Outcome in Patients with Partial and Full-Thickness Cheek Defects following Free Flap Reconstruction—A Multicentric Analysis of 47 Cases
Abstract
1. Introduction
2. Material and Methods
2.1. Study Cohort
2.2. Classification of Cheek Carcinomas
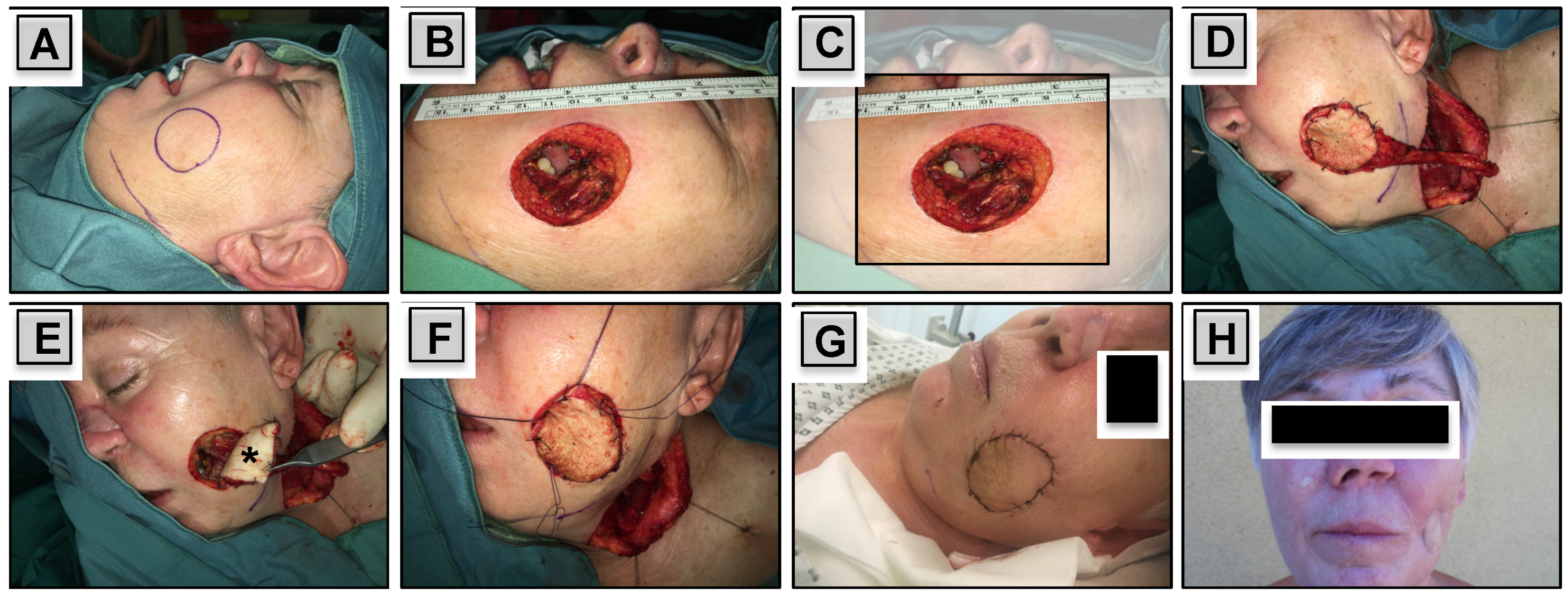
2.3. Free Flap Reconstruction
2.4. Complications and Functional Outcome
2.5. Statistical Methods
3. Results
3.1. Study Cohort
3.2. Tumor Characteristics
3.3. Surgical Resection
3.4. Free Flap Reconstruction
3.5. Complications
3.6. Functional Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mureau, M.A.; Hofer, S.O. Maximizing results in reconstruction of cheek defects. Clin. Plast. Surg. 2009, 36, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Başağaoğlu, B.; Bhadkamkar, M.; Hollier, P.; Reece, E. Approach to Reconstruction of Cheek Defects. Semin. Plast. Surg. 2018, 32, 84–88. [Google Scholar] [CrossRef]
- Ren, Z.H.; Gong, Z.J.; Wu, H.J. Unit resection of buccal squamous cell carcinoma: Description of a new surgical technique. Oncotarget 2016, 8, 52420–52431. [Google Scholar] [CrossRef] [PubMed]
- Rapstine, E.D.; Knaus, W.J., 2nd; Thornton, J.F. Simplifying cheek reconstruction: A review of over 400 cases. Plast. Reconstr. Surg. 2012, 129, 1291–1299. [Google Scholar] [CrossRef]
- Fang, F.M.; Leung, S.W.; Huang, C.C.; Liu, Y.T.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Huang, D.T. Combined-modality therapy for squamous carcinoma of the buccal mucosa: Treatment results and prognostic factors. Head Neck 1997, 19, 506–512. [Google Scholar] [CrossRef]
- Yilmaz, M.; Eskiizmir, G.; Friedman, O. Cutaneous squamous cell carcinoma of the head and neck: Management of the parotid and neck. Facial Plast. Surg. Clin. N. Am. 2012, 20, 473–481. [Google Scholar] [CrossRef]
- Heller, L.; Cole, P.; Kaufman, Y. Cheek reconstruction: Current concepts in managing facial soft tissue loss. Semin. Plast. Surg. 2008, 22, 294–305. [Google Scholar] [CrossRef]
- Gong, Z.J.; Ren, Z.H.; Wang, K.; Tan, H.Y.; Zhang, S.; Wu, H.J. Reconstruction design before tumour resection: A new concept of through-and-through cheek defect reconstruction. Oral. Oncol. 2017, 74, 123–129. [Google Scholar] [CrossRef]
- Millard, D.R. Principlization of Plastic Surgery; Little Brown and Company: Boston, MA, USA, 1986. [Google Scholar]
- Lin, C.S.; Jen, Y.M.; Cheng, M.F.; Lin, Y.S.; Su, W.F.; Hwang, J.M.; Chang, L.P.; Chao, H.L.; Liu, D.W.; Lin, H.Y.; et al. Squamous cell carcinoma of the buccal mucosa: An aggressive cancer requiring multimodality treatment. Head Neck 2006, 28, 150–157. [Google Scholar] [CrossRef]
- Diaz, E.M., Jr.; Holsinger, F.C.; Zuniga, E.R.; Roberts, D.B.; Sorensen, D.M. Squamous cell carcinoma of the buccal mucosa: One institution’s experience with 119 previously untreated patients. Head Neck 2003, 25, 267–273. [Google Scholar] [CrossRef]
