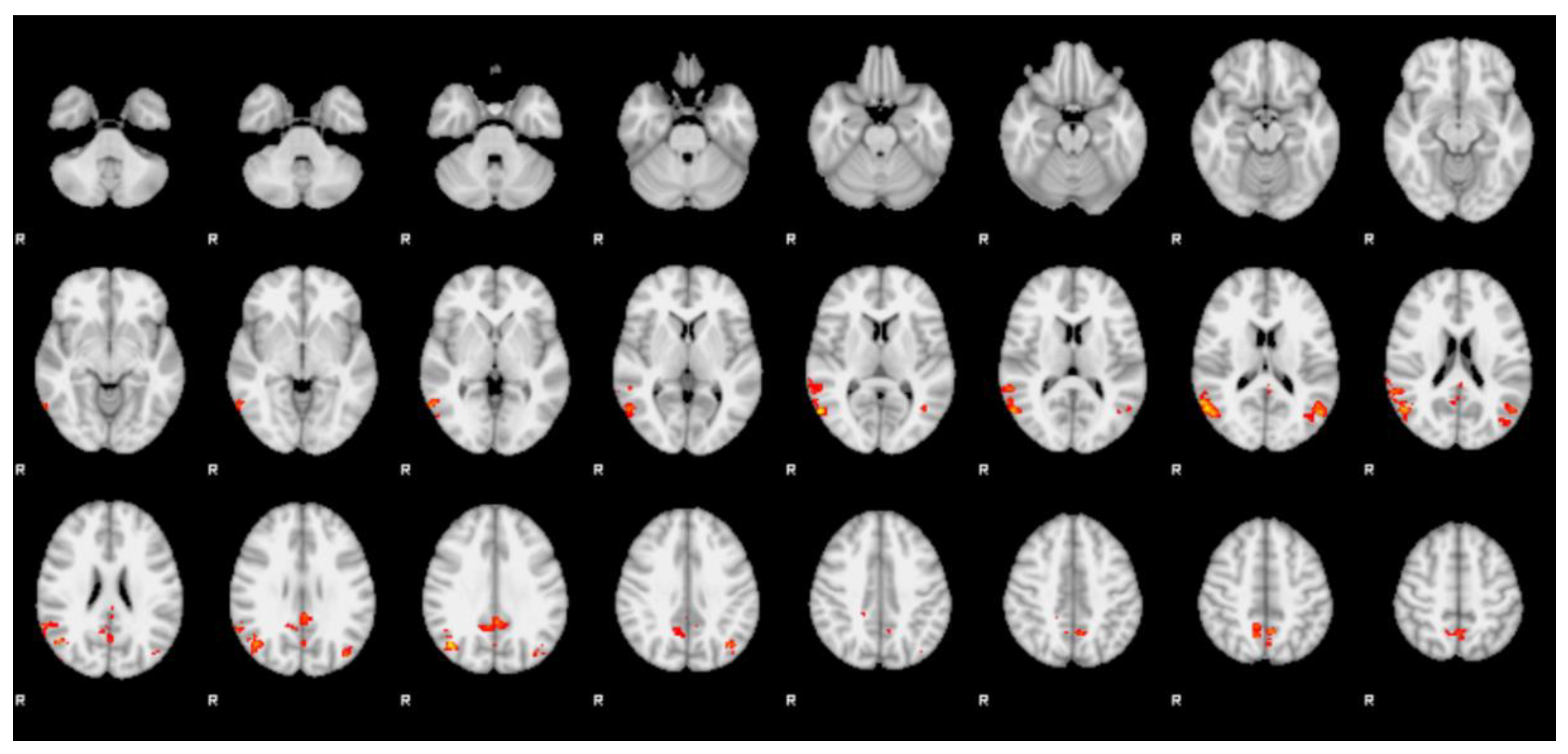
| Cluster | Z-stat | x (mm) | y (mm) | z (mm) | Region (Harvard) |
|---|---|---|---|---|---|
| 1 | 3.01 | −14 | −54 | 26 | left precuneus cortex |
| 3.01 | 0 | −56 | 14 | left precuneus cortex | |
| 3 | −6 | −56 | 16 | left precuneus cortex | |
| 2.89 | −2 | −60 | 16 | left precuneus cortex | |
| 2.82 | −2 | −62 | 8 | left precuneus cortex | |
| 2.74 | 4 | −56 | 22 | right precuneus cortex |
| Cluster | Z-stat | x (mm) | y (mm) | z (mm) | Region (Harvard) |
|---|---|---|---|---|---|
| 1 | 3.99 | 58 | −66 | 8 | right lateral occipital cortex |
| 3.9 | 44 | −68 | 30 | right lateral occipital cortex | |
| 3.71 | 56 | −62 | 16 | right lateral occipital cortex | |
| 3.64 | 60 | −56 | 16 | right angular gyrus | |
| 3.56 | 48 | −64 | 22 | right lateral occipital cortex | |
| 3.52 | 50 | −68 | 16 | right lateral occipital cortex | |
| 2 | 3.44 | −2 | −46 | 30 | left cingulate gyrus, posterior division |
| 3.06 | −2 | −40 | 28 | left cingulate gyrus, posterior division | |
| 2.83 | 6 | −52 | 32 | right cingulate gyrus, posterior division | |
| 2.82 | 12 | −54 | 34 | right precuneus cortex | |
| 2.78 | 0 | −66 | 26 | left precuneus cortex | |
| 2.75 | 8 | −56 | 24 | right precuneus cortex | |
| 3 | 3.58 | −52 | −66 | 16 | left lateral occipital cortex |
| 3.14 | −40 | −68 | 36 | left lateral occipital cortex | |
| 3.06 | −48 | −58 | 16 | left angular gyrus | |
| 3.02 | −38 | −76 | 30 | left lateral occipital cortex | |
| 2.84 | −42 | −64 | 10 | left lateral occipital cortex (l-OFA) | |
| 2.8 | −40 | −72 | 18 | left lateral occipital cortex (l-OFA) | |
| 4 | 3.38 | −2 | −56 | 50 | left precuneus cortex |
| 3.09 | 10 | −58 | 46 | right precuneus cortex | |
| 3.06 | 10 | −50 | 48 | right precuneus cortex | |
| 3.01 | 12 | −58 | 50 | right precuneus cortex | |
| 2.74 | −12 | −62 | 60 | left lateral occipital cortex | |
| 2.72 | −10 | −68 | 58 | left lateral occipital cortex |
