Long-Term Effects in Bone Mineral Density after Different Bariatric Procedures in Patients with Type 2 Diabetes: Outcomes of a Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Randomization
2.2. Anthropometric Parameters
2.3. Standard Meal Test
2.4. Laboratory Determinations
2.5. Surgical Procedures
2.6. Statistical Analysis
3. Results
3.1. Changes in BMD after Bariatric Surgery
3.2. Correlation of BMD with Anthropometrics, Biochemical and Hormonal Parameters
3.3. Changes in BMD Regarding 5 Year T2D Outcomes after Surgery
3.4. Predicting Factors of BMD Reduction after Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuno, T.; Tanimoto, E.; Morita, S.; Shimada, Y.J. Effects of bariatric surgery on cardiovascular disease: A concise update of recent advances. Front. Cardiovasc. Med. 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Castanha, C.R.; Tcbc-Pe, Á.A.B.F.; Castanha, A.R.; Belo, G.Q.M.B.; Lacerda, R.M.R.; Vilar, L. Evaluation of quality of life, weight loss and comorbidities of patients undergoing bariatric surgery. Rev. Col. Bras. Cir. 2018, 45, e1864. [Google Scholar] [PubMed]
- Yu, E.W.; Kim, S.C.; Sturgeon, D.J.; Lindeman, K.G.; Weissman, J.S. Fracture risk after Roux-en-Y gastric bypass vs adjustable gastric banding among medicare beneficiaries. JAMA Surg. 2019, 154, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.M.; Haglind, E.G.C.; Clowes, J.A.; Achenbach, S.J.; Atkinson, E.J.; Melton, L.J.; Kennel, K.A. Fracture risk following bariatric surgery: A population-based study. Osteoporos. Int. 2013, 25, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.; Jean, S.; Gamache, P.; Lebel, S.; Mac-Way, F.; Biertho, L.; Michou, L.; Gagnon, C. Change in fracture risk and fracture pattern after bariatric surgery: Nested Case-Control study. BMJ 2016, 354, i3794. [Google Scholar] [CrossRef] [Green Version]
- Compston, J. Obesity and bone. Curr. Osteoporos. Rep. 2013, 11, 30–35. [Google Scholar] [CrossRef]
- Gnudi, S.; Sitta, E.; Lisi, L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J. Bone Miner. Metab. 2009, 27, 479–484. [Google Scholar] [CrossRef]
- Ong, T.; Sahota, O.; Tan, W.; Marshall, L. A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: A cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone 2014, 59, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Premaor, M.O.; Pilbrow, L.; Tonkin, C.; A Parker, R.; Compston, J. Obesity and fractures in postmenopausal women. J. Bone Miner. Res. 2009, 25, 292–297. [Google Scholar] [CrossRef]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Diez-Perez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: Glow. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Lespessailles, E.; Paccou, J.; Javier, R.