High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection
2.2. Microarray Analysis
2.3. Bioinformatic Analysis and Statistical Analysis
3. Results
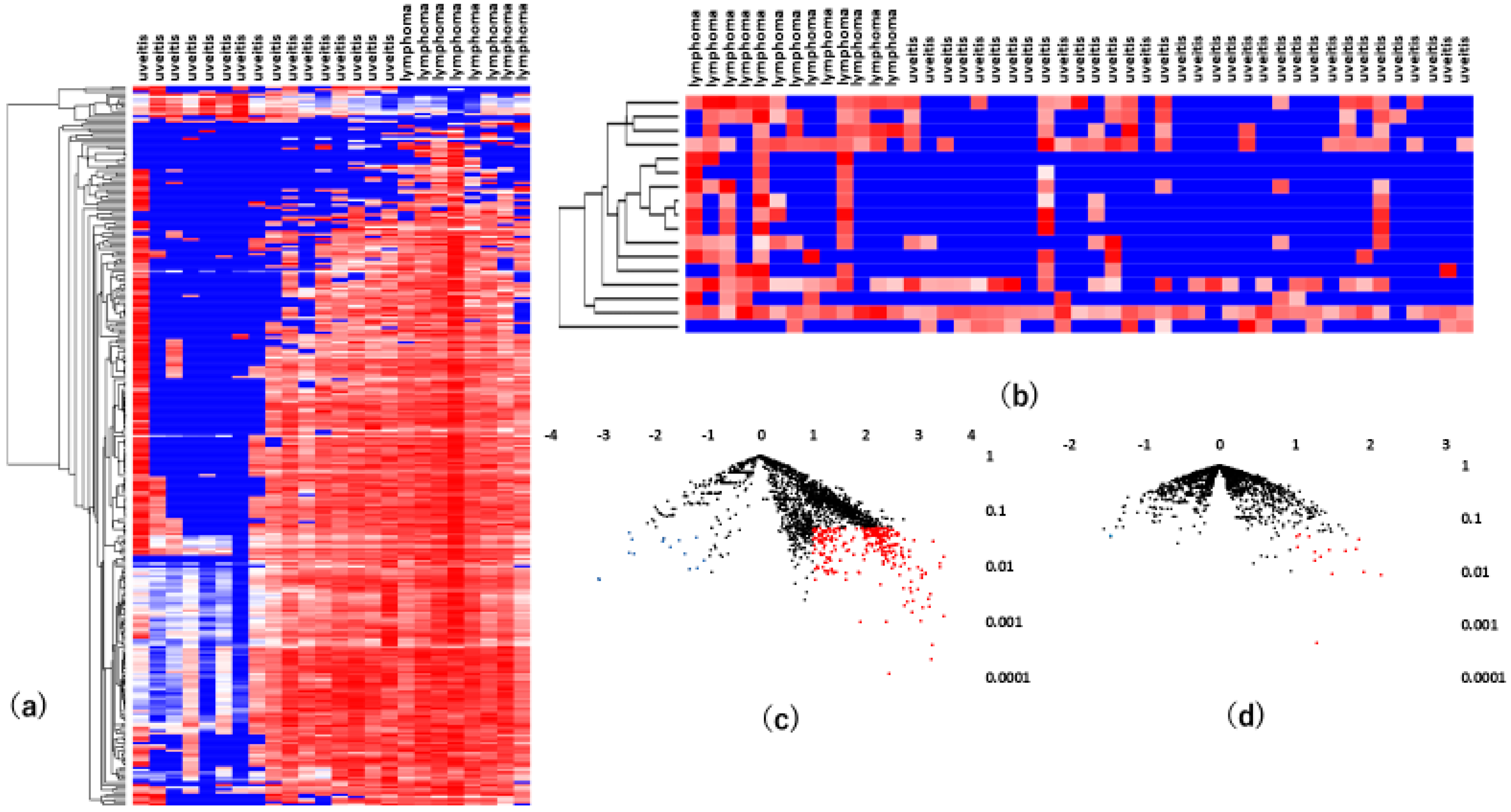
3.1. High-Throughput Vitreous and Serum miRNA Profiling in VRL Patients
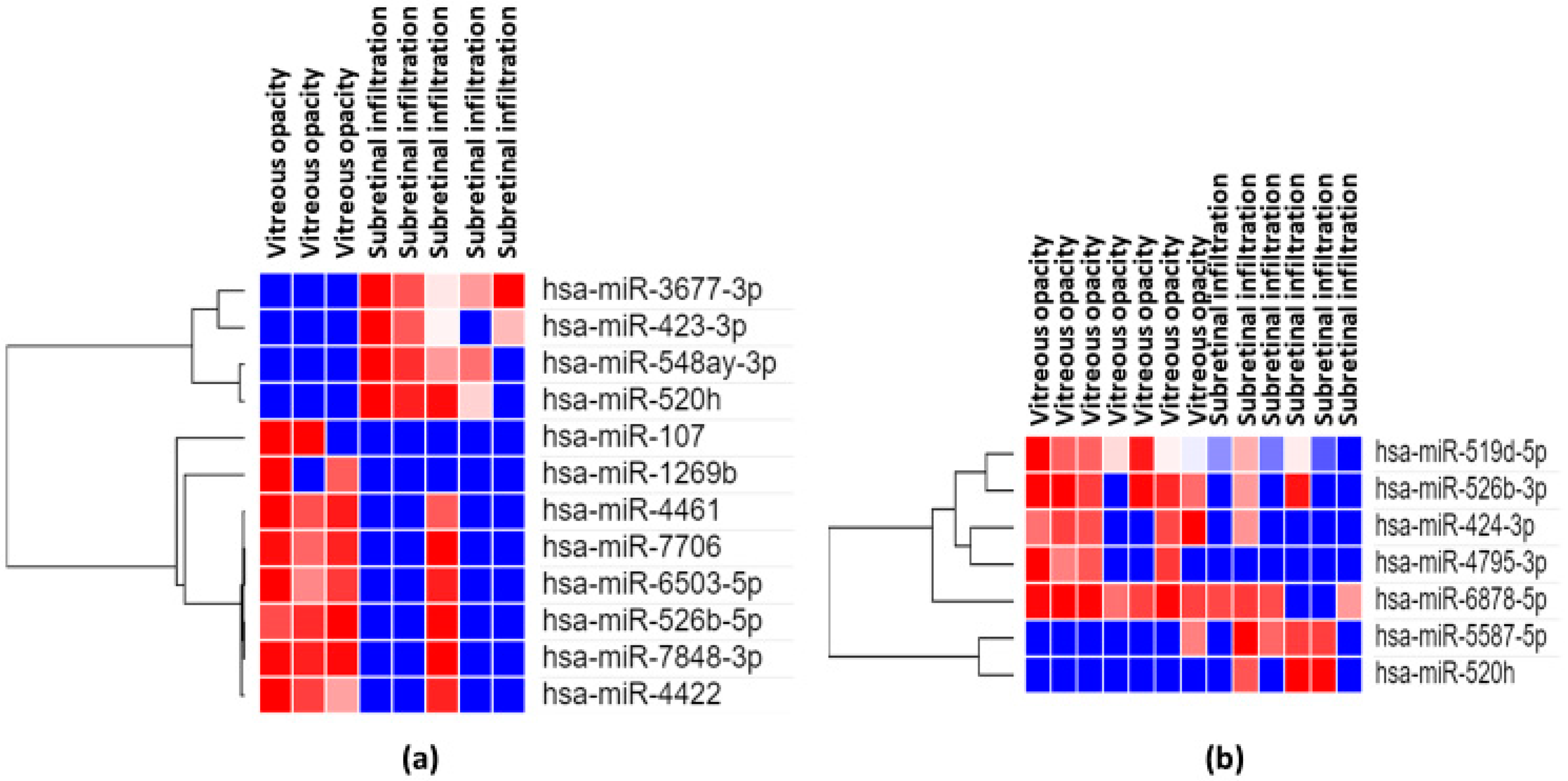
3.2. Vitreous miRNAs
3.3. Serum miRNAs
3.4. Comparison Between Vitreous and Serum miRNA Profiles of VRL Versus Uveitis
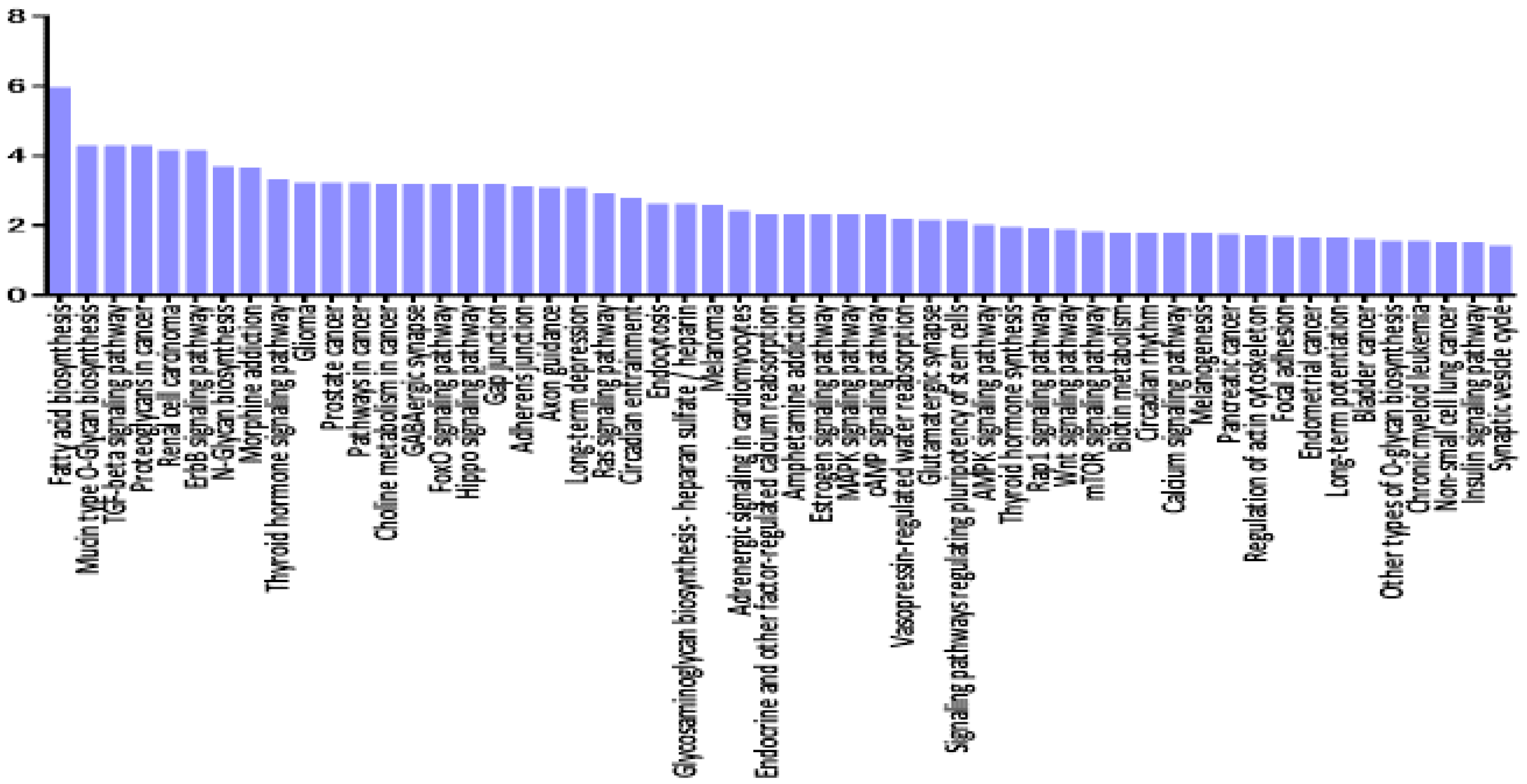
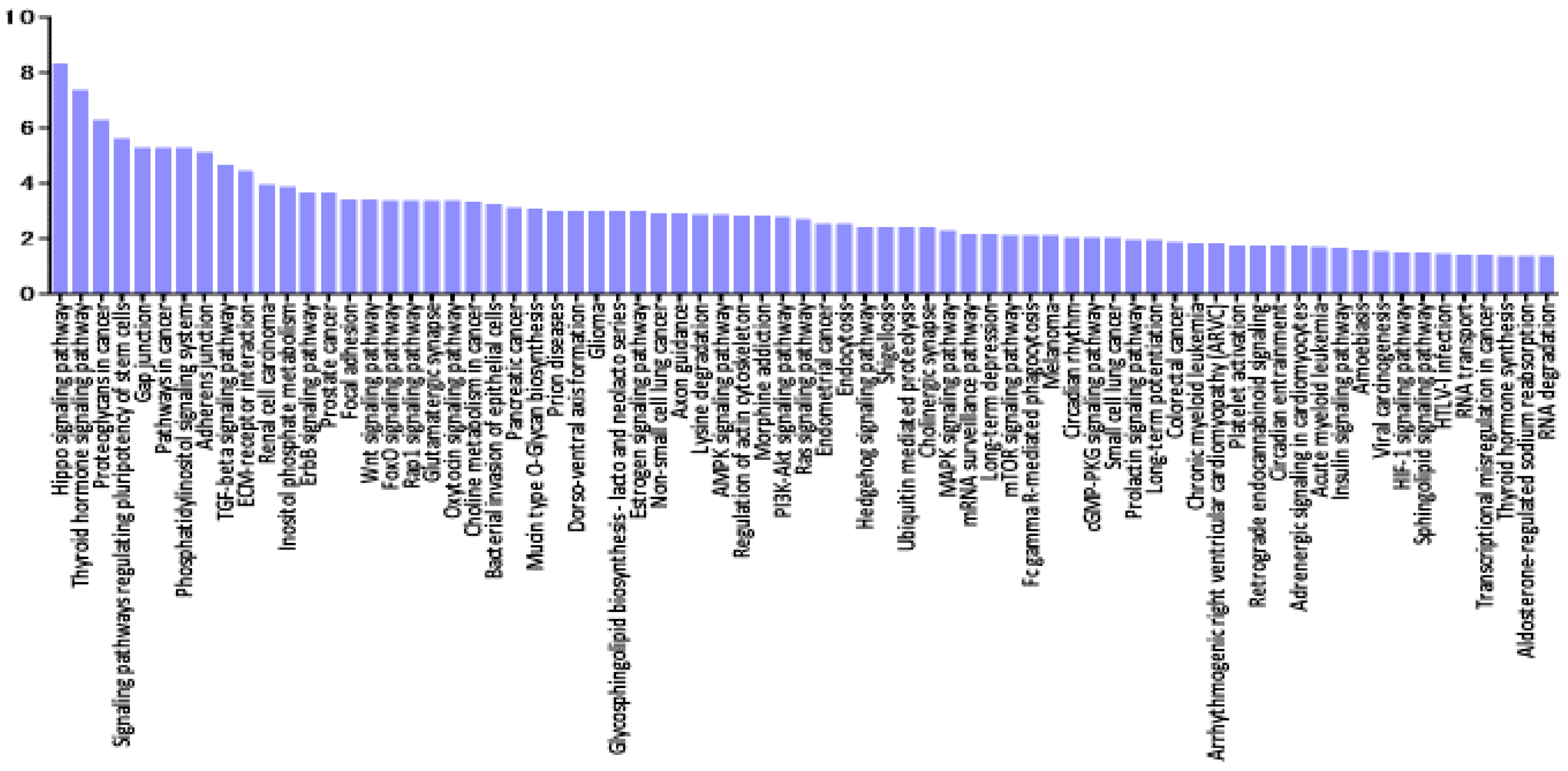
3.5. Pathway Enrichment Analysis of miRNAs Deregulated in VRL
3.6. Comparative Analysis of Selected miRNA Gene Targets and Differentially Expressed Genes in VRL
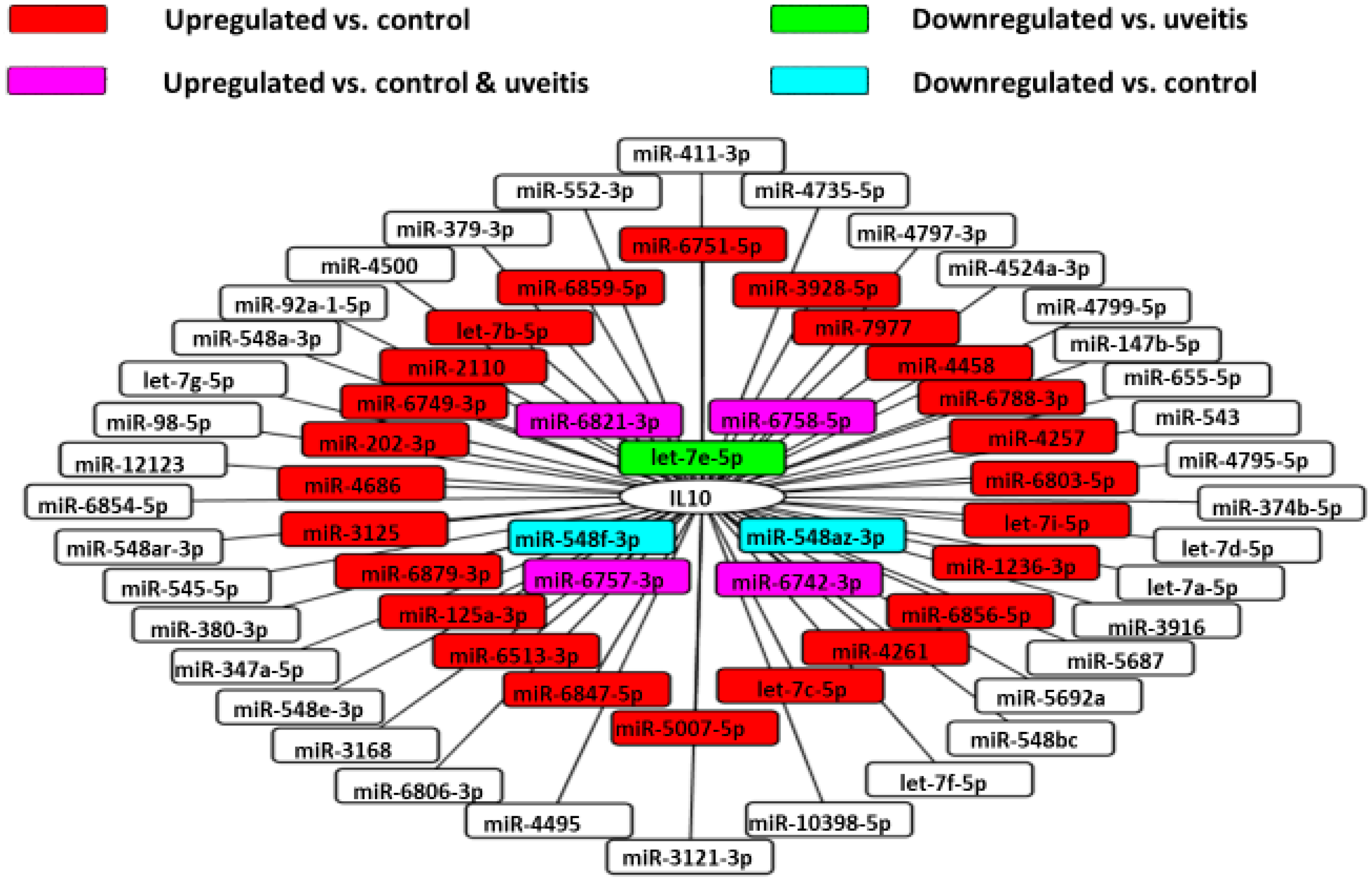
3.7. Vitreous IL-10 Levels and miRNAs
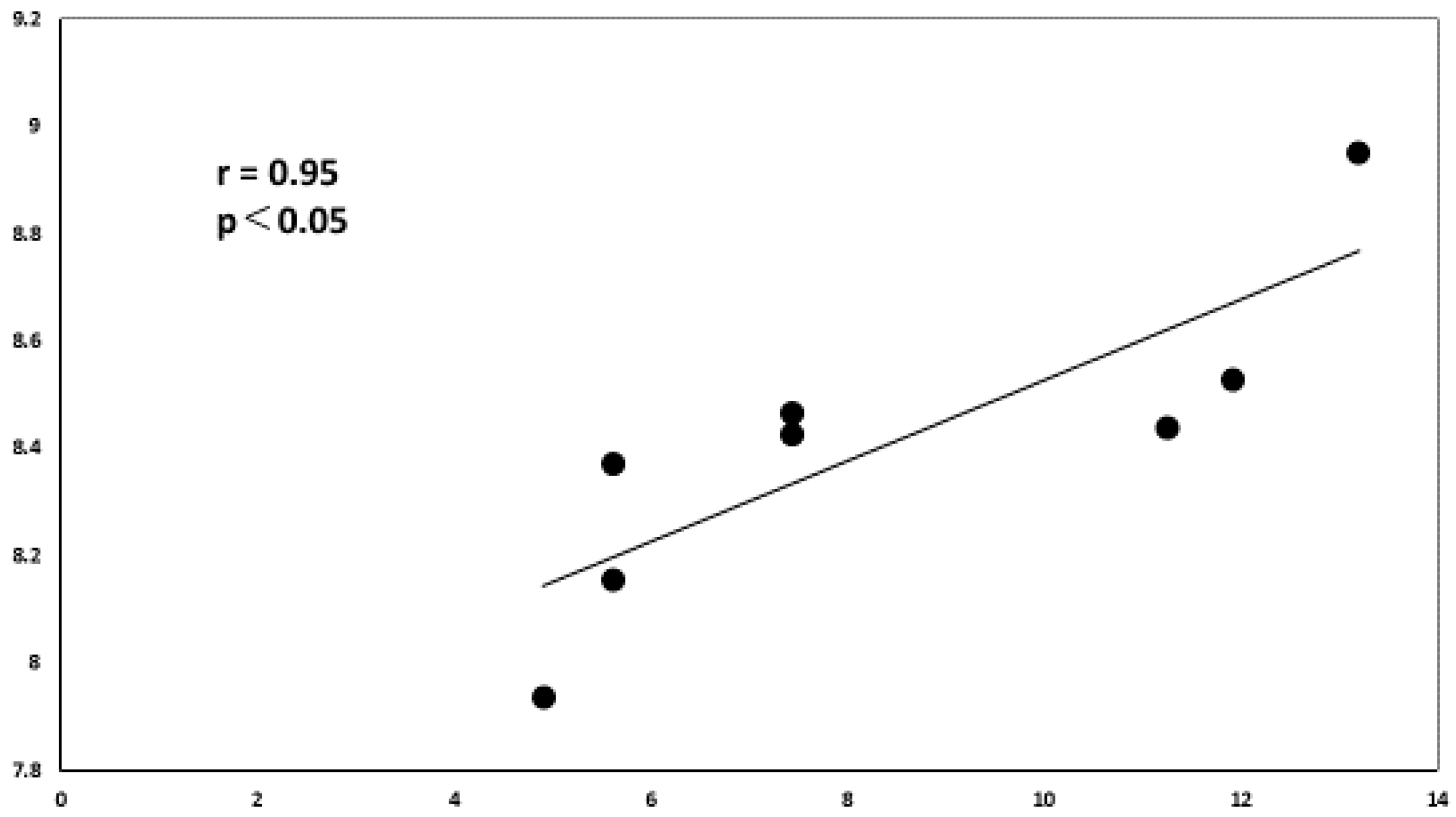
3.8. Association between Clinical Features and miRNAs
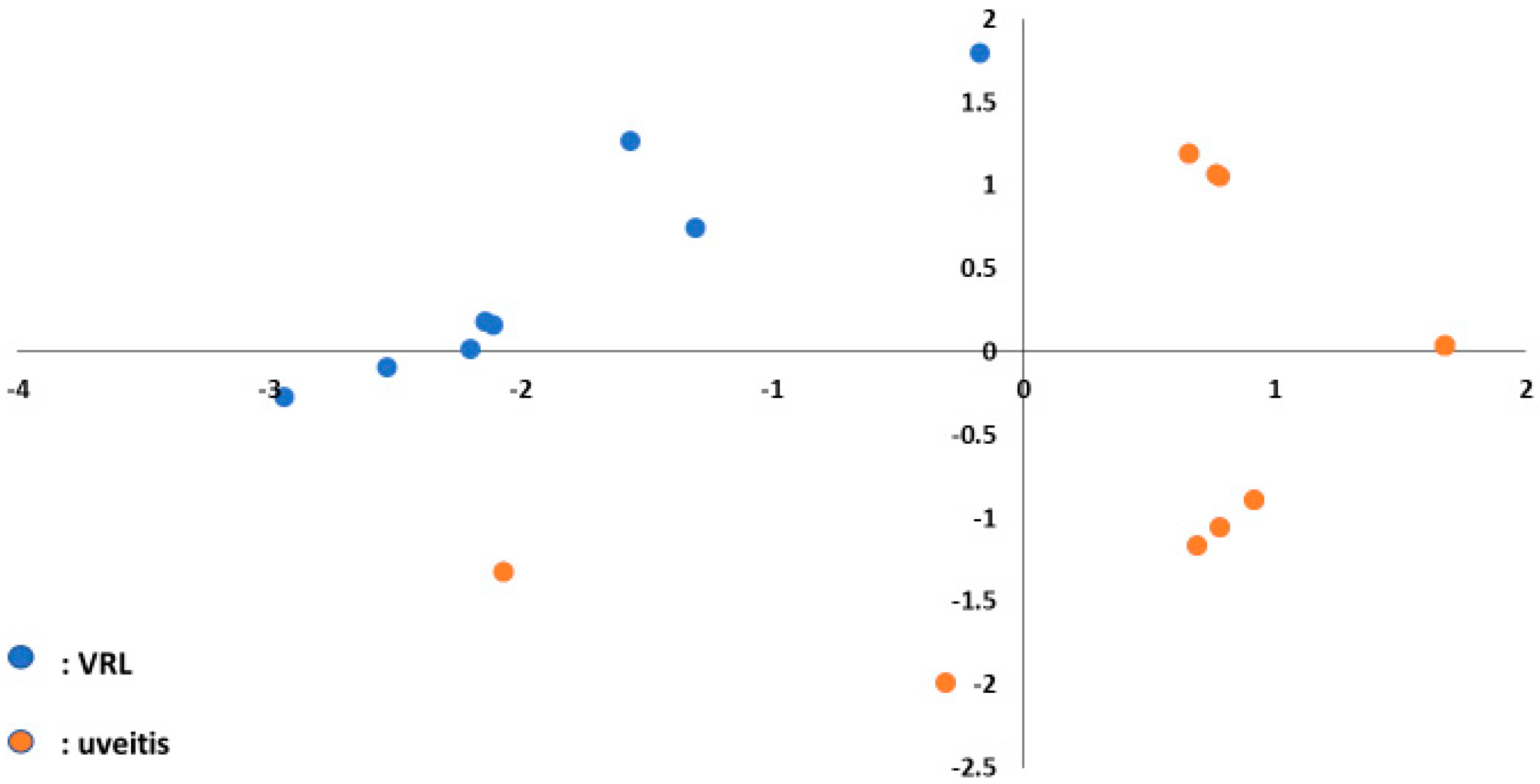
3.9. Machine Learning and miRNA Expression Validation in VRL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goto, H.; Mochizuki, M.; Yamaki, K.; Kotake, S.; Usui, M.; Ohno, S. Epidemiological Survey of Intraocular Inflammation in Japan. Jpn. J. Ophthalmol. 2007, 51, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, N.; Sonoda, K.-H.; Takeuchi, M.; Matsumura, M.; Mochizuki, M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn. J. Ophthalmol. 2012, 56, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L. Diagnosis of intraocular lymphoma. Ocul. Immunol. Inflamm. 2004, 12, 7–16. [Google Scholar] [CrossRef]
- Sen, H.N.; Bodaghi, B.; Le Hoang, P.; Nussenblatt, R. Primary intraocular lymphoma: Diagnosis and differential diagnosis. Ocul. Immunol. Inflamm. 2009, 17, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; The Japanese Intraocular Lymphoma Study Group; Usui, Y.; Goto, H. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn. J. Ophthalmol. 2012, 56, 383–389. [Google Scholar] [CrossRef]
- Akpek, E.K.; Maca, S.M.; Christen, W.G.; Foster, C. Elevated vitreous interleukin-10 level is not diagnostic of intraocular-central nervous system lymphoma. Ophthalmology 1999, 106, 2291–2295. [Google Scholar] [CrossRef]
- Pochat-Cotilloux, C.; Bienvenu, J.; Nguyen, A.-M.; Ohanessian, R.; Ghesquières, H.; Sève, P.; Garnier, L.; Kodjikian, L. Use of a threshold of interleukin-10 and il-10/il-6 ratio in ocular samples for the screening of vitreoretinal lymphoma. Retina 2018, 38, 773–781. [Google Scholar] [CrossRef]
- Yonese, I.; Takase, H.; Yoshimori, M.; Onozawa, E.; Tsuzura, A.; Miki, T.; Mochizuki, M.; Miura, O.; Arai, A. CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential. Eur. J. Haematol. 2018, 102, 191–196. [Google Scholar] [CrossRef]