| Cismen (n = 10) | Ciswomen (n = 10) | Transmen (n = 10) | Transwomen (n = 10) | F | p | |
|---|---|---|---|---|---|---|
| SCL-90 R GSI | 0.30 ± 0.21 | 0.62 ± 0.42 | 0.79 ± 0.57 | 0.97 ± 0.68 | 2.16 | 0.16 |
| GIDYQ-AA GLOBAL SCORE | 4.74 ± 0.11 | 4.80 ± 0.14 | 2.35 ± 0.24∞ | 2.20 ± 0.24∞ | 518.82 | <0.0001 |
| GIDYQ-AA SUBJECTIVE INDICATOR | 4.86 ± 0.17 | 4.78 ± 0.17 | 2.03 ± 0.17∞ | 2.05 ± 0.32∞ | 487.58 | <0.0001 |
| GIDYQ-AA SOCIAL INDICATOR | 4.42 ± 0.20 | 4.77 ± 0.30 | 2.92 ± 0.55¥ | 2.46 ± 0.54§ | 82.29 | <0.0001 |
| GIDYQ-AA SOMATIC INDICATOR | 5.00 ± 0.00 | 4.97 ± 0.11 | 1.40 ± 0.54∞ | 1.50 ± 0.95∞ | 141.62 | <0.0001 |
| GIDYQ-AA SOCIO-LEGAL INDICATOR | 5.00 ± 0.00 | 4.95 ± 0.16 | 3.20 ± 0.42∞ | 3.05 ± 0.60∞ | 70.30 | <0.0001 |
| BUT GSI | 0.50 ± 0.47 | 1.17 ± 0.79 | 2.57 ± 0.82 * | 2.65 ± 1.10 * | 14.94 | <0.0001 |
| BUT WP | 0.90 ± 0.78 | 1.96 ± 1.43 | 2.58 ± 1.12 * | 2.60 ± 1.13 * | 3.61 | 0.013 |
| BUT BIC | 0.49 ± 0.42 | 1.34 ± 0.93 | 3.54 ± 0.85 * | 3.35 ± 1.32 * | 23.11 | <0.0001 |
| BUT AV | 0.83 ± 0.21 | 0.23 ± 0.20 | 2.33 ± 0.88 * | 1.90 ± 1.31 * | 23.72 | <0.0001 |
| BUT CSM | 0.67 ± 0.96 | 1.52 ± 1.03 | 1.24 ± 1.08 | 2.27 ± 1.02 § | 4.21 | 0.012 |
| BUT DEP | 0.15 ± 0.27 | 0.30 ± 0.39 | 2.62 ± 1.00 * | 2.82 ± 1.53 * | 24.39 | <0.0001 |
| BUT PSDI | 1.56 ± 0.84 | 1.81 ± 1.08 | 2.90 ± 0.87 * | 3.26 ± 0.65 * | 9.45 | <0.0001 |
| BUT Body Parts | Cismen | Ciswomen | Transmen | Transwomen | F | p |
|---|---|---|---|---|---|---|
| Height | 0.80 ± 1.48 | 1.20 ± 1.62 | 2.70 ± 2.16 | 1.10 ± 1.52 | 2.76 | 0.057 |
| Head shape | 0.00 ± 0.00 | 0.50 ± 0.85 | 0.50 ± 0.71 | 0.90 ± 1.29 | 1.89 | 0.327 |
| Skin | 0.80 ± 1.32 | 1.30 ± 1.42 | 0.60 ± 0.84 | 1.60 ± 1.64 | 1.40 | 0.257 |
| Hair | 0.80 ± 1.57 | 0.70 ± 1.06 | 0.20 ± 0.42 | 1.90 ± 2.18 | 2.45 | 0.064 |
| Face shape | 0.10 ± 0.32 | 0.90 ± 1.29 | 1.30 ± 1.34 | 1.80 ± 1.40 | 2.65 | 0.113 |
| Forehead | 0.60 ± 1.26 | 0.00 ± 0.00 | 0.20 ± 0.42 | 2.20 ± 2.10§ | 4.52 | 0.009 |