-M.; Thomas, T.; Cortet, B. GRIO scientific committee obesity, bariatric surgery, and fractures. J. Clin. Endocrinol. Metab. 2019, 104, 4756–4768. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E. Type 2 diabetes mellitus and bone. J. Intern. Med. 2018, 283, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S. Diabetes and bone. Calcif. Tissue Int. 2017, 100, 107–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Abrahamsen, B.; Napoli, N.; Akesson, K.; Chandran, M.; Eastell, R.; Fuleihan, G.E.-H.; Josse, R.; Kendler, D.; Kraenzlin, M.; et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018, 29, 2585–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnon, C.; Schafer, A.L. Bone health after bariatric surgery. JBMR Plus 2018, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Silverberg, S.J. Bone loss after bariatric surgery: Causes, consequences, and management. Lancet Diabetes Endocrinol. 2014, 2, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Martínez, S.B.; Jauregui, E.P.; Martín, J.E.S. Impact of bariatric surgery on bone tissue. Endocrinol. Diabetes Nutr. 2019, 66, 62–68. [Google Scholar]
- Mieczkowska, A.; Irwin, N.; Flatt, P.; Chappard, D.; Mabilleau, G. Glucose-Dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone 2013, 56, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Wong, I.P.L.; Driessler, F.; Khor, E.C.; Shi, Y.-C.; Hörmer, B.; Nguyen, A.D.; Enriquez, R.F.; Eisman, J.A.; Sainsbury, A.; Herzog, H.; et al. Peptide YY regulates bone remodeling in mice: A link between gut and skeletal biology. PLoS ONE 2012, 7, e40038. [Google Scholar] [CrossRef]
- Nuche-Berenguer, B.; Moreno, P.; Esbrit, P.; Dapía, S.; Caeiro, J.R.; Cancelas, J.; Haro-Mora, J.J.; Villanueva-Peñacarrillo, M.L. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif. Tissue Int. 2009, 84, 453–461. [Google Scholar] [CrossRef]
- Carrasco, F.; Basfi-Fer, K.; Rojas, P.; Valencia, A.; Csendes, A.; Codoceo, J.; Inostroza, J.; Ruz, M. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: Relationships with variations in vitamin d, ghrelin, and adiponectin levels. Obes. Surg. 2014, 24, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Wewalka, M.; Ding, S.-A.; Simonson, D.C.; Foster, K.; Holst, J.J.; Vernon, A.; Goldfine, A.B.; Halperin, F. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2015, 101, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Maghrabi, A.H.; Wolski, K.; Abood, B.; Licata, A.; Pothier, C.; Bhatt, D.L.; Nissen, S.; Brethauer, S.A.; Kirwan, J.P.; Schauer, P.R.; et al. Two-Year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring) 2015, 23, 2344–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilarrasa, N.; José, P.S.; Garcia, I.; Gomez-Vaquero, C.; Miras, P.M.; De Gordejuela, A.G.R.; Masdevall, C.; Pujol, J.; Soler, J.; Gómez, J.M. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-Year Follow-Up. Obes. Surg. 2010, 21, 465–472. [Google Scholar] [CrossRef]
