Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding
Abstract
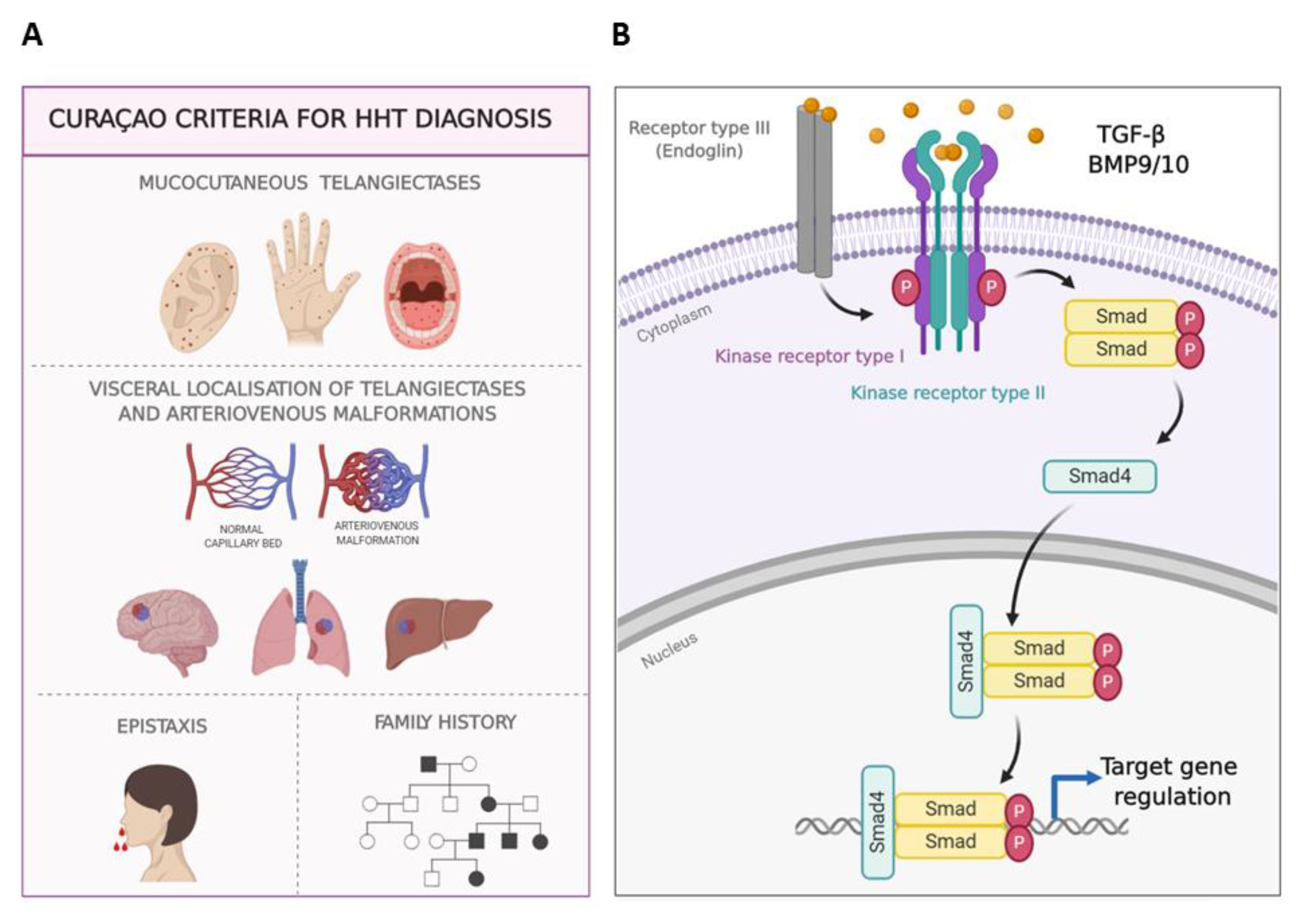
:1. Introduction
2. General Care and Control of Anemia
3. Therapeutic Pathways/Strategies of Pharmacological Treatments for HHT
- -
- Strategy 1. Although HHT does not result from a clotting failure, the use of antifibrinolytics to restore the balance between coagulation versus fibrinolysis would help to promote a quicker coagulation and to stabilize the fibrin network. Among the antifibrinolytics used for epistaxis treatment, tranexamic acid (TA) and ε-aminocaproic acid (AC) stand out [23,24].
- -
- Strategy 2. HHT is associated with haploinsufficiency in ENG or ALK1 genes, therefore stimulating their protein expression is thought to revert the HHT phenotype. At this point, raloxifene hydrochloride and bazedoxifene acetate, two specific estrogen receptor modulators (SERMs), have proven efficiency and safety, and have been designated as orphan drugs for HHT (2010 EU/3/10/730 and 2014 EU/3/14/1367; respectively) [25,26].
- -
- Strategy 3. Antiangiogenic therapies tackle the excess of abnormal vasculature present on the nasal mucosa in HHT. Therefore, bevacizumab (BZ) (Avastin®), a humanized monoclonal antibody against the main angiogenic factor, the vascular endothelial growth factor (VEGF), has been widely used and tested on HHT. Its systemic administration has improved hepatic function, delaying the liver transplant [27] but it has not shown consistent results when tested to decrease epistaxis events by topical spray administration [28,29].