- Strome, S.E.; To, W.; Strawderman, M.; Gersten, K.; Devaney, K.O.; Bradford, C.R.; Esclamado, R.M. Squamous cell carcinoma of the buccal mucosa. Otolaryngol. Head Neck Surg. 1999, 120, 375–379. [Google Scholar] [CrossRef]
- Trivedi, N.P.; Kekatpure, V.; Kuriakose, M.A. Radical (compartment) resection for advanced buccal cancer involving masticator space (T4b): Our experience in thirty patients. Clin. Otolaryngol. 2012, 37, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.F.; Kuo, Y.R.; Wei, F.C.; Su, C.Y.; Chien, C.Y. Reconstruction of concomitant lip and cheek through-and-through defects with combined free flap and an advancement flap from the remaining lip. Plast. Reconstr. Surg. 2004, 113, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Jeng, S.F.; Wei, F.C.; Su, C.Y.; Chien, C.Y. Functional reconstruction of complex lip and cheek defect with free composite anterolateral thigh flap and vascularized fascia. Head Neck 2008, 30, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Silva, A.K.; Yu, P.; Skoracki, R.J. A comprehensive algorithm for oncologic maxillary reconstruction. Plast. Reconstr. Surg. 2013, 131, 47–60. [Google Scholar] [CrossRef]
- Jena, A.; Patnayak, R.; Sharan, R.; Reddy, S.K.; Manilal, B.; Rao, L.M. Outcomes of pectoralis major myocutaneous flap in female patients for oral cavity defect reconstruction. J. Oral. Maxillofac. Surg. 2014, 72, 222–231. [Google Scholar] [CrossRef]
- Al Deek, N.F.; Wei, F.C.; Tsao, C.K. Fistulae After Successful Free Tissue Transfer to Head and Neck: Its Prevention and Treatment. Clin. Plast. Surg. 2016, 43, 739–745. [Google Scholar] [CrossRef]
- Lin, C.H.; Chiu, Y.H.; Perng, C.K.; Liao, W.C.; Ma, H. Experience With the Use of Free Fasciocutaneous Flap in Through-and-Through Cheek-Buccal Defect Reconstruction: Surgical Outcome and Quality of Life Analysis. Ann. Plast. Surg. 2016, 76, 74–79. [Google Scholar] [CrossRef]
- Leclère, F.M.; Bosc, R.; Temam, S.; Leymarie, N.; Mirghani, H.; Sarfati, B.; Kolb, F. Reconstruction of large mandibulofacial defects with the composed double skin paddle fibula free flap: A review of 32 procedures. Laryngoscope 2014, 124, 1336–1343. [Google Scholar] [CrossRef]
- Brown, J.S.; Shaw, R.J. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010, 11, 1001–1008. [Google Scholar] [CrossRef]
- Khan, M.N.; Rodriguez, L.G.; Pool, C.D.; Laitman, B.; Hernandez, C.; Erovic, B.M.; Teng, M.S.; Genden, E.M.; Miles, B.A. The versatility of the serratus anterior free flap in head and neck reconstruction. Laryngoscope 2017, 127, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Janik, S.; Pyka, J.; Stanisz, I.; Wachholbinger, T.; Leonhard, M.; Roesner, I.; Denk-Linnert, D.M.; Miles, B.A.; Schneider-Stickler, B.; Erovic, B.M. Use of the myocutaneous serratus anterior free flap for reconstruction after salvage glossectomy. Eur. Arch. Otorhinolaryngol. 2019, 276, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Osborn, H.A.; Goldsmith, T.A.; Varvares, M.A. Assessing functional outcomes in head and neck surgical oncology. Head Neck 2019, 41, 2051–2057. [Google Scholar] [CrossRef] [PubMed]