- Muschitz, C.; Kocijan, R.; Marterer, C.; Nia, A.R.; Muschitz, G.K.; Resch, H.; Pietschmann, P. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015, 100, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.; Cauley, J.A. The role of bone marrow and visceral fat on bone metabolism. Curr. Osteoporos. Rep. 2011, 9, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Pérez, F.; Casajoana, A.; Gómez-Vaquero, C.; Virgili, N.; López-Urdiales, R.; Hernández-Montoliu, L.; Pujol-Gebelli, J.; Osorio, J.; Alves, C.; Perez-Maraver, M.; et al. Changes in bone mineral density in patients with type 2 diabetes after different bariatric surgery procedures and the role of gastrointestinal hormones. Obes. Surg. 2019, 30, 180–188. [Google Scholar] [CrossRef]
- Bredella, M.A.; Greenblatt, L.; Eajazi, A.; Torriani, M.; Yu, E.W. Effects of Roux-En-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone 2016, 95, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Coupaye, M.; Rivière, P.; Breuil, M.C.; Castel, B.; Bogard, C.; Dupré, T.; Flamant, M.; Msika, S.; LeDoux, S. Comparison of nutritional status during the first year after sleeve gastrectomy and Roux-En-Y gastric bypass. Obes. Surg. 2013, 24, 276–283. [Google Scholar] [CrossRef]
- Hsin, M.-C.; Huang, C.-K.; Tai, C.-M.; Yeh, L.-R.; Kuo, H.-C.; Garg, A. A Case-Matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg. Obes. Relat. Dis. 2015, 11, 181–185. [Google Scholar] [CrossRef]
- Lanzarini, E.; Nogues, X.; Goday, A.; Benaiges, D.; De Ramón, M.; Villatoro, M.; Pera, M.; Grande, L.; Ramón, J.M. High-Dose vitamin D supplementation is necessary after bariatric surgery: A prospective 2-Year Follow-up study. Obes. Surg. 2015, 25, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Vix, M.; Liu, K.-H.; Diana, M.; D’Urso, A.; Mutter, D.; Marescaux, J. Impact of Roux-En-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: Short-Term results from a prospective randomized clinical trial. Surg. Endosc. 2013, 28, 821–826. [Google Scholar] [CrossRef]
- Casajoana, A.; Pujol, J.; Garcia, A.; Elvira, J.; Virgili, N.; De Oca, F.J.; Duran, X.; Fernández-Veledo, S.; Vendrell, J.; Vilarrasa, N. Predictive value of gut peptides in T2D remission: Randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes. Surg. 2017, 27, 2235–2245. [Google Scholar] [CrossRef]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L., 2nd; Robertson, R.P.; Rubino, F.; et al. How do we define cure of diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Pothuaud, L.; Barthe, N.; Krieg, M.-A.; Mehsen, N.; Carceller, P.; Hans, D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: A preliminary spine BMD–Matched, Case-Control study. J. Clin. Densitom. 2009, 12, 170–176. [Google Scholar] [CrossRef]