3.1. Strategy 1. Antifibrinolysis: ε-Aminocaproic and Tranexamic Acids
3.2. Strategy 2. Upregulating ENG and ACVRL1
3.2.1. Hormonal Therapy: Specific Estrogen Receptor Modulators (SERMs)
3.2.2. Immunosuppressor Tacrolimus (FK506)
3.2.3. N-Acetylcysteine
3.3. Strategy 3. Antiangiogenesis
3.3.1. Anti-VEGF and Tyrosine Kinase Inhibitors (TKI)
3.3.2. Non-Selective Adrenergic β-blockers
3.3.3. Antiangiogenesis by FGF Ligand Blocking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Aminocaproic acid |
| ACVRL1/ALK1 | Activin Receptor-Like Kinase 1 |
| AVM | Arteriovenous Malformation |
| BMP9 | Bone Morphogenetic Protein 9 |
| BZ | Bevacizumab |
| CM | Capillary Malformation |
| CYP2D6 | Cytochrome P450 2D6 |
| ECs | Endothelial Cells |
| EMA | European Medicine Agency |
| ENG | Endoglin |
| EPHB4 | Ephrin Type-B Receptor 4 |
| ER | Estrogen Receptor |
| ESS | Epistaxis Severity Score |
| FDA | Food and Drug Administration |
| FGF | Fibroblast Growth Factor |
| FK506 | Tacrolimus |
| GI | Gastrointestinal |
| HHT | Hereditary Hemorrhagic Telangiectasia |
| iKO | inducible Knockout |
| JPHT | Juvenile Polyposis/HHT |
| MADH4/Smad4 | Mothers Against Decapentaplegic Homolog 4 |
| mRNA | messenger Ribonucleic Acid |
| NAC | N-acetylcysteine |
| OLT | Orthotopic Liver Transplant |
| OPG | Osteoprotegerin |
| PDGF | Platelet Derived Growth Factor |
| RANK | Receptor Activator of Nuclear Factor κ B |
| RANK-L | Receptor Activator of Nuclear Factor κ B Ligand |
| RASA1 | RAS P21 Protein Activator 1 |
| SERMs | Selective Estrogen Receptor Modulator |
| TA | Tranexamic Acid |
| TFRE | Transcription Factor Regulatory Element |
| TGF-β | Transforming Growth Factor β |
| VEGF | Vascular Endothelial Growth Factor |
| VM | Venous Malformation |
References
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for Hereditary Hemorrhagic Telangiectasia (Rendu- Osler-Weber Syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Shovlin, C.L. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010, 24, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Shovlin, C.L. Pulmonary arteriovenous malformations. Am. J. Respir. Crit. Care Med. 2014, 190, 1217–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldsen, A.D.; Vase, P.; Green, A. Hereditary haemorrhagic telangiectasia: A population-based study of prevalence and mortality in Danish patients. J. Intern. Med. 1999, 245, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jessurun, G.A.J.; Kamphuis, D.J.; Van der Zande, F.H.R.; Nossent, J.C. Cerebral arteriovenous malformations in the Netherlands Antilles. High prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin. Neurol. Neurosurg. 1993, 95, 193–198. [Google Scholar] [CrossRef]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrel, J.; et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.W.; Berg, J.N.; Baldwin, M.A.; Gallione, C.J.; Marondel, I.; Yoon, S.J.; Stenzel, T.T.; Speer, M.; Pericak-Vance, M.A.; Diamond, A.; et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type. Nat. Genet. 1996, 13, 189–195. [Google Scholar] [CrossRef]
- Gallione, C.J.; Repetto, G.M.; Legius, E.; Rustgi, A.K.; Schelley, S.L.; Tejpar, S.; Mitchell, G.; Drouin, É.; Westermann, C.J.J.; Marchuk, D.A. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004, 363, 852–859. [Google Scholar] [CrossRef]
- Duan, X.Y.; Guo, D.C.; Regalado, E.S.; Shen, H.; Coselli, J.S.; Estrera, A.L.; Safi, H.J.; Bamshad, M.J.; Nickerson, D.A.; LeMaire, S.A.; et al. SMAD4 rare variants in individuals and families with thoracic aortic aneurysms and dissections. Eur. J. Hum. Genet. 2019, 27, 1054–1060. [Google Scholar] [CrossRef]
- Cole, S.G.; Begbie, M.E.; Wallace, G.M.F.; Shovlin, C.L.L. A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J. Med. Genet. 2005, 42, 577–582. [Google Scholar] [CrossRef]
- Bayrak-Toydemir, P.; McDonald, J.; Akarsu, N.; Toydemir, R.M.; Calderon, F.; Tuncali, T.; Tang, W.; Miller, F.; Mao, R. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am. J. Med. Genet. A 2006, 140, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Wooderchak-Donahue, W.L.; McDonald, J.; O’Fallon, B.; Upton, P.D.; Li, W.; Roman, B.L.; Young, S.; Plant, P.; Fülöp, G.T.; Langa, C.; et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 2013, 93, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayrak-Toydemir, P.; Stevenson, D. Capillary Malformation-Arteriovenous Malformation Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Morales-Angulo, C.; Del Valle-Zapico, A. Hereditary hemorrhagic telangiectasia. Otolaryngol. Head Neck Surg. 1998, 119, 293. [Google Scholar] [PubMed]
- AAssar, O.S.; Friedman, C.M.; White, R.I. The Natural History of Epistaxis in Hereditary Hemorrhagic Telangiectasia. Laryngoscope 1991, 101, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Geisthoff, U.W.; Schneider, G.; Fischinger, J.; Plinkert, P.K. Hereditäre hämorrhagische teleangiektasie (Morbus Osler). Eine interdisziplinäre herausforderung. HNO 2002, 50, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher, A.E.; Marchuk, D.A.; White, R.I. Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 1995, 333, 918–924. [Google Scholar] [CrossRef]