- Hiemcke-Jiwa, L.S.; Loon, N.H.T.D.-V.; Leguit, R.J.; Nierkens, S.; Norel, J.O.-V.; De Boer, J.; Roholl, F.F.; De Weger, R.A.; Huibers, M.M.H.; De Groot-Mijnes, J.D.; et al. Potential Diagnosis of Vitreoretinal Lymphoma by Detection of MYD88 Mutation in Aqueous Humor With Ultrasensitive Droplet Digital Polymerase Chain Reaction. JAMA Ophthalmol. 2018, 136, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.-L.; Hou, H.-A.; Hsu, Y.-J.; Chen, Y.-K.; Tang, J.-L.; Tsay, W.; Yeh, P.-T.; Yang, C.; Lin, C.-P.; Tien, H.-F. Clinical outcomes of primary intraocular lymphoma patients treated with front-line systemic high-dose methotrexate and intravitreal methotrexate injection. Ann. Hematol. 2016, 95, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.S.; Johnston, P.B.; Nowakowski, G.S.; Castellino, A.; Raja, H. The diagnosis and treatment of primary vitreoretinal lymphoma: A review. Int. J. Retin. Vitr. 2018, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Baehring, J.M.; Androudi, S.; Longtine, J.J.; Betensky, R.A.; Sklar, J.; Foster, C.S.; Hochberg, F.H. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer 2005, 104, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Bavaharan, B.; Mahendradas, P.; Yadav, N.K. Primary vitreoretinal lymphoma: Prevalence, impact, and management challenges. Clin. Ophthalmol. 2019, 13, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkassery, V.; Schroers, R.; Coupland, S.; Wunderlich, M.-I.; Schargus, M.; Heinz, C.; Wasmuth, S.; Heiligenhaus, A.; Ahle, G.; Lenoble, P.; et al. Vitreous microRNA levels as diagnostic biomarkers for vitreoretinal lymphoma. Blood 2017, 129, 3130–3133. [Google Scholar] [CrossRef]
- Tuo, J.; Shen, D.; Yang, H.H.; Chan, C.-C. Distinct microRNA-155 expression in the vitreous of patients with primary vitreoretinal lymphoma and uveitis. Am. J. Ophthalmol. 2013, 157, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Song, M.-K.; Park, B.-B.; Uhm, J.-E. Understanding Immune Evasion and Therapeutic Targeting Associated with PD-1/PD-L1 Pathway in Diffuse Large B-cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 1326. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Cui, Z.; Liu, X.; Wu, S.; Wu, Y.; Fang, F.; Zhao, H. LncRNA FIRRE is activated by MYC and promotes the development of diffuse large B-cell lymphoma via Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2019, 510, 594–600. [Google Scholar] [CrossRef]
- Dobashi, A. Molecular Pathogenesis of Diffuse Large B-Cell Lymphoma. J. Clin. Exp. Hematop. 2016, 56, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Usui, Y.; Wakabayashi, Y.; Okunuki, Y.; Kimura, K.; Tajima, K.; Matsuda, R.; Ueda, S.; Ma, J.; Nagai, T.; Mori, H.; et al. Immune Mediators in Vitreous Fluids from Patients with Vitreoretinal B-Cell Lymphoma. Investig. Opthalmol. Vis. Sci. 2012, 53, 5395–5402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, K.; Kato, K.; Matsuzaki, J.; Boku, N.; Abe, S.; Saito, Y.; Daiko, H.; Takizawa, S.; Aoki, Y.; Sakamoto, H.; et al. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw. Open 2019, 2, e194573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugie, T.; Halvorson, I.; Thornhill, J. Brown adipose tissue temperature responses following electrical stimulation of ventromedial hypothalamic and lateral preoptic areas or after norepinephrine infusion to long evans or sprague-dawley rats. Brain Res. 1992, 575, 57–62. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, L.; Shi, L.; Yun, F.; Liu, X.; Chen, Y.; Chen, C.; Ren, Y.; Jia, Y. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell. Mol. Biol. Lett. 2019, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, J.; Xiao, Z.; Wang, X.; Luo, J. BBOX1-AS1 contributes to colorectal cancer progression by sponging hsa-miR-361-3p and targeting SH2B1. FEBS Open Bio 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Nan, A.; Zhang, N.; Jia, Y.; Li, X.; Ling, Y.; Dai, J.; Zhang, S.; Yang, Q.; Yi, Y.; et al. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol. Cancer 2019, 18, 13. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Chen, H.; Zhang, G.; Liu, H.; Kong, R.; Chen, H.; Wang, Y.; Li, Y.; Tian, F.; et al. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 807. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, H.; Liang, Z. circ-MYBL2 Serves As A Sponge For miR-361-3p Promoting Cervical Cancer Cells Proliferation And Invasion. OncoTargets Ther. 2019, 12, 9957–9964. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Chen, Y.; Jiang, B.; Bai, N.; Li, X. Hsa_circ_0011385 accelerates the progression of thyroid cancer by targeting miR-361-3p. Cancer Cell Int. 2020, 20, 49. [Google Scholar] [CrossRef]
- Zhao, D.; Cui, Z. MicroRNA-361-3p regulates retinoblastoma cell proliferation and stemness by targeting hedgehog signaling. Exp. Ther. Med. 2018, 17, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Zhou, Z.; Huang, H. miR-30b-3p Inhibits Proliferation and Invasion of Hepatocellular Carcinoma Cells via Suppressing PI3K/Akt Pathway. Front. Genet. 2019, 10, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Deng, Y.; Chen, G.; Yang, H. Investigation of the clinical significance and molecular mechanism of miR-21-5p in hepatocellular carcinoma: A systematic review based on 24 studies and bioinformatics investigation. Oncol. Lett. 2018, 17, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.; Bae, S.; Choi, Y.; Cha, H.; Kim, S.; Lee, J.; Jeon, S.; Jung, H.; Ahn, K.; et al. MicroRNA-1290 promotes asiatic acidinduced induced apoptosis by decreasing BCL2 protein level in A549 nonsmall cell lung carcinoma cells. Oncol. Rep. 2014, 32, 1029–1036. [Google Scholar] [CrossRef]
- Li, M.; He, X.-Y.; Zhang, Z.-M.; Li, S.; Ren, L.-H.; Cao, R.-S.; Feng, Y.-D.; Ji, Y.-L.; Zhao, Y.; Shi, R. MicroRNA-1290 promotes esophageal squamous cell carcinoma cell proliferation and metastasis. World J. Gastroenterol. 2015, 21, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Pe’Er, J.; Kaufman, R.; Maly, B.; Habot-Wilner, Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum MicroRNAs Are Promising Novel Biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.P.; Bruse, S.E.; David-Rus, R.; Buyske, S.; Brzustowicz, L.M. Altered MicroRNA Expression Profiles in Postmortem Brain Samples from Individuals with Schizophrenia and Bipolar Disorder. Biol. Psychiatry 2011, 69, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Sourvinou, I.S.; Markou, A.; Lianidou, E.S. Quantification of Circulating miRNAs in Plasma. J. Mol. Diagn. 