- Kanis, J.; McCloskey, E.V.; Johansson, H.; Oden, A.; Strom, O.; Borgström, F. Development and use of FRAX® in osteoporosis. Osteoporos. Int. 2010, 21, 407–413. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Kanis, J. Adjusting fracture probability by trabecular bone score. Calcif. Tissue Int. 2015, 96, 500–509. [Google Scholar] [CrossRef]
- Azagra, R.; Zwart, M.; Aguyé, A.; Martín-Sánchez, J.C.; Casado, E.; Díaz-Herrera, M.A.; Moriña, D.; Cooper, C.; Díez-Pérez, A.; Dennison, E.M.; et al. Fracture experience among participants from the FROCAT study: What thresholding is appropriate using the FRAX tool? Maturitas 2016, 83, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Brzozowska, M.; Sainsbury, A.; Eisman, J.A.; Baldock, P.A.; Center, J.R. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes. Rev. 2012, 14, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muschitz, C.; Kocijan, R.; Haschka, J.; Zendeli, A.; Pirker, T.; Geiger, C.; Müller, A.; Tschinder, B.; Kocijan, A.; Marterer, C.; et al. The impact of vitamin d, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: The BABS study. J. Bone Miner. Res. 2015, 31, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Tangalakis, L.L.; Tabone, L.; Spagnoli, A.; Muehlbauer, M.; Omotosho, P.; Torquati, A. Effects of Roux-En-Y gastric bypass on osteoclast activity and bone density in morbidly obese patients with type 2 diabetes. Obes. Surg. 2019, 30, 290–295. [Google Scholar] [CrossRef]
- Liu, C.; Wu, D.; Zhang, J.-F.; Xu, D.; Xu, W.-F.; Chen, Y.; Liu, B.-Y.; Li, P.; Li, L. Changes in bone metabolism in morbidly obese patients after bariatric surgery: A Meta-Analysis. Obes. Surg. 2015, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Näslund, I.; Rask, E.; Szabo, E. Effect of gastric bypass on bone mineral density, parathyroid hormone and vitamin d: 5 Years Follow-up. Obes. Surg. 2016, 26, 1141–1145. [Google Scholar] [CrossRef]
- Lindeman, K.G.; Greenblatt, L.; Rourke, C.; Bouxsein, M.L.; Finkelstein, J.S.; Yu, E.W. Longitudinal 5-Year evaluation of bone density and microarchitecture after Roux-En-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 2018, 103, 4104–4112. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.; Jørgensen, N.R.; Hermann, A.P.; Støving, R.K. Continuos decline in bone mineral density and deterioration of bone microarchitecture 7 years after Roux-En-Y gastric bypass surgery. Eur. J. Endocrinol. 2020, 182, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Fan, X.-T.; Li, S.-Z.; Zhai, T.; Dong, J. Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: A Meta-Analysis. Obes. Surg. 2019, 30, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Crawford, M.R.; Pham, N.; Khan, L.; Bena, J.F.; Schauer, P.R.; Kashyap, S.R. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr. Pract. 2018, 24, 256–264. [Google Scholar] [CrossRef]