| Brows | 0.30 ± 0.95 | 0.50 ± 0.71 | 0.50 ± 0.97 | 2.40 ± 1.78§ | 5.03 | 0.005 |
| Eyes | 0.30 ± 0.95 | 0.50 ± 0.97 | 0.90 ± 1.29 | 1.60 ± 1.65 | 1.47 | 0.24 |
| Nose | 0.50 ± 0.97 | 1.30 ± 1.34 | 1.00 ± 1.15 | 3.50 ± 1.58§ | 5.97 | 0.002 |
| Lips | 0.20 ± 0.63 | 0.20 ± 0.42 | 0.40 ± 1.52 | 1.40 ± 1.45 | 2.71 | 0.060 |
| Mouth | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.40 ± 0.70 | 1.40 ± 1.35§ | 5.15 | 0.005 |
| Teeth | 0.40 ± 0.70 | 1.30 ± 1.49 | 1.00 ± 1.25 | 2.50 ± 2.07 | 2.61 | 0.067 |
| Ears | 0.20 ± 0.63 | 0.40 ± 0.70 | 0.80 ± 1.03 | 1.40 ± 1.84 | 1.49 | 0.235 |
| Neck | 0.00 ± 0.00 | 0.60 ± 1.58 | 0.90 ± 1.45 | 1.78 ± 1.56 | 2.31 | 0.095 |
| Chin | 0.30 ± 0.95 | 0.40 ± 1.26 | 0.70 ± 1.25 | 2.22 ± 1.79§ | 3.66 | 0.022 |
| Moustache | 0.10 ± 0.32 | 1.10 ± 2.8 | 0.11 ± 0.33 | 4.60 ± 1.26§ | 23.99 | <0.0001 |
| Beard | 0.20 ± 0.42 | 0.00 ± 0.00 | 0.22 ± 0.67 | 5.00 ± 0.00§ | 335.78 | <0.0001 |
| Body Hair | 0.80 ± 1.48 | 1.60 ± 2.37 | 0.44 ± 1.33 | 4.80 ± 0.63§ | 12.48 | <0.0001 |
| Shoulders | 0.40 ± 0.70 | 0.10 ± 0.32 | 1.40 ± 1.51 | 2.80 ± 1.87§ | 7.31 | 0.001 |
| Arms | 0.50 ± 0.71 | 0.90 ± 1.52 | 1.50 ± 1.64 | 2.30 ± 1.83§ | 3.26 | 0.033 |
| Hands | 0.00 ± 0.00 | 0.90 ± 1.37 | 0.90 ± 1.37 | 3.20 ± 1.93§ | 8.86 | <0.0001 |
| Chest | 0.50 ± 0.85 | 0.20 ± 0.42 | 2.90 ± 2.13∞ | 2.80 ± 1.93∞ | 9.48 | <0.0001 |
| Breast | 0.00 ± 0.00 | 1.00 ± 1.56 | 4.90 ± 0.32£ | 2.00 ± 2.40∞∞ | 18.24 | <0.0001 |
| Stomach | 0.50 ± 1.27 | 0.10 ± 0.32 | 1.10 ± 0.32 | 1.30 ± 1.64 | 1.94 | 0.141 |
| Belly | 0.80 ± 1.23 | 1.20 ± 1.48 | 2.70 ± 1.77∞ | 2.40 ± 1.90∞ | 3.68 | 0.021 |
| Genitals | 0.20 ± 0.42 | 0.30 ± 1.08 | 4.50 ± 0.85∞ | 4.40 ± 1.35∞ | 49.76 | <0.0001 |
| Buttocks | 0.40 ± 0.84 | 1.00 ± 1.34 | 2.60 ± 1.65∞ | 3.10 ± 1.73∞ | 7.69 | <0.0001 |
| Hips | 0.10 ± 0.32 | 11.10 ± 1.29 | 3.50 ± 1.84££ | 2.40 ± 2.07∞∞ | 13.23 | <0.0001 |
| Thighs | 0.10 ± 0.32¥ | 2.40 ± 2.01 | 3.50 ± 1.58 | 2.40 ± 1.71 | 11.93 | <0.0001 |
| Knees | 0.00 ± 0.00 | 1.50 ± 2.01 | 0.80 ± 0.92 | 2.00 ± 2.05$$ | 4.07 | 0.014 |