- Ingrosso, M.; Sabbà, C.; Pisani, A.; Principi, M.; Gallitelli, M.; Cirulli, A.; Francavilla, A. Evidence of small-bowel involvement in hereditary hemorrhagic telangiectasia: A capsule-endoscopic study. Endoscopy 2004, 36, 1074–1079. [Google Scholar] [CrossRef]
- Vase, P.; Grove, O. Gastrointestinal Lesions in Hereditary Hemorrhagic Telangiectasia. Gastroenterology 1986, 91, 1079–1083. [Google Scholar] [CrossRef]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef]
- Kasthuri, R.S.; Montifar, M.; Nelson, J.; Kim, H.; Lawton, M.T.; Faughnan, M.E. Prevalence and predictors of anemia in hereditary hemorrhagic telangiectasia. Am. J. Hematol. 2017, 92, E591–E593. [Google Scholar] [CrossRef]
- Robert, F.; Desroches-Castan, A.; Bailly, S.; Dupuis-Girod, S.; Feige, J.J. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J. Rare. Dis. 2020, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Pérez Del Molino, A.; Zarrabeitia, R.; Fernández-L, A.; Botella, L.M. Efficacy of tranexamic acid in a patient with hereditary haemorrhagic telangiectasia and massive epistaxis. Med. Clin. (Barc.) 2004, 123, 118–119. [Google Scholar] [CrossRef]
- Fernandez-L, A.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; Ramirez, J.R.; Moralez-Angulo, C.; Zarrabeltia, R.; Perez-Molino, A.; Bernabéu, C.; Botella, L.M. Therapeutic action of tranexamic acid in hereditary haemorrhagic telangiectasia (HHT): Regulation of ALK-I/endoglin pathway in endothelial cells. Thromb. Haemost. 2007, 97, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiñana, V.; Bernabeu-Herrero, M.E.; Zarrabeitia, R.; Bernabeu, C.; Botella, L.M. Estrogen therapy for hereditary haemorrhagic telangiectasia (HHT): Effects of raloxifene, on Endoglin and ALK1 expression in endothelial cells. Thromb. Haemost. 2010, 103, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrabeitia, R.; Ojeda-Fernandez, L.; Recio, L.; Bernabéu, C.; Parra, J.A.; Albiñana, V.; Botella, L.M. Bazedoxifene, a new orphan drug for the treatment of bleeding in hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2016, 115, 1167–1177. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ginon, I.; Saurin, J.C.; Marion, D.; Guillot, E.; Decullier, E.; Roux, A.; Carette, M.F.; Gilbert-Dussardier, B.; Hatron, P.Y.; et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA J. Am. Med. Assoc. 2012, 307, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.J.; Sautter, N.B.; McWilliams, J.P.; Chakinala, M.M.; Merlo, C.A.; Johnson, M.H.; James, M.; Everett, E.M.; Clancy, M.S.; Faughnan, M.E.; et al. Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 316, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Fargeton, A.E.; Roux, A.; Bréant, V.; Colombet, B.; Rivière, S.; Cartier, C.; Lacombe, P.; et al. Effect of bevacizumab nasal spray on epistaxis duration in hereditary hemorrhagic telangectasia: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 316, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Albiñana, V.; Recio-Poveda, L.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2012, 108, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Albiñana, V.; Giménez-Gallego, G.; García-Mato, A.; Palacios, P.; Recio-Poveda, L.; Cuesta, A.-M.; Patier, J.-L.; Botella, L.-M. Topically Applied Etamsylate: A New Orphan Drug for HHT-Derived Epistaxis (Antiangiogenesis through FGF Pathway Inhibition). TH Open 2019, 3, e230–e243. [Google Scholar] [CrossRef] [Green Version]
- Geisthoff, U.W.; Seyfert, U.T.; Kübler, M.; Bieg, B.; Plinkert, P.K.; König, J. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid—A double blind placebo-controlled cross-over phase IIIB study. Thromb. Res. 2014, 134, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, E.; Preis, M.; Hadar, T.; Shvero, J.; Haddad, M. Antiestrogen therapy for Hereditary hemorrhagic telangiectasia: A double-blind placebo-controlled clinical trial. Laryngoscope 2009, 119, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Dupuis-Girod, S.; Boutitie, F.; Rivière, S.; Morinière, S.; Hatron, P.Y.; Manfredi, G.; Kaminsky, P.; Capitaine, A.L.; Roy, P.; et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: A European cross-over controlled trial in a rare disease. J. Thromb. Haemost. 2014, 12, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Lebrin, F.; Srun, S.; Raymond, K.; Martin, S.; Van Den Brink, S.; Freitas, C.; Bréant, C.; Mathivet, T.; Larrivée, B.; Thomas, J.L.; et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 2010, 16, 420–428. [Google Scholar] [CrossRef]
- Riss, D.; Burian, M.; Wolf, A.; Kranebitter, V.; Kaider, A.; Arnoldner, C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: A double-blind, randomized, placebo-controlled trial. Head Neck. 2015, 37, 783–787. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Samson, G.; Roux, A.; Fargeton, A.E.; Rioufol, C.; Schwiertz, V.; Disant, F.; Chapuis, F.; et al. ELLIPSE Study: A Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. MAbs 2014, 6, 794–799. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Gilson, C.; Busbridge, M.; Patel, D.; Shi, C.; Dina, R.; Abdulla, F.N.; Awan, I. Can Iron Treatments Aggravate Epistaxis in Some Patients With Hereditary Hemorrhagic Telangiectasia? Laryngoscope 2016, 126, 2468–2474. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Wu, V.; Faughnan, M.E.; Lasso, A.; Figol, A.; Kilty, S.J. Prospective pilot study of Floseal® for the treatment of anterior epistaxis in patients with hereditary hemorrhagic telangiectasia (HHT). J. Otolaryngol. Head Neck Surg. 2019, 48, 48. [Google Scholar] [CrossRef] [Green Version]
