High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Measurements
2.4. Statistical Analysis
3. Results
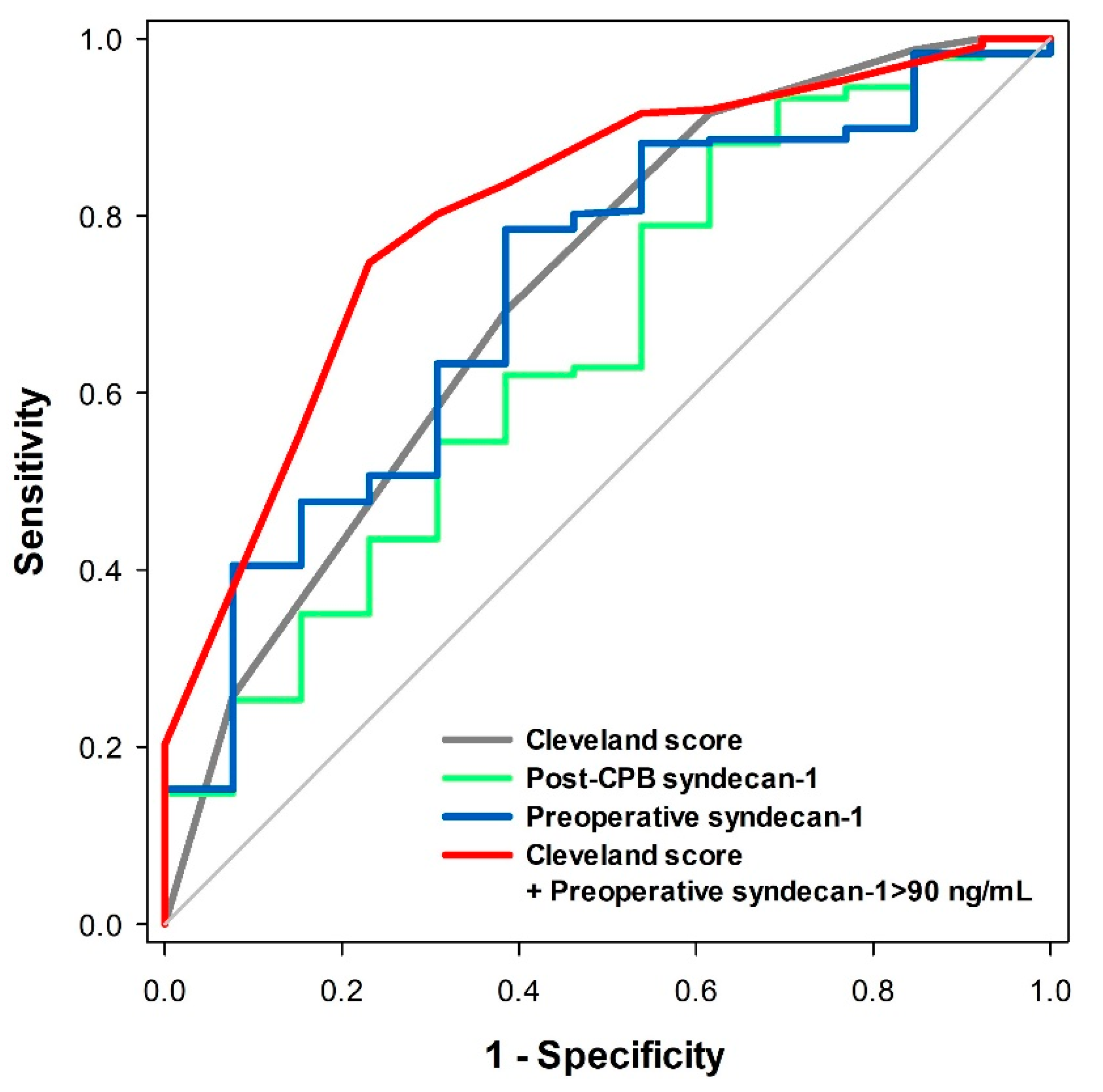
3.1. Prediction of Postoperative Severe AKI
3.2. Comparison Between Low and High Preoperative Syndecan-1 Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thiele, R.H.; Isbell, J.M.; Rosner, M.H. AKI associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2015, 10, 500–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liotta, M.; Olsson, D.; Sartipy, U.; Holzmann, M.J. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Robert, A.M.; Kramer, R.S.; Dacey, L.J.; Charlesworth, D.C.; Leavitt, B.J.; Helm, R.E.; Hernandez, F.; Sardella, G.L.; Frumiento, C.; Likosky, D.S.; et al. Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann. Thorac. Surg. 2010, 90, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Shim, J.K.; Lee, S.; Song, J.W.; Choi, N.; Lee, S.; Kwak, Y.L. Chronic progression of cardiac surgery associated acute kidney injury: Intermediary role of acute kidney disease. J. Thorac. Cardiovasc. Surg. 2019. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflügers Archiv. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.W.; Zullo, J.; Lipphardt, M.; Dragovich, M.; Zhang, F.X.; Fu, B.; Goligorsky, M.S. Endothelial glycocalyx-the battleground for complications of sepsis and kidney injury. Nephrol. Dial. Transplant. 2018, 33, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolarova, H.; Ambruzova, B.; Svihalkova Sindlerova, L.; Klinke, A.; Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat. Inflamm. 2014, 2014, 694312. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein c depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Ray, S.; Srivastava, L.M.; Bhargava, S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016, 49, 768–776. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Berkestedt, I.; Schmidtchen, A.; Ljunggren, L.; Bodelsson, M. Increased levels of glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock 2008, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svennevig, K.; Hoel, T.; Thiara, A.; Kolset, S.; Castelheim, A.; Mollnes, T.; Brosstad, F.; Fosse, E.; Svennevig, J. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion 2008, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Rehm, M.; Abicht, J.; Paul, J.O.; Stoeckelhuber, M.; Pfirrmann, M.; Reichart, B.; Becker, B.F.; Christ, F. Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1445–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruegger, D.; Brettner, F.; Rossberg, I.; Nussbaum, C.; Kowalski, C.; Januszewska, K.; Becker, B.F.; Chappell, D. Acute degradation of the endothelial glycocalyx in infants undergoing cardiac surgical procedures. Ann. Thorac. Surg. 2015, 99, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Haberer, A.; Tiefenthaller, A.; Januszewska, K.; Chappell, D.; Brettner, F.; Mayer, P.; Dalla Pozza, R.; Genzel-Boroviczeny, O. Perturbation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2015, 150, 1474–1481.e1471. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.P.; Paganini, E.P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, S.H.; Patel, N.N.; Murphy, G.J. Vascular endothelial cell changes in postcardiac surgery acute kidney injury. Am. J. Physiol. Ren. Physiol. 2018, 314, F726–F735. [Google Scholar] [CrossRef]
- de Melo Bezerra Cavalcante, C.T.; Castelo Branco, K.M.; Pinto Junior, V.C.; Meneses, G.C.; de Oliveira Neves, F.M.; de Souza, N.M.; Penaforte, K.L.; Martins, A.M.; Liborio, A.B. Syndecan-1 improves severe acute kidney injury prediction after pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2016, 152, 178–186.e172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesonen, E.; Passov, A.; Andersson, S.; Suojaranta, R.; Niemi, T.; Raivio, P.; Salmenpera, M.; Schramko, A. Glycocalyx degradation and inflammation in cardiac surgery. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 341–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padberg, J.S.; Wiesinger, A.; di Marco, G.S.; Reuter, S.; Grabner, A.; Kentrup, D.; Lukasz, A.; Oberleithner, H.; Pavenstadt, H.; Brand, M.; et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014, 234, 335–343. [Google Scholar] [CrossRef]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ards and other organ dysfunction. Ann. Intensive Care 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Holst, D.P.; Kaye, D.M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 2008, 14, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Jacob, M.; Rehm, M.; Loetsch, M.; Welsch, U.; Conzen, P.; Becker, B.F. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1993–H1999. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.R.; Jennings, C.A. Neutralisation of heparan sulphate and low molecular weight heparin by protamine. Thromb. Haemost. 1985, 53, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Passov, A.; Schramko, A.; Makisalo, H.; Nordin, A.; Andersson, S.; Pesonen, E.; Ilmakunnas, M. Graft glycocalyx degradation in human liver transplantation. PLoS ONE 2019, 14, e0221010. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Chen, R.; Liu, S.; Yu, X.; Zou, J.; Ding, X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesthesia 2016, 30, 82–89. [Google Scholar] [CrossRef]
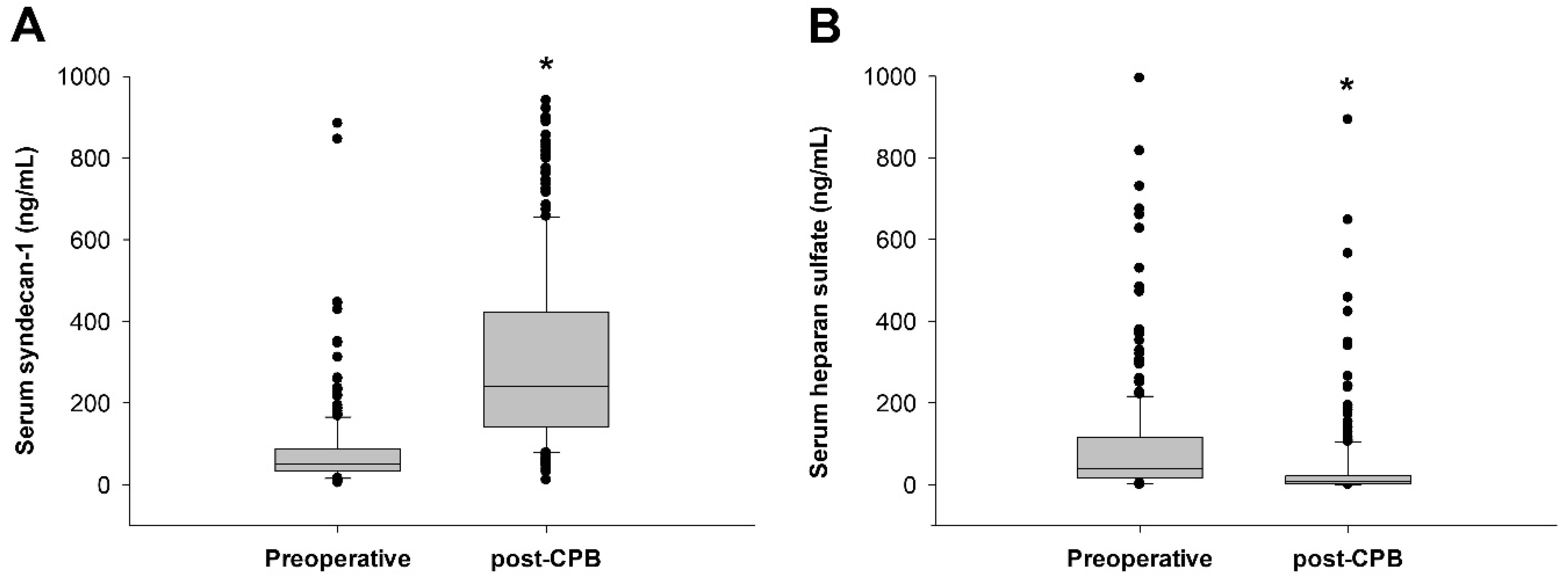
| Total (5–884 ng/mL) (N = 250) | Low SDC-1 Group (0–89 ng/mL) (N = 191) | High SDC-1 Group (90–884 ng/mL) (N = 59) | p Value | |
|---|---|---|---|---|
| Age (years) | 66 (57–73) | 66 (57–73) | 65 (56–74) | 0.648 |
| Male (n (%)) | 118 (47.2) | 87 (45.5) | 31 (52.5) | 0.347 |
| Body mass index (kg/m2) | 23.9 ± 3.8 | 24.0 ± 4.0 | 23.7 ± 2.9 | 0.567 |
| Hypertension | 133 (53.2) | 108 (56.5) | 25 (42.4) | 0.057 |
| Diabetes mellitus | 48 (19.2) | 36 (18.8) | 12 (20.3) | 0.799 |
| Chronic obstructive lung disease | 9 (3.6) | 6 (3.1) | 3 (5.1) | 0.690 |
| Preoperative serum Cr >1.2 mg/dL | 15 (6.0) | 7 (3.7) | 8 (13.6) | 0.010 |
| Prior myocardial infarction | 8 (3.2) | 6 (3.1) | 2 (3.4) | 0.924 |
| Congestive heart failure | 55 (22.0) | 38 (19.9) | 17 (28.8) | 0.148 |
| Coronary artery occlusive disease | 35 (14.0) | 28 (14.7) | 7 (11.9) | 0.589 |
| Peripheral artery occlusive disease | 3 (1.2) | 2 (1.0) | 1 (1.7) | >0.999 |
| Liver cirrhosis | 9 (3.6) | 4 (2.1) | 5 (8.5) | 0.036 |
| Preoperative steroid use | 5 (2.0) | 5 (2.6) | 0 (0.0) | 0.344 |
| Preoperative inotrope use | 9 (3.6) | 5 (2.6) | 4 (6.8) | 0.221 |
| Severe aortic stenosis | 62 (24.8) | 47 (24.6) | 15 (25.4) | 0.899 |
| Severe aortic regurgitation | 54 (21.6) | 44 (23.0) | 10 (16.9) | 0.321 |
| Severe mitral stenosis | 25 (10.0) | 15 (7.9) | 10 (16.9) | 0.042 |
| Severe mitral regurgitation | 75 (30) | 65 (34.0) | 10 (16.9) | 0.012 |
| Severe tricuspid regurgitation | 59 (23.6) | 34 (17.8) | 25 (42.4) | <0.001 |
| Type of surgery | 0.238 | |||
| Mitral valve repair/replacement | 116 (46.4) | 84 (44.0) | 32 (54.2) | |
| Aortic valve replacement | 72 (28.8) | 62 (32.5) | 10 (16.9) | |
| Double valve surgery | 23 (9.2) | 16 (8.4) | 7 (11.9) | |
| Valve + CABG | 22 (8.8) | 16 (8.4) | 6 (10.2) | |