2013, 15, 827–834. [Google Scholar] [CrossRef]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Go, H.; Jang, J.-Y.; Kim, P.-J.; Kim, Y.-G.; Nam, S.J.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.-W.; Jeon, Y.K. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget 2015, 6, 15035–15049. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shao, Q.; Li, C.; Liu, H.; Li, J.; Wang, Y.; Song, W.; Li, L.; Wang, G.; Shao, Z.; et al. Effects of microRNA-21 on apoptosis by regulating the expression of PTEN in diffuse large B-cell lymphoma. Medicine 2017, 96, e7952. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Du, J.; Ruan, L. MicroRNA-21 regulates the viability and apoptosis of diffuse large B-cell lymphoma cells by upregulating B cell lymphoma-2. Exp. Ther. Med. 2017, 14, 4489–4496. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Soneji, S.; Marafioti, T.; Cooper, C.D.; Palazzo, S.; Paterson, J.C.; Cattan, H.; Enver, T.; Mager, R.; Boultwood, J.; et al. Microrna expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int. J. Cancer 2007, 121, 1156–1161. [Google Scholar] [CrossRef]
- Jia, Y.J.; Liu, Z.B.; Wang, W.G.; Sun, C.B.; Wei, P.; Yang, Y.L.; You, M.J.; Yu, B.H.; Li, X.Q.; Zhou, X.Y. HDAC6 regulates microRNA-27b that suppresses proliferation, promotes apoptosis and target MET in diffuse large B-cell lymphoma. Leukemia 2017, 32, 703–711. [Google Scholar] [CrossRef]
- Mazan-Mamczarz, K.; Gartenhaus, R.B. Role of microRNA deregulation in the pathogenesis of diffuse large B-cell lymphoma (DLBCL). Leuk. Res. 2013, 37, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, X.; Li, J.; Yun, X.; Huang, R.; Deng, X.; Wang, Y.; Chen, Y.; Xiao, G. miR-181a-5p, an inducer of Wnt-signaling, facilitates cell proliferation in acute lymphoblastic leukemia. Oncol. Rep. 2017, 37, 1469–1476. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xiusheng, H.; Xiao, X.; Wang, Y. Overexpression of miR-422a inhibits cell proliferation and invasion, and enhances chemosensitivity in osteosarcoma cells. Oncol. Rep. 2016, 36, 3371–3378. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, S.; Jiao, J.-W.; Sun, K.-X.; Zong, Z.-H. MicroRNA-133b targets glutathione S-transferase π expression to increase ovarian cancer cell sensitivity to chemotherapy drugs. Drug Des. Dev. Ther. 2015, 9, 5225–5235. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shao, X.; Gao, W.; Zhang, Z.; Liu, P.; Wang, R.; Huang, P.; Yin, Y.; Shu, Y. MicroRNA-133b inhibits the growth of non-small-cell lung cancer by targeting the epidermal growth factor receptor. FEBS J. 2012, 279, 3800–3812. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Jiang, H.; Liu, Y.; Huang, Y.; Xiong, X.; Wu, H.; Dai, X. MicroRNA-133b inhibits hepatocellular carcinoma cell progression by targeting Sirt1. Exp. Cell Res. 2016, 343, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, Z.; Liu, Y.; Xu, B.; Tang, J. MicroRNA-133b Inhibits Proliferation, Cellular Migration, and Invasion via Targeting LASP1 in Hepatocarcinoma Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Ayeldeen, G.; Nassar, Y.; Ahmed, H.; Shaker, O.; Gheita, T. Possible use of miRNAs-146a and -499 expression and their polymorphisms as diagnostic markers for rheumatoid arthritis. Mol. Cell. Biochem. 2018, 449, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chang, W.-A.; Hsu, Y.-L.; Chen, C.-H.; Kuo, P.-L. Deduction of Novel Genes Potentially Involved in Osteoblasts of Rheumatoid Arthritis Using Next-Generation Sequencing and Bioinformatic Approaches. Int. J. Mol. Sci. 2017, 18, 2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormseth, M.J.; Solus, J.F.; Vickers, K.C.; Oeser, A.M.; Raggi, P.; Stein, C.M. Utility of Select Plasma MicroRNA for Disease and Cardiovascular Risk Assessment in Patients with Rheumatoid Arthritis. J. Rheumatol. 2015, 42, 1746–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Si, Z.-Z.; Li, T.; Li, J.-Q.; Zhang, Z.-Q.; Chen, G.-S.; Qi, H.-Z.; Yao, H. MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3-induced activation of the DNA repair pathway. Am. J. Physiol. Physiol. 2019, 316, C299–C311. [Google Scholar] [CrossRef]
- Zhuang, H.; Shen, J.; Zheng, Z.; Luo, X.; Gao, R.; Zhuang, X. MicroRNA-146a rs2910164 polymorphism and the risk of diffuse large B cell lymphoma in the Chinese Han population. Med. Oncol. 2014, 31, 306. [Google Scholar] [CrossRef]
- Javanmard, S.H.; Zare, N.; Eskandari, N.; Mehrzad, V. The expression level of hsa-miR-146a-5p in plasma-derived exosomes of patients with diffuse large B-cell lymphoma. J. Res. Med. Sci. 2019, 24, 10. [Google Scholar] [CrossRef]
- Zhu, T.-Y.; Zhang, H.-J.; Tao, J.; Sheng, L.; Hu, X.; Rong, R.-M.; Xu, M. Twist2 promotes kidney cancer cell proliferation and invasion by regulating ITGA6 and CD44 expression in the ECM-receptor interaction pathway. OncoTargets Ther. 2016, 9, 1801–1812. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.; Jin, W.; Fu, S.; Li, N.; Zhang, Y.; Sun, G.; Jiang, R.; Han, R.; Li, Z.; et al. Analyses of MicroRNA and mRNA Expression Profiles Reveal the Crucial Interaction Networks and Pathways for Regulation of Chicken Breast Muscle Development. Front. Genet. 2019, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Drillenburg, P.; Wielenga, V.; Kramer, M.; Van Krieken, J.; Kluin-Nelemans, H.C.; Hermans, J.; Heisterkamp, S.; Noordijk, E.; Kluin, P.; Pals, S. CD44 expression predicts disease outcome in localized large B cell lymphoma. Leukemia 1999, 13, 1448–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzankov, A. Prognostic significance of CD44 expression in diffuse large B cell lymphoma of activated and germinal centre B cell-like types: A tissue microarray analysis of 90 cases. J. Clin. Pathol. 