- Dupuis-Girod, S.; Pitiot, V.; Bergerot, C.; Fargeton, A.E.; Beaudoin, M.; Decullier, E.; Bréant, V.; Colombet, B.; Philouze, P.; Faure, F.; et al. Efficacy of TIMOLOL nasal spray as a treatment for epistaxis in hereditary hemorrhagic telangiectasia. A double-blind, randomized, placebo-controlled trial. Sci. Rep. 2019, 9, 1986. [Google Scholar] [CrossRef]
- Kroon, S.; Snijder, R.J.; Mager, J.J.; Post, M.C.; Tenthof van Noorden, J.; Van Geenen, E.J.M.; Drenth, J.P.H.; Grooteman, K.V. Octreotide for gastrointestinal bleeding in hereditary hemorrhagic telangiectasia: A prospective case series. Am. J. Hematol. 2019, 94, E247–E249. [Google Scholar] [CrossRef] [Green Version]
- Dupuis-Girod, S.; Fargeton, A.-E.; Grobost, V.; Rivière, S.; Beaudoin, M.; Decullier, E.; Bernard, L.; Bréant, V.; Colombet, B.; Philouze, P.; et al. Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. J. Clin. Med. 2020, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Masoudi-Sobhanzadeh, Y.; Omidi, Y.; Amanlou, M.; Masoudi-Nejad, A. Drug databases and their contributions to drug repurposing. Genomics 2020, 112, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Silverman, S. Fibrinolytic Activity in Lesions of Hereditary Hemorrhagic Telangiectasia. Arch. Dermatol. 1973, 107, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hanawa, S.; Morishima, T. Fibrinolytic activity in cutaneous lesions of hereditary hemorrhagic telangiectasia. Nippon Hifuka Gakkai Zasshi. Jpn. J. Dermatol. 1985, 95, 11–16. [Google Scholar]
- Saba, H.I.; Morelli, G.A.; Logrono, L.A. Treatment of bleeding in hereditary hemorrhagic telangiectasia with aminocaproic acid. N. Engl. J. Med. 1994, 330, 1789–1790. [Google Scholar] [CrossRef]
- Annichino-Bizzacchi, J.M.; Facchini, R.M.; Torresan, M.Z.; Arruda, V.R. Hereditary hemorrhagic telangiectasia response to aminocaproic acid treatment. Thromb. Res. 1999, 96, 73–76. [Google Scholar] [CrossRef]
- Korzenik, J.R.; Topazian, M.D.; White, R.; Saba, H.I.; Morelli, G.; Logrono, L. Treatment of bleeding in hereditary hemorrhagic telangiectasia with aminocaproic acid. N. Engl. J. Med. 1994, 331, 1236. [Google Scholar]
- Mannucci, P.M. Hemostatic drugs. N. Engl. J. Med. 1998, 339, 245–253. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Sulaiman, N.L.; Govani, F.S.; Jackson, J.K.; Begbie, M.E. Elevated factor VIII in hereditary haemorrhagic telangiectasia (HHT): Association with venous thromboembolism. Thromb. Haemost. 2007, 98, 1031–1039. [Google Scholar]
- Sabba, C.; Gallitelli, M.; Palasciano, G. Efficacy of unusually high doses of tranexamic acid for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 2001, 345, 926. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Longacre, A.V.; Gross, C.P.; Gallitelli, M.; Henderson, K.J.; White, R.I.; Proctor, D.D. Diagnosis and management of gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am. J. Gastroenterol. 2003, 98, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, E.; Preis, M.; Shvero, J.; Nageris, B.; Hadar, T. Anti-estrogen therapy for hereditary hemorrhagic telangiectasia—A long-term clinical trial. Rhinology 2011, 49, 214–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, A.U.; Glass, J.; Netchvolodoff, C.V.; Bowen, L.M. Hereditary hemorrhagic telangiectasia and danazol. Ann. Intern. Med. 1988, 109, 171. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Dunbar, S.D.; Newsom, W.A. Hemostatic effects of tamoxifen in hereditary hemorrhagic telangiectasia. Thromb. Haemost. 2001, 85, 371–372. [Google Scholar] [PubMed]
- Nelson, E.R.; Wardell, S.E.; McDonnell, D.P. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: Implications for the treatment and prevention of osteoporosis. Bone 2013, 53, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Lee, S.M.; Jung, I.K.; Lim, Y.; Kim, J.H. Effects of genistein on early-stage cutaneous wound healing. Biochem. Biophys. Res. Commun. 2011, 410, 514–519. [Google Scholar] [CrossRef]
- Albiñana, V.; Sanz-Rodríguez, F.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Immunosuppressor FK506 increases endoglin and activin receptor-like kinase 1 expression and modulates transforming growth factor-β1 signaling in endothelial cells. Mol. Pharmacol. 2011, 79, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Albiñana, V.; Velasco, L.; Zarrabeitia, R.; Botella, L.M. Tacrolimus as a therapeutic drug in hereditary hemorrhagic telangiectasia (HHT). In Tacrolimus: Effectiveness, Safety and Drug Interactions; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 163–172. ISBN 9781628083668. [Google Scholar]
- Skaro, A.I.; Marotta, P.J. Regression of cutaneous and gastrointestinal telangiectasia with sirolimus and aspirin in a patient with hereditary hemorrhagic telangiectasia. Ann. Intern. Med. 2006, 144, 226–227. [Google Scholar]