| Legs | 0.30 ± 0.95 | 1.60 ± 1.96 | 1.90 ± 1.37 | 2.00 ± 1.76 | 2.54 | 0.073 |
| Ankles | 0.20 ± 0.63 | 1.20 ± 1.87 | 0.80 ± 1.35 | 1.90 ± 1.73§ | 3.19 | 0.036 |
| Feet | 0.20 ± 0.63 | 0.60 ± 0.84 | 1.20 ± 1.40 | 3.10 ± 1.73§ | 7.92 | <0.0001 |
| Smell | 0.20 ± 0.63 | 0.30 ± 0.48 | 0.60 ± 0.43 | 2.70 ± 0.67§ | 9.82 | <0.0001 |
| Body sounds | 0.00 ± 0.000 | 0.30 ± 0.48 | 0.50 ± 0.97 | 2.50 ± 2.07§ | 9.08 | <0.0001 |
| Sweating | 0.70 ± 1.16 | 1.00 ± 1.86 | 1.20 ± 1.40 | 3.10 ± 1.97§ | 4.48 | 0.009 |
| Blushing | 0.20 ± 0.63 | 0.60 ± 0.84 | 1.60 ± 2.01 | 0.80 ± 1.55 | 1.38 | 0.267 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, A.D.; Ristori, J.; Castellini, G.; Cocchetti, C.; Cassioli, E.; Orsolini, S.; Sensi, C.; Romani, A.; Mazzoli, F.; Cipriani, A.; et al. Neural Correlates of Gender Face Perception in Transgender People. J. Clin. Med. 2020, 9, 1731. https://doi.org/10.3390/jcm9061731
Fisher AD, Ristori J, Castellini G, Cocchetti C, Cassioli E, Orsolini S, Sensi C, Romani A, Mazzoli F, Cipriani A, et al. Neural Correlates of Gender Face Perception in Transgender People. Journal of Clinical Medicine. 2020; 9(6):1731. https://doi.org/10.3390/jcm9061731
Chicago/Turabian StyleFisher, Alessandra Daphne, Jiska Ristori, Giovanni Castellini, Carlotta Cocchetti, Emanuele Cassioli, Stefano Orsolini, Carolina Sensi, Alessia Romani, Francesca Mazzoli, Agnese Cipriani, and et al. 2020. "Neural Correlates of Gender Face Perception in Transgender People" Journal of Clinical Medicine 9, no. 6: 1731. https://doi.org/10.3390/jcm9061731
APA StyleFisher, A. D., Ristori, J., Castellini, G., Cocchetti, C., Cassioli, E., Orsolini, S., Sensi, C., Romani, A., Mazzoli, F., Cipriani, A., Ricca, V., Vignozzi, L., Viggiano, M. P., Mascalchi, M., Maggi, M., & Gavazzi, G. (2020). Neural Correlates of Gender Face Perception in Transgender People. Journal of Clinical Medicine, 9(6), 1731. https://doi.org/10.3390/jcm9061731