2003, 56, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Zuo, C.; Wang, J.; Liu, J.; Jiao, B.; Zheng, J.; Cai, Z. Prognostic significance of the aggregative perivascular growth pattern of tumor cells in primary central nervous system diffuse large B-cell lymphoma. Neuro Oncol. 2013, 15, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tullio, G.; De Fazio, V.; Sgherza, N.; Minoia, C.; Serratì, S.; Merchionne, F.; Loseto, G.; Iacobazzi, A.; Rana, A.; Petrillo, P.; et al. Challenges and Opportunities of MicroRNAs in Lymphomas. Molecules 2014, 19, 14723–14781. [Google Scholar] [CrossRef] [Green Version]
- Nagel, S.; Hirschmann, P.; Dirnhofer, S.; Günthert, U.; Tzankov, A. Coexpression of CD44 variant isoforms and receptor for hyaluronic acid-mediated motility (RHAMM, CD168) is an International Prognostic Index and C-MYC gene status-independent predictor of poor outcome in diffuse large B-cell lymphomas. Exp. Hematol. 2010, 38, 38–45. [Google Scholar] [CrossRef]
- Cacemiro, M.D.C.; Berzoti-Coelho, M.G.; Cominal, J.G.; Burin, S.M.; De Castro, F.A. Hippo pathway deregulation: Implications in the pathogenesis of haematological malignancies. J. Clin. Pathol. 2016, 70, 9–14. [Google Scholar] [CrossRef]
- Cottini, F.; Hideshima, T.; Xu, C.; Sattler, M.; Dori, M.; Agnelli, L.; Hacken, E.T.; Bertilaccio, M.T.; Antonini, E.; Neri, A.; et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat. Med. 2014, 20, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Chorzalska, A.; Kim, J.F.; Roder, K.; Tepper, A.; Ahsan, N.; Rao, R.S.P.; Olszewski, A.J.; Yu, X.; Terentyev, D.; Morgan, J.; et al. Long-Term Exposure to Imatinib Mesylate Downregulates Hippo Pathway and Activates YAP in a Model of Chronic Myelogenous Leukemia. Stem Cells Dev. 2017, 26, 656–677. [Google Scholar] [CrossRef]
- Missotten, T.; Tielemans, D.; Bromberg, J.E.; Van Hagen, P.M.; Van Lochem, E.G.; Van Dongen, J.J.; Baarsma, G.S.; Langerak, A.W. Multicolor Flowcytometric Immunophenotyping Is a Valuable Tool for Detection of Intraocular Lymphoma. Ophthalmology 2013, 120, 991–996. [Google Scholar] [CrossRef]
- Tan, W.J.; Wang, M.M.; Ricciardi-Castagnoli, P.; Tang, T.; Chee, S.-P.; Lim, T.S.; Chan, A. Single-cell MYD88 sequencing of isolated B cells from vitreous biopsies aids vitreoretinal lymphoma diagnosis. Blood 2019, 134, 709–712. [Google Scholar] [CrossRef]
- Bonzheim, I.; Giese, S.; Deuter, C.; Süsskind, D.; Zierhut, M.; Waizel, M.; Szurman, P.; Federmann, B.; Schmidt, J.; Quintanilla-Martinez, L.; et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: A valuable tool to improve diagnostic yield of vitreous aspirates. Blood 2015, 126, 76–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Shi, F.; Qiu, H.; Tong, Y.; Gao, X. Identification of potential key genes associated with diffuse large B-cell lymphoma based on microarray gene expression profiling. Neoplasma 2017, 64, 824–833. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Fang, Y.; Lu, F.; Pan, J.; Wang, L.; Gong, W.; Fei, F.; Cui, J.; Zhong, J.; Hu, R.; et al. Analysis of differential expression profile of miRNA in peripheral blood of patients with lung cancer. J. Clin. Lab. Anal. 2019, 33, e23003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.-X.; Ma, M.-H.; Zhang, C.-D.; Shao, S.; Zhou, N.-M.; Dai, D.-Q. miR-1236-3p inhibits invasion and metastasis in gastric cancer by targeting MTA2. Cancer Cell Int. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, T.; Jiang, D.; Liu, J.; Yuan, X.; Feng, J.; Li, Q.; Zhang, Q.; Li, X.; Liu, Y.; Zhang, J. miR-1236-3p suppresses the migration and invasion by targeting KLF8 in lung adenocarcinoma A549 cells. Biochem. Biophys. Res. Commun. 2017, 492, 461–467. [Google Scholar] [CrossRef]
- Zhou, K.; Feng, X.; Wang, Y.; Liu, Y.; Tian, L.; Zuo, W.; Yi, S.; Wei, X.; Song, Y.; Qiu, L. miR-223 is repressed and correlates with inferior clinical features in mantle cell lymphoma through targeting SOX11. Exp. Hematol. 2018, 58, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Yin, Q.; Zhang, M.; Li, S.; Chen, S. Leukocyte miR-223-3p is not associated with altered platelet responses to clopidogrel in patients with coronary artery disease. J. Cent. South Univ. Med. Sci. 2018, 43, 421–427. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, Y.; Xu, J.; Li, W.-J.; Chen, Y.; Sun, H.-J. NFE2/miR-423-5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol. Cell Biol. 2019, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, G.; He, F.; Zhang, L.; Yang, K.; Yu, H.; Zhou, J.; Gan, H. MicroRNA 375 modulates hyperglycemia-induced enteric glial cell apoptosis and Diabetes-induced gastrointestinal dysfunction by targeting Pdk1 and repressing PI3K/Akt pathway. Sci. Rep. 2018, 8, 12681. [Google Scholar] [CrossRef] [Green Version]




| Vitreoretinal Lymphoma | Uveitis with Vitreous Opacity | Macular Hole/Epiretinal Membrane | Healthy Individuals | |||
|---|---|---|---|---|---|---|
| N | 14 | 40 | 26 | 12 | ||
| Age | 66.6 ± 12.0 | 68.1 ± 14.1 | 68.7 ± 9.5 | 53.9 ± 20.5 | ||
| Sex | Male | 5 | 8 | 12 | 6 | |
| Female | 9 | 32 | 14 | 6 | ||
| Clinical features | Main ocular lesion: vitreous | 7 | 40 | NA | NA | |
| sub-retina | 7 | 0 | NA | NA | ||
| Primary organ: eye | 7 | NA | NA | NA | ||
| Brain involvement | 10 | NA | NA | NA | ||
| Cytopathology (>class IV) | 1 | NA | NA | NA | ||
| IgH positivity | 11 | NA | NA | NA | ||
| IL-10/IL-6 ratio (>1) | 14 | NA | NA | NA | ||
| White blood cell (/μL) | 6392 | 6626 | 5952 | 6562 | ||
| IL-2 receptor (U/mL) | 323 | 416 | NA | NA | ||
| vs. Control; Up | vs. Uveitis; Up | vs. Control; Down | vs. Uveitis: Down |
|---|---|---|---|
| miR-629-3p | miR-3677-3p | miR-519c-5p, miR-523-5p miR-518e-5p, miR-522-5p miR-519a-5p, miR-519b-5p | miR-519c-5p, miR-523-5p miR-518e-5p, miR-522-5p miR-519a-5p, miR-519b-5p |
| miR-6740-5p | miR-6513-3p | miR-106a-3p | miR-27a-3p |
| miR-4647 | miR-5094 | miR-516a-5p | miR-3160-5p |
| miR-6780a-3p | miR-152-5p | miR-6869-3p | miR-2467-3p |