- Ruiz, S.; Chandakkar, P.; Zhao, H.; Papoin, J.; Chatterjee, P.K.; Christen, E.; Metz, C.N.; Blanc, L.; Campagne, F.; Marambaud, P. Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Hum. Mol. Genet. 2017, 26, 4786–4798. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Droege, F.; Gamen, K.E.; Geisthoff, U.; Gall, H.; Tello, K.; Richter, M.J.; Deubner, L.M.; Schmiedel, R.; Hecker, M.; et al. Treatment with low-dose tacrolimus inhibits bleeding complications in a patient with hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894018805406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosman, A.; Kroon, S.; Vorselaars, V.; Doef, H.; Post, M.; Snijder, R.; Mager, J. Tacrolimus treatment for two rare complications caused by hereditary haemorrhagic telangiectasia: A description of two cases. Angiogenesis 2019, 22, 629. [Google Scholar]
- De Gussem, E.M.; Snijder, R.J.; Disch, F.J.; Zanen, P.; Westermann, C.J.J.; Mager, J.J. The effect of N-acetylcysteine on epistaxis and quality of life in patients with HHT: A pilot study. Rhinology 2009, 47, 85–88. [Google Scholar] [PubMed]
- Albiñana, V. Ensayos Preclínicos In Vitro con Células Endoteliales, Como Aproximaciones Terapéuticas Farmacológicas Para la Telangiectasia Hemorrágica Hereditaria (HHT). Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 28 June 2012. [Google Scholar]
- Roy, H.; Bhardwaj, S.; Ylä-Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett. 2006, 580, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Cirulli, A.; Liso, A.; D’Ovidio, F.; Mestice, A.; Pasculli, G.; Gallitelli, M.; Rizzi, R.; Specchia, G.; Sabbà, C. Vascular endothelial growth factor serum levels are elevated in patients with hereditary hemorrhagic telangiectasia. Acta Haematol. 2003, 110, 29–32. [Google Scholar] [CrossRef]
- Sadick, H.; Riedel, F.; Naim, R.; Goessler, U.; Hörmann, K.; Hafner, M.; Lux, A. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-β1 as well as high ALK1 tissue expression. Haematologica 2005, 90, 818–828. [Google Scholar]
- Peng, H.L.; Hu, G.Y.; Zhang, G.S.; Gong, F.J. Analysis of angiogenesis related proteins and its implication in type-2 hereditary hemorrhagic telangiectasia. Zhonghua Xue Ye Xue Za Zhi 2006, 27, 616–620. [Google Scholar]
- Botella, L.M.; Albiñana, V.; Ojeda-Fernandez, L.; Recio-Poveda, L.; Bernabéu, C. Research on potential biomarkers in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Michels, S.; Rosenfeld, P.J.; Puliafito, C.A.; Marcus, E.N.; Venkatraman, A.S. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: Twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005, 112, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Adams, L.A.; MacQuillan, G.; Tibballs, J.; Vanden Driesen, R.; Delriviere, L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transplant. 2008, 14, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Flieger, D.; Hainke, S.; Fischbach, W. Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann. Hematol. 2006, 85, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Girod, S.; Buscarini, E. Hereditary hemorrhagic telangiectasia: To transplant or not to transplant? Liver Int. 2016, 36, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Buscarini, E.; Botella, L.M.; Geisthoff, U.; Kjeldsen, A.D.; Mager, H.J.; Pagella, F.; Suppressa, P.; Zarrabeitia, R.; Dupuis-Girod, S.; Shovlin, C.L.; et al. Safety of thalidomide and bevacizumab in patients with hereditary hemorrhagic telangiectasia. Orphanet. J. Rare Dis. 2019, 14, 28. [Google Scholar] [CrossRef] [Green Version]
- Albitar, H.A.; Choby, G.; Pruthi, R.; O’Brien, E.; Stokken, J.; Kamath, P.; Leise, M.; Gallo De Moraes, A.; Cajigas, H.; Dubrock, H.; et al. Intravenous bevacizumab in HHT-related bleeding: Significant inter-individual variability in the need for maintenance therapy. Angiogenesis 2019, 22, 589–590. [Google Scholar]
- Kim, Y.H.; Kim, M.J.; Choe, S.W.; Sprecher, D.; Lee, Y.J.; Oh, S.P. Selective effects of oral antiangiogenic tyrosine kinase inhibitors on an animal model of hereditary hemorrhagic telangiectasia. J. Thromb. Haemost. 2017, 15, 1095–1102. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Gossage, J.R.; Chakinala, M.M.; Oh, S.P.; Kasthuri, R.; Hughes, C.C.W.; McWilliams, J.P.; Parambil, J.G.; Vozoris, N.; Donaldson, J.; et al. Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis 2019, 22, 145–155. [Google Scholar] [CrossRef]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Papoin, J.; Choi, H.; Nomura-Kitabayashi, A.; Patel, R.; Gillen, M.; Diao, L.; Chatterjee, P.K.; et al. Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. Clin. Investig. 2020, 130, 942–957. [Google Scholar] [CrossRef] [Green Version]
- Kovacs-Sipos, E.; Holzmann, D.; Scherer, T.; Soyka, M.B. Nintedanib as a novel treatment option in hereditary haemorrhagic telangiectasia. BMJ. Case. Rep. 2017, 2017, bcr2017219393. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; De La Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carpintero, I.; Ruiz-Rodriguez, R.; López-Gutiérrez, J.C. Propranolol in the treatment of infantile hemangioma: Clinical effectiveness, risks, and recommendations. Actas Dermosifiliogr. 2011, 102, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Olitsky, S.E. Topical timolol for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. Am. J. Otolaryngol. Head Neck Med. Surg. 2012, 33, 375–376. [Google Scholar] [CrossRef]
- Ichimura, K.; Kikuchi, H.; Imayoshi, S.; Dias, M.S. Topical application of timolol decreases the severity and frequency of epistaxis in patients who have previously undergone nasal dermoplasty for hereditary hemorrhagic telangiectasia. Auris Nasus Larynx. 2016, 43, 429–432. [Google Scholar] [CrossRef]