- Scibora, L.M.; Ikramuddin, S.; Buchwald, H.; Petit, M.A. Examining the link between bariatric surgery, bone loss, and osteoporosis: A review of bone density studies. Obes. Surg. 2012, 22, 654–667. [Google Scholar] [CrossRef]
- Vilarrasa, N.; Gómez, J.M.; Elio, I.; Gómez-Vaquero, C.; Masdevall, C.; Pujol, J.; Virgili, N.; Burgos, R.; Sánchez-Santos, R.; de Gordejuela, A.G.; et al. Evaluation of bone disease in morbidily obese women after gastric bypass and risk factors implicated in bone loss. Obes. Surg. 2009, 19, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.L.; Paganotto, M.; Radominski, R.B.; Kulak, C.M.; Borba, V.C. Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporos. Int. 2014, 26, 757–764. [Google Scholar] [CrossRef]
- Coen, P.M.; Carnero, E.A.; Goodpaster, B.H. Exercise and bariatric surgery. Exerc. Sport Sci. Rev. 2018, 46, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.-Y. Age-Related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korner, J.; Inabnet, W.; Febres, G.; Conwell, I.M.; McMahon, N.J.; Salas, R.; Taveras, C.; Schrope, B.; Bessler, M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-En-Y gastric bypass. Int. J. Obes. 2009, 33, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, F.; Basfi-Fer, K.; Rojas, P.; Csendes, A.; Papapietro, K.; Codoceo, J.; Inostroza, J.; Krebs, N.F.; Westcott, J.L.; Miller, L.V.; et al. Calcium absorption may be affected after either sleeve gastrectomy or Roux-En-Y gastric bypass in premenopausal women: A 2-Y prospective study. Am. J. Clin. Nutr. 2018, 108, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Meng, J.; Jia, M.; Bi, L.; Zhou, Y.; Wang, Y.; Hu, J.; He, G.; Luo, X. Exendin-4, a Glucagon-Like Peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J. Bone Miner. Res. 2013, 28, 1641–1652. [Google Scholar] [CrossRef] [Green Version]
- Misra, M.; Miller, K.K.; Tsai, P.; Gallagher, K.; Lin, A.; Lee, N.; Herzog, D.B.; Klibanski, A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J. Clin. Endocrinol. Metab. 2006, 91, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, J.; Yu, H.; Zhang, P.; Han, J.; Bao, Y. Unacylated ghrelin is correlated with the decline of bone mineral density after Roux-En-Y gastric bypass in obese Chinese with type 2 diabetes. Surg. Obes. Relat. Dis. 2019, 15, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, Z.; Wang, L.; Fu, Z.; Ji, L.; Wu, X. The prevalence of osteoporosis tested by quantitative computed tomography in patients with different glucose tolerances. J. Clin. Endocrinol. Metab. 2019, 105, dgz036. [Google Scholar] [CrossRef]