| miR-657 | miR-892a | miR-548s | miR-4710 |
| miR-1266-3p | miR-300 | miR-614 | miR-4718 |
| miR-4714-5p | miR-433-3p | miR-548f-3p | let-7e-5p |
| miR-3160-3p | miR-4650-5p | miR-4718 | miR-504-5p |
| miR-605-5p | miR-3927-5p | miR-3194-3p | miR-6070 |
| miR-4330 | miR-6841-5p | miR-3160-5p | miR-4755-3p |
| miR-7155-3p | miR-3973 | miR-4480 | miR-3194-3p |
| miR-3120-5p | miR-4524a-5p | miR-2467-3p | miR-191-5p |
| miR-216b-3p | miR-654-3p | miR-29b-2-5p | miR-526a, miR-520c-5p miR-518d-5p |
| miR-4756-3p | miR-6505-3p | miR-29b-3p | miR-4480 |
| miR-4733-3p | miR-4756-3p | miR-646 | miR-4789-5p |
| miR-6716-3p | miR-146b-3p | miR-515-5p | |
| miR-1184 | miR-603 | miR-6070 | |
| miR-8070 | miR-3126-3p | miR-4755-3p | |
| miR-4692 | miR-5194 | miR-31-3p | |
| miR-30b-5p | miR-7973 | miR-6835-3p |
| vs. Uveitis Up (Vit) | vs. Uveitis Up (Serum) | vs. Uveitis Down (Vit) | vs. Uveitis Down (Serum) |
|---|---|---|---|
| miR-3677-3p | miR-4475 | miR-519c-5p, miR-523-5p miR-518e-5p, miR-522-5p miR-519a-5p, miR-519b-5p | miR-548p |
| miR-6513-3p | miR-27b-3p | miR-27a-3p | |
| miR-5094 | miR-4684-5p | miR-3160-5p | |
| miR-152-5p | miR-1286 | miR-2467-3p | |
| miR-892a | miR-7848-3p | miR-4710 | |
| miR-300 | miR-6500-5p | miR-4718 | |
| miR-433-3p | miR-6513-3p | let-7e-5p | |
| miR-4650-5p | miR-5684 | miR-504-5p | |
| miR-3927-5p | miR-369-5p | miR-6070 | |
| miR-6841-5p | miR-500a-3p | miR-4755-3p | |
| miR-3973 | miR-4445-3p | miR-3194-3p | |
| miR-4524a-5p | miR-4439 | miR-191-5p | |
| miR-654-3p | miR-4434 | miR-526a, miR-520c-5p miR-518d-5p | |
| miR-6505-3p | miR-138-2-3p | miR-4480 | |
| miR-4756-3p | miR-181c-5p | miR-4789-5p | |
| miR-146b-3p | |||
| miR-603 | |||
| miR-3126-3p | |||
| miR-5194 | |||
| miR-7973 |
| microRNA | p-Value | Target Genes |
|---|---|---|
| miR-6817-5p | 6.17E-12 | COL6A5, THBS1, COLA6A, ITGA2, LAMC1, FN1 |
| miR-6799-3p | 0.02 | COL6A6, COL4A1 |
| miR-196a-5p | 4.98E-07 | COL24A1, COL3A1, COL1A2 |
| let-7c-5p | 7.78E-19 | COL27A1, ITGB6, COL3A1, COL1A1, COL1A2, ITGA7, COL4A6, COL5A2, COL4A1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minezaki, T.; Usui, Y.; Asakage, M.; Takanashi, M.; Shimizu, H.; Nezu, N.; Narimatsu, A.; Tsubota, K.; Umazume, K.; Yamakawa, N.; et al. High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis. J. Clin. Med. 2020, 9, 1844. https://doi.org/10.3390/jcm9061844
Minezaki T, Usui Y, Asakage M, Takanashi M, Shimizu H, Nezu N, Narimatsu A, Tsubota K, Umazume K, Yamakawa N, et al. High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis. Journal of Clinical Medicine. 2020; 9(6):1844. https://doi.org/10.3390/jcm9061844
Chicago/Turabian StyleMinezaki, Teruumi, Yoshihiko Usui, Masaki Asakage, Masakatsu Takanashi, Hiroyuki Shimizu, Naoya Nezu, Akitomo Narimatsu, Kinya Tsubota, Kazuhiko Umazume, Naoyuki Yamakawa, and et al. 2020. "High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis" Journal of Clinical Medicine 9, no. 6: 1844. https://doi.org/10.3390/jcm9061844