- Epperla, N.; Brilliant, M.H.; Vidaillet, H. Topical timolol for treatment of epistaxis in hereditary haemorrhagic telangiectasia associated with bradycardia: A look at CYP2D6 metabolising variants. BMJ Case. Rep. 2014, 2014, bcr2013203056. [Google Scholar] [CrossRef] [Green Version]
- Volotinen, M.; Hakkola, J.; Pelkonen, O.; Vapaatalo, H.; Mäenpää, J. Metabolism of ophthalmic timolol: New aspects of an old drug. Basic Clin. Pharmacol. Toxicol. 2011, 108, 297–303. [Google Scholar] [CrossRef]
- Botella, L.M.; Zarrabeitia, R.; Albinana, V.; Ojeda-Fernández, M.L.; Diez-Gonzalez, V.; Parra-Blanco, J.A. Efficacy of topical timolol for the treatment of mucocutaneous telangiectasias in patients with hereditary haemorrhagic telangiectasia. Angiogenesis 2015, 18, 594. [Google Scholar]
- Mei-Zahav, M.; Blau, H.; Bruckheimer, E.; Zur, E.; Goldschmidt, N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—A preliminary report. J. Otolaryngol. Head Neck Surg. 2017, 46, 58. [Google Scholar] [CrossRef] [Green Version]
- Mei-Zahav, M.; Goldschmidt, N.; Bruckheimer, E.; Soudri, E. A double-blind placebo-controlled study assessing the safety and efficacy of topical propranolol for moderate–severe epistaxis in patients with hereditary hemorrhagic telangiectasia (HHT). Angiogenesis 2019, 22, 597–598. [Google Scholar]
- Esteban-Casado, S.; Martín de Rosales Cabrera, A.M.; Usarralde Pérez, A.; Martínez Simón, J.J.; Zhan Zhou, E.; Marcos Salazar, M.S.; Pérez Encinas, M.; Botella Cubells, L. Sclerotherapy and Topical Nasal Propranolol: An Effective and Safe Therapy for HHT-Epistaxis. Laryngoscope 2019, 129, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Patier, J.L.; Camacho Aguirre, A.; Sirgo, N.; Gonzalez Nino, I.; Suárez Carantoña, C.; Gonzalez Garcia, A.; López Rodriguez, M.; Botella, L.M. Effectiveness and safety of the treatment with oral propranolol in patients with hereditary hemorrhagic telangiectasia and bloodhypertension or atrial fibrillation: A possible anti-angiogenictreatment in epistaxis. Angiogenesis 2019, 22, 628. [Google Scholar]
- Thomas, K.A.; Rios-Candelore, M.; Gimenez-Gallego, G.; DiSalvo, J.; Bennett, C.; Rodkey, J.; Fitzpatrick, S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc. Natl. Acad. Sci. USA 1985, 82, 6409–6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, F.; Baird, A.; Ling, N.; Ueno, N.; Hill, F.; Denoroy, L.; Klepper, R.; Gospodarowicz, D.; Böhlen, P.; Guillemin, R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc. Natl. Acad. Sci. USA 1985, 82, 6507–6511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends. Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Loges, S.; Mazzone, M.; Hohensinner, P.; Carmeliet, P. Silencing or Fueling Metastasis with VEGF Inhibitors: Antiangiogenesis Revisited. Cancer Cell 2009, 15, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Droege, F.; Thangavelu, K.; Lang, S.; Geisthoff, U. Improvement in hereditary hemorrhagic telangiectasia after treatment with the multi-kinase inhibitor Sunitinib. Ann. Hematol. 2016, 95, 2077–2078. [Google Scholar] [CrossRef]
- Geisthoff, U.W.; Nguyen, H.L.P.; Hess, D. Improvement in hereditary hemorrhagic telangiectasia after treatment with the phosphoinositide 3-kinase inhibitor BKM120. Ann. Hematol. 2014, 93, 703–704. [Google Scholar] [CrossRef]


| 1 | Decrease hemorrhages stabilizing the fibrin network with antifibrinolytics |
| |
| 2 | Stimulate ENG and ALK1 transcription to increase protein expression to partially overcome haploinsufficiency |
| |
| 3 | Decrease the abnormal excessive vasculature of the nose mucosa through antiangiogenesis |
|
| Trial Registration # | Country | Phase | Title | Intervention | Number of Patients | Trial Design | Outcome | Start Date | Status |
|---|---|---|---|---|---|---|---|---|---|
| EudraCT 2010-020545-26 | IT | 2 | Bevacizumab, an anti-angiogenic monoclonal antibody effective for prevention of hemorrhage in patients with HHT: possible regression of visceral arteriovenous malformations | Bevacizumab | 18 | Single-arm, controlled | Frequency of Epistaxis | 2008 | O |
| EudraCT 2008-006755-44 | FR | 2 | METAFORE: Maladie de Rendu-Osler: Etude de l’Efficacité et de la tolérance du Bevacizumab utilisé pour le traitement des formes hépatiques sévères. Etude de phase II | Bevacizumab | 25 | Information not available | Effect on cardiac output in patients with severe liver damage | 2009 | O |
| NCT02389959 | US | 4 | Intranasal Bevacizumab for HHT-Related Epistaxis | Bevacizumab | 40 | Two-arm, randomized, double-blind, placebo-controlled | Improvement in ESS | 2014 | R |
| NCT02287558 | US | 1 | Pomalidomide in HHT and Transfusion-Dependent Vascular Ectasia: a Phase I Study | Pomalidomide | 9 | Single-arm, open-label | Transfusion requirement measure | 2015 | R |
| NCT04167085 | US | 4 | NOrth American Study for the Treatment of Recurrent epIstaxis With DoxycycLine: The NOSTRIL Trial | Doxycycline | 24 | Two-arm, randomized, double-blind, crossover | Frequency of epistaxis | 2017 | A |
| NCT02963129 | AR | 3 | Treatment of Nasal Staphylococcus Aureus Colonization in Patients With HHT | Mupirocin | 40 | Two-arm, randomized, triple-blind, placebo | Nosebleed by Sadick scale | 2017 | U |