| Valve + aorta | 17 (6.8) | 13 (6.8) | 4 (6.8) | |
| Left ventricular ejection fraction (%) | 62 ± 11 | 62 ± 12 | 60 ± 9 | 0.175 |
| LA volume index (mL/m2) | 64 (43–94) | 59 (39–88) | 67 (52–112) | 0.067 |
| RV systolic pressure (mmHg) | 36 (28–46) | 33 (27–43) | 41 (33–51) | 0.001 |
| Cleveland score | 2 (2–3) | 2 (1–3) | 2 (2–3) | 0.088 |
| EuroSCORE | 5 (3–7) | 5 (3–7) | 5 (3–8) | 0.371 |
| Pre-operative medication | ||||
| Beta-blockers | 95 (38.0) | 70 (36.6) | 25 (42.4) | 0.429 |
| RAS antagonists | 137 (54.8) | 109 (57.1) | 28 (47.5) | 0.195 |
| Calcium-channel blockers | 59 (23.6) | 51 (26.7) | 8 (13.6) | 0.053 |
| Antiplatelet agent | 57 (22.8) | 49 (25.7) | 8 (13.6) | 0.075 |
| Heparin | 77 (30.8) | 45 (23.6) | 32 (54.2) | <0.001 |
| Diuretics | 154 (61.6) | 110 (57.6) | 44 (74.6) | 0.019 |
| Statins | 94 (37.6) | 75 (39.3) | 19 (32.2) | 0.328 |
| Digoxin | 41 (16.4) | 28 (14.7) | 13 (22.0) | 0.181 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Acute kidney injury | 47 (18.8) | 28 (14.7) | 19 (32.2) | 0.003 |
| Stage 1 | 34 (13.6) | 23 (12.0) | 11 (18.6) | |
| Stage 2 | 6 (2.4) | 2 (1.0) | 4 (6.8) | |
| Stage 3 | 7 (2.8) | 3 (1.6) | 4 (6.8) | |
| Oliguria | 7 (2.8) | 3 (1.5) | 4 (6.8) | 0.025 |
| Renal replacement therapy | 3 (1.2) | 0 (0.0) | 3 (5.1) | 0.013 |
| Serum creatinine (mg/dL) | ||||
| Baseline | 0.83 ± 0.23 | 0.81 ± 0.20 | 0.92 ± 0.27 | 0.024 |
| Postoperative 6 h | 0.74 ± 0.28 | 0.71 ± 0.26 | 0.84 ± 0.32 | 0.024 |
| Postoperative 24 h | 0.97 ± 0.71 | 0.91 ± 0.75 | 1.14 ± 0.52 | 0.048 |
| Postoperative 48 h | 0.93 ± 1.21 | 0.89 ± 1.36 | 1.05 ± 0.43 | 0.688 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Duration of CPB (min) | 95 (70–120) | 90 (70–120) | 105 (75–136) | 0.131 |
| Crystalloid (mL) | 5859 (4784–6982) | 5791 (4703–5791) | 6406 (5248–7554) | 0.010 |
| Colloid (mL) | 600 (450–1000) | 600 (450–980) | 650 (450–1100) | 0.260 |
| Patients requiring pRBC transfusion (n (%)) | 148 (59.2) | 111 (58.1) | 37 (62.7) | 0.530 |
| Amount of pRBC transfusion (mL) | 280 (0–560) | 280 (0–280) | 280 (0–560) | 0.107 |
| Patients requiring FFP/cryoprecipitate/platelet transfusion (n (%)) | 91 (36.4) | 58 (30.3) | 33 (55.9) | <0.001 |
| Amount of FFP/cryoprecipitate/platelet transfusion (mL) | 0 (0–608) | 0 (0–260) | 260 (0–1280) | <0.001 |
| Urine output (mL) | 6358 (5430–7408) | 6175 (5340–7230) | 7000 (6165–7650) | 0.007 |