- Madsen, L.R.; Espersen, R.; Ornstrup, M.J.; Jørgensen, N.R.; Langdahl, B.L.; Richelsen, B. Bone health in patients with type 2 diabetes treated by Roux-En-Y gastric bypass and the role of diabetes remission. Obes. Surg. 2019, 29, 1823–1831. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Li, J.; Chen, D.; Cheng, Z.; Xu, S.; Huang, Y.; Wang, Q. A Meta-Analysis of the effects of bariatric surgery on fracture risk. Obes. Rev. 2018, 19, 728–736. [Google Scholar] [CrossRef]
- Ablett, A.D.; Boyle, B.R.; Avenell, A. Fractures in adults after weight loss from bariatric surgery and weight management programs for obesity: Systematic review and Meta-analysis. Obes. Surg. 2019, 29, 1327–1342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Dong, J.; Zhou, D.; Liu, F. Comparative risk of fracture for bariatric procedures in patients with obesity: A systematic review and Bayesian network Meta-Analysis. Int. J. Surg. 2020, 75, 13–23. [Google Scholar] [CrossRef]
- Ahlin, S.; Peltonen, M.; Sjöholm, K.; Anveden, Å.; Jacobson, P.; Andersson-Assarsson, J.C.; Taube, M.; Larsson, I.; Lohmander, L.S.; Näslund, I.; et al. Fracture risk after three bariatric surgery procedures in Swedish obese subjects: Up to 26 years Follow-Up of a controlled intervention study. J. Intern. Med. 2020, 287, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Leib, E.; Winzenrieth, R.; Lamy, O.; Hans, D. Comparing bone microarchitecture by trabecular bone score (TBS) in Caucasian American women with and without osteoporotic fractures. Calcif. Tissue Int. 2014, 95, 201–208. [Google Scholar] [CrossRef]
- Marengo, A.P.; Pérez, F.G.; Martín, L.S.; Monseny, R.; Casajoana, A.; Yepes, R.V.; Virgili, N.; Simó-Servat, A.; Prats, A.; Gómez-Vaquero, C.; et al. Is trabecular bone score valuable in bone microstructure assessment after gastric bypass in women with morbid obesity? Nutrients 2017, 9, 1314. [Google Scholar] [CrossRef] [Green Version]
- Tothill, P.; Hannan, W.J.; Cowen, S.; Freeman, C.P. Anomalies in the measurement of changes in Total-Body bone mineral by Dual-Energy X-Ray absorptiometry during weight change. J. Bone Miner. Res. 1997, 12, 1908–1921. [Google Scholar] [CrossRef]
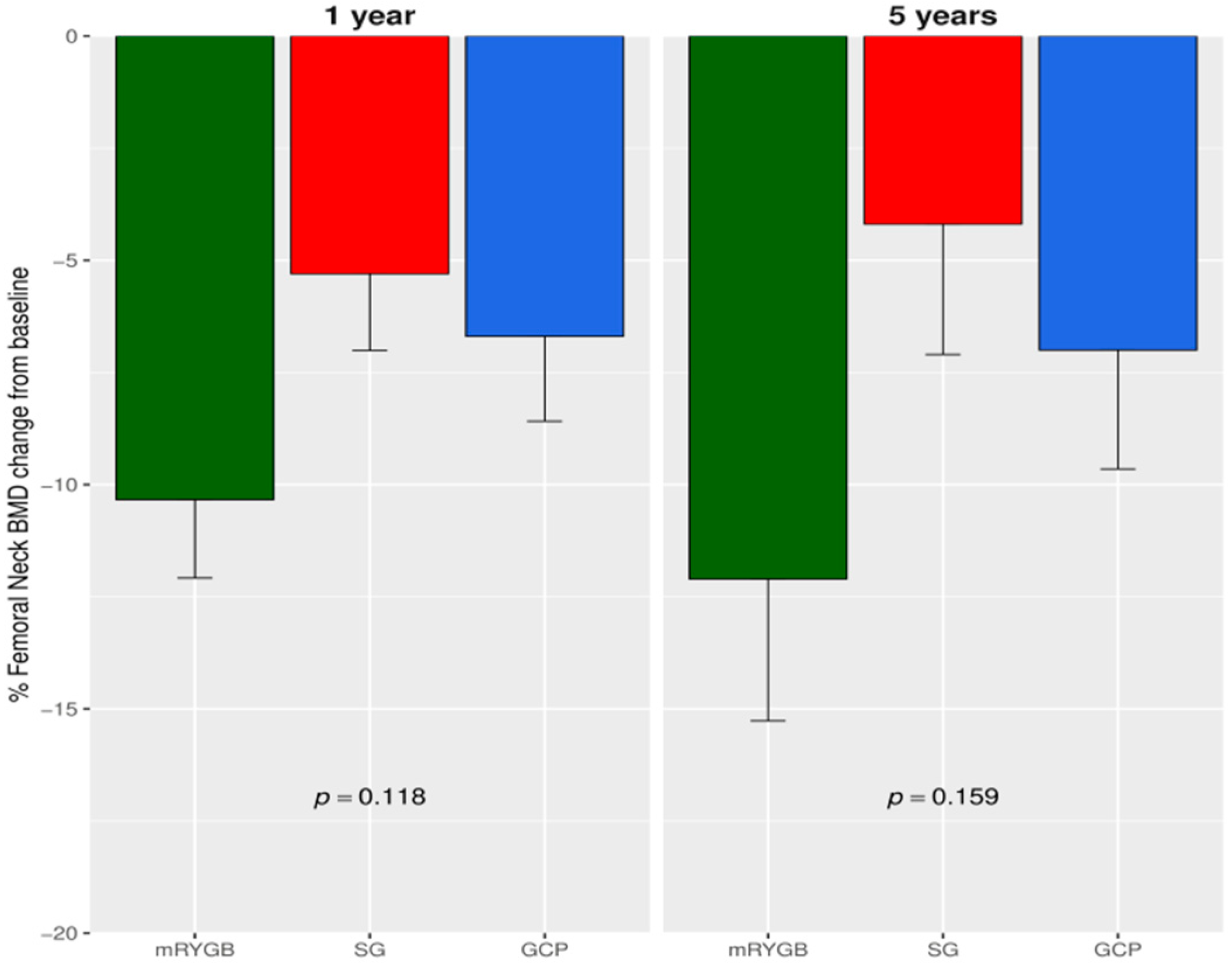
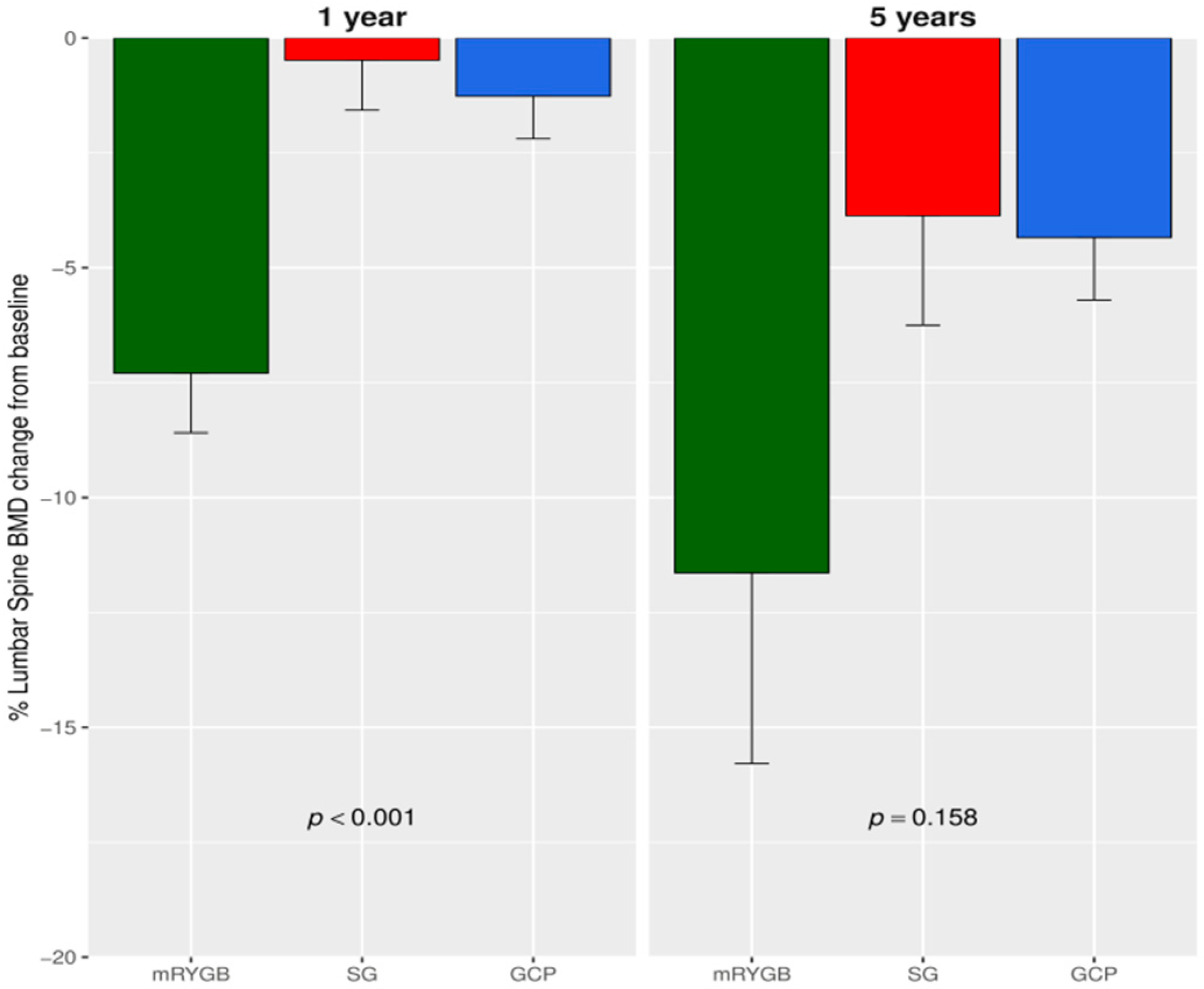
| Parameter | Metabolic Gastric Bypass | Sleeve Gastrectomy | Greater Curvature Plication | p |
|---|---|---|---|---|
| Sex (male/female) | 7/8 | 5/10 | 3/12 | 0.301 |
| Age (years) | 51.1 (7.70) | 49.2 (9.16) | 49.7 (8.12) | 0.827 |
| Weight (kg) | 103.01 (10.8) | 102.30 (10.7) | 105.53 (11.8) | 0.301 |
| BMI (kg/m2) | 38.73 (2.01) | 39.02 (1.68) | 40.90 (1.44) | 0.004 * |
| HbA1c (%) | 7.39 (1.95) | 7.89 (1.71) | 8.05 (2.15) | 0.498 |