| NCT03981562 | CA | 2 | Vitamin D and HHT | Vit D | 60 | Three-arm, randomized, double-blind, placebo | Change in ESS | 2018 | R |
| EudraCT z017-003272-31 | NL | 2 | Efficacy and safety of oral itraconazole in the reduction of epistaxis severity in HHT | Itraconazole | 25 | Single-arm, open-label | Change in epistaxis severity | 2018 | O |
| NCT03397004 | CA | 2 | Doxycycline for HHT | Doxycycline Hyclate | 30 | Two-arm, randomized, double-blind, placebo-controlled, crossover | Reduction in epistaxis (nose bleeding) severity over 96 weeks | 2018 | R |
| NCT04113187 | FR | 3 | Propranolol for Epistaxis in HHT a Patients | Propranolol | 38 | Two-arm, double-blind | Cumulative duration of epistaxis (in minutes) | 2019 | NR |
| NCT04139018 | US | 2 | Timolol Gel for Epistaxis in HHT | Timolol Gel | 30 | Two-arm, double-blind, randomized controlled | ESS | 2019 | R |
| NCT03910244 | US | 2 | Pomalidomide for the Treatment of Bleeding in HHT | Pomalidomide | 159 | Two-arm, placebo-controlled, double-blind | Change in ESS | 2019 | R |
| NCT03850730 | US | 1–2 | Pazopanib for the Treatment of Epistaxis in HHT | Pazopanib | 30 | Single-arm, open-label | Percent change in epistaxis duration in minutes | 2019 | NR |
| NCT03850964 | US | 2–3 | Pazopanib Effects on Bleeding in HHT | Pazopanib | 45 | Two-arm, double-blind, placebo controlled | Change in epistaxis duration in minutes | 2019 | NR |
| EudraCT 2019-003585-40 | NL | NA | An uncontrolled, open label pilot-study assessing the efficacy in reducing bleeding severity, and the safety of oral tacrolimus in patients with HHT | Tacrolimus | 20 | Uncontrolled, single-arm, open-label | Change in the epistaxis and/or gastrointestinal severity | 2019 | O |
| EudraCT 2019-002593-31 | FR | 2 | Efficacy of Nintedanib per os as a treatment for epistaxis in HHT disease. A national, randomized, multicenter phase II study EPICURE | Nintedanib | 60 | Two-arm, double-blind, randomized controlled, placebo | Frequency of Epistaxis | 2019 | O |
| EudraCT 2018-004179-11 | NL | 2 | Effectiveness of Somatostatin Analogues in patients with HHT and symptomatic gastrointestinal bleeding, the SAIPAN-trial: a multicenter, randomized, open-label, parallel group, superiority trial | Somatostatin Analogues | 38 | Two-arm, open-label, randomized controlled | Decreasing the transfusion requirements | 2019 | O |
| ACTRN 12619001020178 | AU | 2 | A pilot study assessing the effectiveness of oral Propranolol in preventing epistasis in patients with HHT | Propranolol | 15 | Single-arm, open-label, non-randomized | Change in Epistasis | 2019 | NR |
| NCT03954782 | FR | 2 | Efficacy of Nintedanib Per os as a Treatment for Epistaxis in HHT Disease | Nintedanib | 60 | Two-arm, triple-blind, randomized | Epistaxis duration assessed on epistaxis grids completed by the patients | 2020 | O |
| Trial Registration # | Country | Phase | Title | Intervention | Number of Patients | Trial Design | Outcome (Summary of Statistically Significant Outcomes) | Start Date | Status | Link to Results |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00004327 | US | 2 | Phase II Pilot Study of Octreotide, a Somatostatin Octapeptide Analog, for Gastrointestinal Hemorrhage in Hormone-Refractory HHT and Senile Ectasia | Octreotide | 8 | Information not available | - | 1995 | C | - |
| NCT00004654 | US | 3 | Phase III Randomized, Placebo-Controlled, Crossover Study of Soy Protein Isolate for HHT | Soy protein | 60 | Randomized | - | 1996 | C | - |
| NCT01031992 | GE | 3 | Tranexamic Acid and Epistaxis in HHT | Tranexamic acid | 23 | Two-arm, double-blind, controlled | No changes in hemoglobin levels. Significant reduction in ESS | 2002 | C | Article [32] |
| NCT00375622 | IL | 2 | Anti-Estrogen Therapy for HHT A Double-Blind Placebo-Controlled Clinical Trial | Tamoxifen | 25 (60) * | Double-blind, placebo-controlled | Significant reduction in ESS (frequency and severity). | 2005 | C | Article [33] |
| NCT00355108 | FR | 3 | ATERO: A Randomized Study with Tranexamic Acid in Epistaxis of Rendu Osler Syndrome | Tranexamic acid | 118 (170) * | Single-arm, double-blind, randomized, crossover | Significant decrease in the duration of epistaxis | 2006 | C | Article [34] |
| NCT00389935 | US | 2 | Thalidomide Reduces Arteriovenous Malformation Related Gastrointestinal Bleeding | Thalidomide | 14 | Single-arm, open-label | - | 2006 | C | - |
| NCT00588146 | US | 2 | Phase 2 Study of PEG-Intron in HHT | Pegylated IFN-α2B | 10 | Two-arm, open-label, randomized | Adverse events plus discontinuation of the study supply | 2007 | T | Trial file |
| NCT01397695 | US | 2 | Topical Bevacizumab for the Management of Recurrent Epistaxis in Patients with HHT | Bevacizumab | 20 | Single-arm, open-label, non-randomized | - | 2009 | T | Trial file |
| NCT01402531 | US | 2 | Submucosal Bevacizumab for the Management of Recurrent Epistaxis in Patients with HHT | Bevacizumab | 10 | Single-arm, open-label, non-randomized | - | 2010 | T | Trial file |
| EudraCT2009-018049-19 | AT | 2 | A randomized double-blind placebo-controlled trial of intranasal submucosal bevacizumab in hereditary hemorrhagic telangiectasia | Bevacizumab | 30 | Two-arm, double-blind, randomizedcontrolled | - | 2010 | C | - |