| Depth of Defect | ||||
|---|---|---|---|---|
| Total | Partial | Full | ||
| Variables | n (%) | n (%) | n (%) | pa |
| Sex | ||||
| Female | 18 (38.3) | 9 (30.0) | 9 (52.9) | |
| Male | 29 (61.7) | 21 (70.0) | 8 (47.1) | 0.211 |
| Tumor Site | ||||
| Buccal Mucosa | 22 (46.8) | 12 (40.0) | 10 (58.8) | |
| Oral Cavity | 16 (34.0) | 12 (40.0) | 4 (23.5) | |
| Parotid Gland | 4 (8.5) | 4 (13.3) | 0 (0) | |
| Nasal Cavity | 3 (6.4) | 1 (3.3) | 2 (11.8) | |
| Skin | 2 (4.3) | 1 (3.3) | 1 (5.9) | 0.255 |
| Histology | ||||
| SCC | 38 (80.9) | 24 (80.0) | 14 (82.4) | |
| ACC | 3 (6.4) | 3 (10.0) | 0 (0) | |
| Sarcoma | 2 (4.3) | 1 (3.3) | 1 (5.9) | |
| Melanoma | 2 (4.3) | 1 (3.3) | 1 (5.9) | |
| MCC | 1 (2.1) | 1 (3.3) | 0 (0) | |
| Malignant Adnexal Tumor | 1 (2.1) | 0 (0) | 1 (5.9) | 0.497 |
| T—Classification | ||||
| T1 | 3 (6.4) | 2 (6.7) | 1 (5.9) | |
| T2 | 11 (23.4) | 8 (26.7) | 3 (17.6) | |
| T3 | 12 (25.5) | 7 (23.3) | 5 (29.4) | |
| T4 | 21 (44.7) | 13 (43.3) | 8 (47.1) | 0.901 |
| N—Classification | ||||
| Nx | 4 (8.5) | 4 (13.3) | 0 (0) | |
| N0 | 30 (63.8) | 17 (56.7) | 13 (76.5) | |
| N1 | 6 (12.8) | 4 (13.3) | 2 (11.8) | |
| N2 | 7 (14.9) | 5 (16.7) | 2 (11.8) | |
| N3 | 0 (0) | 0 (0) | 0 (0) | 0.372 |
| AJCC Tumor Stage | ||||
| Stage I | 3 (6.4) | 2 (6.7) | 1 (5.9) | |
| Stage II | 8 (17.0) | 6 (20.0) | 2 (11.8) | |
| Stage III | 11 (23.4) | 7 (23.3) | 4 (23.5) | |
| Stage IV | 25 (53.2) | 15 (50.0) | 10 (58.8) | 0.896 |
| Aesthetic Zone | ||||
| Zone I | 13 (27.7) | 7 (23.3) | 6 (35.3) | |
| Zone II | 11 (23.4) | 9 (30.0) | 2 (11.8) | |
| Zone III | 23 (48.9) | 14 (46.7) | 9 (52.9) | 0.334 |
| Used Free Flaps | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Total | RFFF | ALT | Scapular/ Parascapular | FFF | Supraclav. Free Flap | SAFF |
| Depth of Defect | |||||||
| Partial | 30 (63.8) | 8 (26.7) | 11 (36.7) | 5 (16.7) | 4 (13.3) | 2 (6.7) | 0 (0) |
| Full | 17 (36.2) | 7 (41.2) | 2 (11.8) | 5 (29.4) | 2 (11.8) | 0 (0) | 1 (5.9) |
| Type of Reconstruction | |||||||
| Cutaneous | 19 (40.4) | 14 (73.7) | 3 (15.8) | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) |
| Myocutaneous | 12 (25.5) | 1 (8.3) | 10 (83.3) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) |
| Osteocutaneous | 16 (34.0) | 0 (0) | 0 (0) | 10 (62.5) | 6 (37.5) | 0 (0) | 0 (0) |
| Aesthetic Zone | |||||||
| Zone I | 13 (27.7) | 4 (30.4) | 4 (30.4) | 4 (30.4) | 0 (0) | 0 (0) | 1 (7.7) |
| Zone II | 11 (23.4) | 1 (9.1) | 4 (36.4) | 3 (27.3) | 3 (27.3) | 0 (0) | 0 (0) |
| Zone III | 23 (48.9) | 10 (43.5) | 5 (21.7) | 3 (13.0) | 3 (13.0) | 2 (8.7) | 0 (0) |