| Calcium (mmol/L) | 2.35 (0.12) | 2.37 (0.12) | 2.6 (0.12) | 0.978 |
| Phosphate (mmol/L) | 1.06 (0.16) | 1.09 (0.18) | 1.08 (0.15) | 0.856 |
| PTH (pmol/L) | 4.75 (4.46) | 3.66 (1.58) | 5.05 (4.45) | 0.803 |
| Vitamin D (nmol/L) | 54.99 (21.35) | 52.67 (29.78) | 52.78 (25.97 | 0.606 |
| Fat Mass (kg) | 36.53 (8.09) | 34.22 (5.57) | 35.01 (12.27) | 0.414 |
| Lean Mass (kg) | 57.39 (10.80) | 53.78 (8.29) | 50.82 (17.33) | 0.670 |
| FNBMD | 0.89 [0.84;0.96] | 0.90 [0.81;0.96] | 0.95 [0.84;1.07] | 0.344 |
| FN T-score | −0.05 [−0.50;0.40] | 0.08 [−0.40;0.50] | 0.85 [−0.25;1.55] | 0.077 |
| FN Z-score | 0.81 [0.48;1.37] | 0.96 [0.10;1.60] | 1.54 [0.73;2.00] | 0.134 |
| LSBMD | 1.03 [0.98;1.09] | 1.11 [1.04;1.21] | 1.08 [0.99;1.14] | 0.255 |
| LS T-score | −0.59 [−1.15;0.05] | 0.28 [−0.40;1.10] | 0.09 [−0.45;0.50] | 0.082 |
| LS Z-score | 0.01 [−0.50;0.50] | 0.79 [0.10;1.80] | 0.62 [−0.05;1.03] | 0.239 |
| Parameter | Metabolic Gastric Bypass | Sleeve Gastrectomy | Greater Curvature Plication | p |
|---|---|---|---|---|
| Sex (male/female) | 6/8 | 4/8 | 3/10 | 0.552 |
| Age (years) | 55.2 (7.40) | 55.2 (8.30) | 53.6 (8.54) | 0.700 |
| Weight (kg) | 74.7 (9.97) | 84.4 (17.0) | 89.2 (11.7) | 0.014 * |
| BMI (kg/m2) | 28.1(2.99) | 32.0 (4.56) | 34.7 (3.68) | <0.001 * |
| HbA1c (%) | 5.43 (0.69) | 6.97 (1.32) | 7.07 (1.66) | 0.002 * |
| Calcium (mmol/L) | 2.32 (0.10) | 2.38 (0.10) | 2.38 (0.10) | 0.221 |
| Phosphate (mmol/L) | 1.13 (0.21) | 1.08 (0.22) | 1.08 (0.15) | 0.722 |
| PTH (pmol/L) | 8.39 (3.50) | 5.66 (2.19) | 6.73 (2.63) | 0.059 |
| Vitamin D (nmol/L) | 61.9 (46.7) | 65.3 (33.6) | 73.4 (52.3) | 0.801 |
| Fat Mass (kg) | 31.2 (6.37) | 40.0 (8.82) | 41.9 (6.86) | 0.001 * |
| Lean Mass (kg) | 39.4 (6.74) | 43.4 (8.91) | 43.1 (7.98) | 0.352 |
| FNBMD | 0.77 [0.72;0.82] | 0.83 [0.78;0.92] | 0.85 [0.74;0.98] | 0.259 |
| FN T-score | −1.08 [−1.68;−0.80] | −0.50 [−0.92;0.23] | −0.40 [−1.07;0.38] | 0.186 |
| FN Z-score | −0.08 [−0.40;0.20] | 0.60 [−0.05;1.15] | 0.78 [0.00;1.40] | 0.081 |
| LSBMD | 0.89 [0.82;0.94] | 1.04 [0.91;1.16] | 0.99 [0.89;1.12] | 0.020 * |
| LS T-score | −1.55 [−2.05;−1.20] | −0.04 [−1.12;1.21] | −0.83 [−1.68;0.23] | 0.011 * |
| LS Z-score | −0.82 [−1.30;−0.40] | 0.93 [0.15;1.83] | 0.34 [−0.80;1.40] | 0.004 * |
| Characteristic | ΔFN BMD | ΔLS BMD | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| Weightb (kg) | 0.450 | 0.009 * | 0.507 | 0.003 * |
| BMIb | 0.104 | 0.563 | 0.061 | 0.733 |
| Weight5 (kg) | 0.661 | <0.001 * | 0.656 | <0.001 * |
| BMI5 | 0.588 | <0.001 * | 0.499 | 0.003 * |
| Fat mass5 (kg) | 0.596 | <0.001 * | 0.509 | 0.003 * |
| Lean mass5 (kg) | 0.408 | 0.021 * | 0.565 | <0.001 * |
| Vitamin D5 (nmol/L) | −0.58 | 0.753 | 0.009 | 0.963 |