| Chest tube drainage (mL) | 470 (307–645) | 440 (290–617) | 532 (360–721) | 0.022 |
| Furosemide dose (mg) | 55 (25–80) | 50 (20–75) | 60 (40–100) | 0.019 |
| Norepinephrine dose (μg) | 972 (424–3329) | 882 (416–2875) | 1235 (469–3760) | 0.339 |
| Vasopressin dose (unit) | 1.0 (0–3.3) | 0.9 (0–3.2) | 1.0 (0–3.7) | 0.274 |
| Patients requiring inotrope (n (%)) | 112 (44.8) | 80 (41.8) | 32 (54.2) | 0.100 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| TNF-α (pg/mL) | ||||
| Post-induction | 1.58 (1.17–2.06) | 1.45 (1.14–1.92) | 1.85 (1.37–2.43) | <0.001 |
| Post-CPB | 6.00 (3.77–9.59) | 5.92 (3.39–9.61) | 6.20 (3.90–9.55) | 0.738 |
| IL-6 (pg/mL) | ||||
| Post-induction | 3.43 (2.18–6.72) | 3.10 (2.05–6.28) | 4.93 (2.71–9.68) | 0.018 |
| Post-CPB | 92.1 (26.6–283.7) | 84.9 (23.1–247.8) | 198.4 (53.1–308.7) | 0.062 |
| C-reactive protein (mg/L) | ||||
| Preoperative | 1.3 (0.6–2.9) | 1.2 (0.6–2.6) | 1.8 (0.9–5.4) | 0.060 |
| Postoperative 6 h | 10.3 (2.8–18.2) | 9.0 (3.2–19.9) | 10.4 (2.5–18.0) | >0.999 |
| Postoperative 24 h | 75.9 (52.7–105.5) | 75.2 (50.8–98.5) | 86.1 (65.5–147.1) | 0.040 |
| Postoperative 48 h | 164.7 (131.8–204.6) | 162.3 (131.0–206.3) | 175.6 (135.6–204.0) | >0.999 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Stroke | 2 (0.8) | 1 (0.5) | 1 (1.7) | 0.417 |
| Sternal infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Hemostatic reoperation | 5 (2.0) | 4 (2.1) | 1 (1.7) | >0.999 |
| Mechanical ventilation >24 h | 15 (6.0) | 8 (4.2) | 7 (11.9) | 0.053 |
| Mortality | 2 (0.8) | 2 (1.0) | 0 (0.0) | >0.999 |
| Length of ICU stay (day) | 3 (2–3) | 3 (2–3) | 3 (2–4) | 0.019 |
| Length of hospital stay (day) | 14 (11–18) | 13 (11–17) | 16 (12–24) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-B.; Soh, S.; Kwak, Y.-L.; Bae, J.C.; Kang, S.H.; Song, J.W. High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. J. Clin. Med. 2020, 9, 1803. https://doi.org/10.3390/jcm9061803
Kim H-B, Soh S, Kwak Y-L, Bae JC, Kang SH, Song JW. High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. Journal of Clinical Medicine. 2020; 9(6):1803. https://doi.org/10.3390/jcm9061803
Chicago/Turabian StyleKim, Hye-Bin, Sarah Soh, Young-Lan Kwak, Jae Chan Bae, Sang Hwa Kang, and Jong Wook Song. 2020. "High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery" Journal of Clinical Medicine 9, no. 6: 1803. https://doi.org/10.3390/jcm9061803