| NCT01408030 | US | 2 | North American Study of Epistaxis in HHT (NOSE) | Bevacizumab- Estriol- Tranexamic Acid | 121 | Four-arm, double-blind, placebo-controlled, randomized | No significant differences on epistaxis frequency | 2011 | C | Article [28] |
| NCT01408732 | US | 1 | Office-sclerotherapy for Epistaxis Due to HHT | Sclerotherapy with sodium tetradecyl sulfate | 18 | Two-arm, open-label, randomized-controlled | No significant differences on severity of epistaxis | 2011 | C | Trial file |
| NCT01485224 | IT | 2 | Efficacy of Thalidomide in the Treatment of HHT | Thalidomide | 31 | Single-arm, open-label, non-randomized | 100% showed a complete or partial response to epistaxis | 2011 | C | Article [35] |
| NCT01314274 | AT | 2 | Intranasal Submucosal Bevacizumab for Epistaxis in HHT | Bevacizumab | 15 | Two-arm, double-blind, randomized, placebo-controlled | No significant differences on epistaxis frequency | 2011 | C | Article [36] |
| NCT01507480 | FR | 1 | The ELLIPSE Study: A Phase-1 Study Evaluating the Tolerance of Bevacizumab Nasal Spray to Treat Epistaxis in HHT | Bevacizumab | 42 | Single-arm, double-blind, randomized, placebo-controlled | Bevacizumab nasal spray is safe | 2011 | C | Article [37] |
| EudraCT 2011-004096-36 | IT | 2 | Efficacy of thalidomide in the treatment of severe recurrent epistaxis in HHT | Thalidomide | 31 | Single-arm, non-controlled | - | 2011 | C | - |
| NCT01908543 | UK | NA | Iron Deficiency and HHT | Ferrous sulphate | 3 | Single-arm, open-label | - | 2013 | T | Article [38] |
| NCT01752049 | CA | NA | Topical Anti-angiogenic Therapy for Telangiectasia in HHT: Proof of Concept | Timolol maleate | 5 | Two-arm, double-blind, placebo-controlled | - | 2013 | C | - |
| EudraCT 2013-004204-19 & NCT02157987 | FR | 1-2 | Treatment of HHT of the Nasal Mucosa by Intranasal Bevacizumab: Search for Effective Dose | Bevacizumab | 30 | Four-arm, double-blind, randomized, controlled | - | 2014 | C | - |
| NCT02638012 | CA | NA | Prospective Pilot Study of Floseal for the Treatment of Anterior Epistaxis in Patients With HHT | Floseal | 10 | Two-arm, open-label, non-randomized | No statistically significant difference noted in ESS | 2015 | C | Article [39] |
| NCT02204371 | US & CA | 2 | Evaluation of Pazopanib on Bleeding in Subjects with HHT | Pazopanib | 7 | Two-arm, open-label, non-randomized | No statistically significant difference noted in ESS | 2015 | T | Trial file |
| EudraCT 2015-000385-55 & NCT02484716 | FR | 2 | Efficacy of a Timolol Nasal Spray as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia (HHT)—(TEMPO) | Timolol | 58 | Two-arm, double-blind, randomized | No statistically significant difference noted in ESS | 2015 | C | Article [40] |
| EudraCT 2016-003982-24 | ES | 4-2 | A phase IV-II, single-center, open, single arm treatment, low level of intervention, to assess the efficacy clinical trial and safety of intranasal administration of ethamsylate in the treatment of HHT, during for 4 weeks | Ethamsylate | 12 | Single-arm, open-label, non-randomized | Statistically significant difference noted in ESS | 2016 | C | Article [31] |
| EudraCT 2016-001340-19 & NCT02874326 | NL | 2 | Octreotide in Patients With GI Bleeding Due to Rendu-Osler-Weber | Octreotide LAR | 15 | Single-arm, open-label | The median number of RBC units needed decreased significantly. | 2016 | C | Article [41] |
| NCT03152019 | FR | 2 | Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hemorrhagic Hereditary Telangiectasia (HHT) (TACRO) | Tacrolimus | 50 | Two-arm, double-blind, randomized | No statistically significant difference noted in ESS | 2017 | C | Article [42] |
| Active Substance | Date | EU-Designated Number | Sponsor | Degree of Evidence Clinical Trial |
|---|---|---|---|---|
| Etamsylate | 11/2018 | EU/3/10/18/2087 | CSIC, Spain | EudraCT: 2016–003982–24 |
| Thalidomide | 02/2017 | EU/3/17/1845 | PlumeStars, S.R.L., Italy | Several clinical trials, Table 3 |
| Bevacizumab | 12/2014 | EU/3/14/1390 | Dupuis-Girod, France | Several clinical trials, Table 3 |
| Bazedoxifene | 11/2014 | EU/3/14/1367 | CSIC, Spain | Observational study, 5 patients |
| Raloxifene | 06/2010 | EU/3/10/730 | CSIC, Spain | Observational study, 19 patients |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albiñana, V.; Cuesta, A.M.; de Rojas-P, I.; Gallardo-Vara, E.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. J. Clin. Med. 2020, 9, 1766. https://doi.org/10.3390/jcm9061766
Albiñana V, Cuesta AM, de Rojas-P I, Gallardo-Vara E, Recio-Poveda L, Bernabéu C, Botella LM. Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. Journal of Clinical Medicine. 2020; 9(6):1766. https://doi.org/10.3390/jcm9061766
Chicago/Turabian StyleAlbiñana, Virginia, Angel M. Cuesta, Isabel de Rojas-P, Eunate Gallardo-Vara, Lucía Recio-Poveda, Carmelo Bernabéu, and Luisa María Botella. 2020. "Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding" Journal of Clinical Medicine 9, no. 6: 1766. https://doi.org/10.3390/jcm9061766
APA StyleAlbiñana, V., Cuesta, A. M., de Rojas-P, I., Gallardo-Vara, E., Recio-Poveda, L., Bernabéu, C., & Botella, L. M. (2020). Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. Journal of Clinical Medicine, 9(6), 1766. https://doi.org/10.3390/jcm9061766