| PTH5 (pmol/L) | −0.224 | 0.217 | −0.251 | 0.166 |
| APh5 (µkat/L) | −0.260 | 0.143 | −0.418 | 0.016 * |
| ΔOsteocalcinb−1a (µg/L) | −0.241 | 0.191 | −0.360 | 0.047 * |
| HbA1c5 (%) | 0.452 | 0.008 * | 0.495 | 0.003 * |
| ΔGLP-1AUCb−1a | −0.337 | 0.080 | −0.528 | 0.004 * |
| ΔPYYb−1a | −0.114 | 0.586 | −0.124 | 0.553 |
| ΔGlucagonb−1a | −0.096 | 0.646 | 0.045 | 0.830 |
| ΔGhrelinb−1a | 0.142 | 0.453 | 0.241 | 0.199 |
| ΔAUC Insulinb−1a | −0.110 | 0.547 | 0.116 | 0.553 |
| ΔFN BMD | Estimate | Std. Error | p-Value |
| (Intercept) | −40.439 | 8.301 | <0.001 * |
| mRYGB | (1 Ref.) | ||
| SG | 3.584 | 3.636 | 0.333 |
| GCP | −0.559 | 4.028 | 0.891 |
| weight | 0.379 | 0.106 | <0.001 * |
| ΔLS BMD | Estimate | Std. Error | p-Value |
| Intercept | −5.825 | 3.268 | 0.086 |
| mRYGB | (Ref.) | ||
| SG | 9.113 | 4.005 | 0.031 |
| GCP | 9.881 | 4.228 | 0.027 |
| Male | (Ref.) | ||
| Female No Menop | −9.916 | 4.660 | 0.042 |
| Female Menop | −11.463 | 3.831 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Pérez, F.; Casajoana, A.; Gómez-Vaquero, C.; Virgili, N.; López-Urdiales, R.; Hernández-Montoliu, L.; Pujol-Gebelli, J.; Osorio, J.; Prats, A.; Vidal-Alabró, A.; et al. Long-Term Effects in Bone Mineral Density after Different Bariatric Procedures in Patients with Type 2 Diabetes: Outcomes of a Randomized Clinical Trial. J. Clin. Med. 2020, 9, 1830. https://doi.org/10.3390/jcm9061830
Guerrero-Pérez F, Casajoana A, Gómez-Vaquero C, Virgili N, López-Urdiales R, Hernández-Montoliu L, Pujol-Gebelli J, Osorio J, Prats A, Vidal-Alabró A, et al. Long-Term Effects in Bone Mineral Density after Different Bariatric Procedures in Patients with Type 2 Diabetes: Outcomes of a Randomized Clinical Trial. Journal of Clinical Medicine. 2020; 9(6):1830. https://doi.org/10.3390/jcm9061830
Chicago/Turabian StyleGuerrero-Pérez, Fernando, Anna Casajoana, Carmen Gómez-Vaquero, Nuria Virgili, Rafael López-Urdiales, Laura Hernández-Montoliu, Jordi Pujol-Gebelli, Javier Osorio, Anna Prats, Anna Vidal-Alabró, and et al. 2020. "Long-Term Effects in Bone Mineral Density after Different Bariatric Procedures in Patients with Type 2 Diabetes: Outcomes of a Randomized Clinical Trial" Journal of Clinical Medicine 9, no. 6: 1830. https://doi.org/10.3390/jcm9061830
APA StyleGuerrero-Pérez, F., Casajoana, A., Gómez-Vaquero, C., Virgili, N., López-Urdiales, R., Hernández-Montoliu, L., Pujol-Gebelli, J., Osorio, J., Prats, A., Vidal-Alabró, A., Pérez-Maraver, M., Fernández-Veledo, S., Vendrell, J., & Vilarrasa, N. (2020). Long-Term Effects in Bone Mineral Density after Different Bariatric Procedures in Patients with Type 2 Diabetes: Outcomes of a Randomized Clinical Trial. Journal of Clinical Medicine, 9(6), 1830. https://doi.org/10.3390/jcm9061830