| Total | 47 (100) | 15 (31.9) | 13 (27.7) | 10 (21.3) | 6 (12.8) | 2 (4.3) | 1 (2.1) |
| Depth of Defect | Aesthetic Zones | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Partial | Full | Zone I | Zone II | Zone III | |||
| Complications | n (%) | n (%) | n (%) | pa | n (%) | n (%) | n (%) | pa |
| Recipient Site | ||||||||
| No | 21 (44.7) | 16 (53.3) | 5 (29.4) | 4 (30.8) | 7 (63.6) | 10 (43.5) | ||
| Yes | 26 (55.3) | 14 (46.7) | 12 (70.6) | 0.138 | 9 (69.2) | 4 (36.4) | 13 (56.5) | 0.268 |
| Infection | 9 (19.1) | 5 (16.7) | 4 (23.5) | 0.704 | 3 (23.1) | 0 (0) | 6 (26.1) | 0.178 |
| Dehiscence | 14 (29.8) | 6 (20.0) | 8 (47.1) | 0.095 | 5 (38.5) | 2 (18.2) | 7 (30.4) | 0.554 |
| Fistula | 10 (21.3) | 4 (13.3) | 6 (35.3) | 0.136 | 6 (46.2) | 1 (9.1) | 3 (13.0) | 0.035 |
| Flap Failure | 2 (4.3) | 1 (3.3) | 1 (5.9) | 1 | 1 (7.7) | 1 (9.1) | 0 (0) | 0.362 |
| Functional Outcome | ||||||||
| Functional Impairment | ||||||||
| No | 29 (61.7) | 19 (63.3) | 10 (58.8) | 9 (69.2) | 7 (63.6) | 13 (56.5) | ||
| Yes | 18 (38.3) | 13 (36.7) | 7 (41.2) | 0.766 | 4 (30.8) | 4 (36.4) | 10 (43.5) | 0.744 |
| Ectropion | 8 (17.0) | 5 (16.7) | 3 (17.6) | 1 | 2 (15.4) | 2 (18.2) | 4 (17.4) | 0.981 |
| Oral Incompetence | 9 (19.1) | 4 (13.3) | 5 (29.4) | 0.252 | 2 (15.4) | 1 (9.1) | 6 (26.1) | 0.46 |
| Trismus | 6 (12.8) | 3 (10.0) | 3 (17.6) | 0.653 | 2 (15.4) | 1 (9.1) | 3 (13.0) | 0.898 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, S.; Eljazzar, R.; Faisal, M.; Grasl, S.; Vyskocil, E.; Miles, B.A.; Brunner, M.; Seemann, R.; Erovic, B.M. Outcome in Patients with Partial and Full-Thickness Cheek Defects following Free Flap Reconstruction—A Multicentric Analysis of 47 Cases. J. Clin. Med. 2020, 9, 1740. https://doi.org/10.3390/jcm9061740
Janik S, Eljazzar R, Faisal M, Grasl S, Vyskocil E, Miles BA, Brunner M, Seemann R, Erovic BM. Outcome in Patients with Partial and Full-Thickness Cheek Defects following Free Flap Reconstruction—A Multicentric Analysis of 47 Cases. Journal of Clinical Medicine. 2020; 9(6):1740. https://doi.org/10.3390/jcm9061740
Chicago/Turabian StyleJanik, Stefan, Rachelle Eljazzar, Muhammad Faisal, Stefan Grasl, Erich Vyskocil, Brett A. Miles, Markus Brunner, Rudolf Seemann, and Boban M. Erovic. 2020. "Outcome in Patients with Partial and Full-Thickness Cheek Defects following Free Flap Reconstruction—A Multicentric Analysis of 47 Cases" Journal of Clinical Medicine 9, no. 6: 1740. https://doi.org/10.3390/jcm9061740
APA StyleJanik, S., Eljazzar, R., Faisal, M., Grasl, S., Vyskocil, E., Miles, B. A., Brunner, M., Seemann, R., & Erovic, B. M. (2020). Outcome in Patients with Partial and Full-Thickness Cheek Defects following Free Flap Reconstruction—A Multicentric Analysis of 47 Cases. Journal of Clinical Medicine, 9(6), 1740. https://doi.org/10.3390/jcm9061740






