Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. OCT Measurements
2.3. VD Measurement Using OCTA
2.4. Statistical Analysis
3. Results
3.1. Demographics
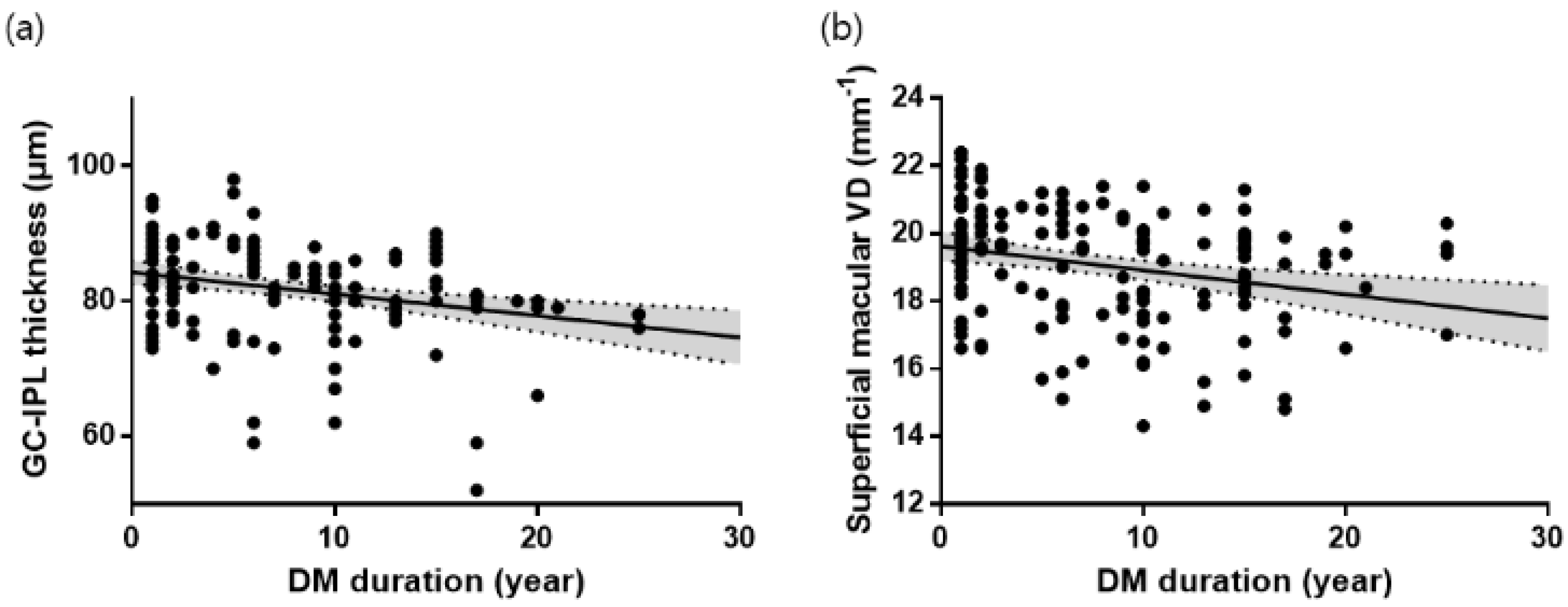
3.2. GC-IPL Thickness in Each Group
3.3. Superficial Macular VD in Each Group
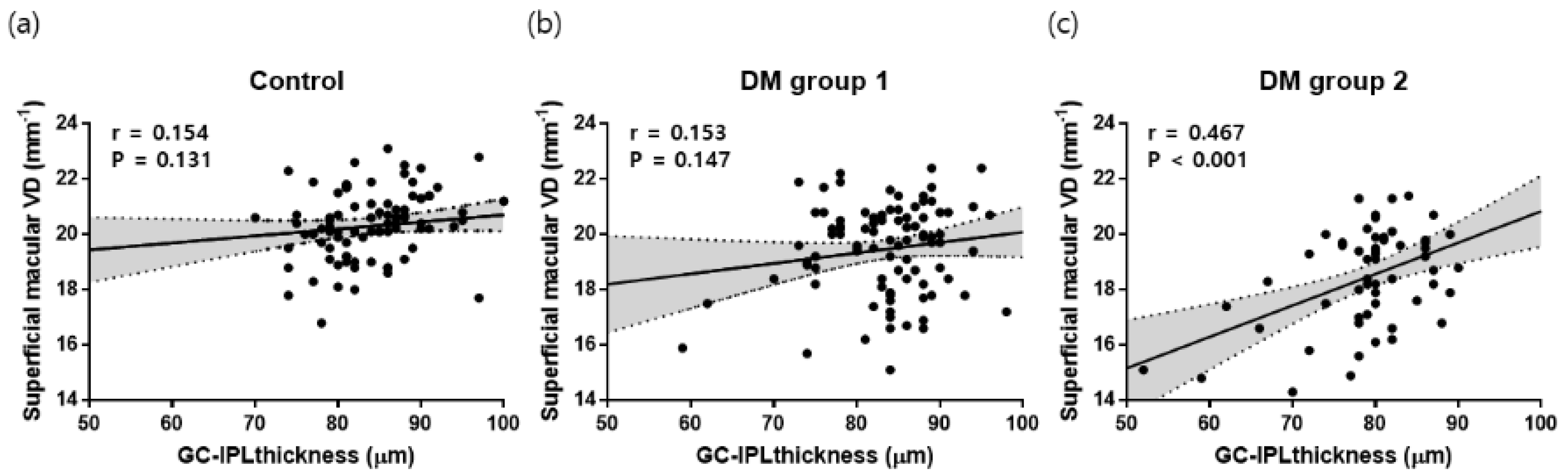
3.4. Factors Associated with Superficial Macular VD in T2DM Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Lu, Y.; Singh, G.M.; Carnahan, E.; Stevens, G.A.; Cowan, M.J.; Farzadfar, F.; Lin, J.K.; Finucane, M.M.; Rao, M.; et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984, 102, 527–532. [Google Scholar] [CrossRef]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef]
- Barot, M.; Gokulgandhi, M.R.; Patel, S.; Mitra, A.K. Microvascular complications and diabetic retinopathy: Recent advances and future implications. Future Med. Chem. 2013, 5, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef]
- Carpineto, P.; Toto, L.; Aloia, R.; Ciciarelli, V.; Borrelli, E.; Vitacolonna, E.; di Nicola, M.; di Antonio, L.; Mastropasqua, R. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye 2016, 30, 673. [Google Scholar] [CrossRef] [Green Version]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simó, R.; Hernández, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Yang, D.; Huang, Z.; Zeng, Y.; Wang, J.; Hu, Y.; Zhang, L. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018, 55, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Alzogool, M.; Xiao, J.; Zhang, S.; Zeng, P.; Lan, Y. Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol. 2018, 55, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, D.; Yu, H.; Yang, D.; Zhuang, X.; Hu, Y.; Li, J.; Yang, J.; Wu, Q.; Liu, B.; et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br. J. Ophthalmol. 2019, 103, 1747–1752. [Google Scholar] [PubMed]
- Alibhai, A.Y.; Moult, E.M.; Shahzad, R.; Rebhun, C.B.; Moreira-Neto, C.; McGowan, M.; Lee, D.; Lee, B.; Baumal, C.R.; Witkin, A.J.; et al. Quantifying microvascular changes using OCT angiography in diabetic eyes without clinical evidence of retinopathy. Ophthalmol. Retin. 2018, 2, 418–427. [Google Scholar] [CrossRef]
- Arend, O.; Wolf, S.; Jung, F.; Bertram, B.; Pöstgens, H.; Toonen, H.; Reim, M. Retinal microcirculation in patients with diabetes mellitus: Dynamic and morphological analysis of perifoveal capillary network. Br. J. Ophthalmol. 1991, 75, 514–518. [Google Scholar] [CrossRef]
- Blair, N.P.; Wanek, J.; Felder, A.E.; Joslin, C.E.; Kresovich, J.K.; Lim, J.I.; Chau, F.Y.; Leiderman, Y.; Shahidi, M. Retinal oximetry and vessel diameter measurements with a commercially available scanning laser ophthalmoscope in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5556–5563. [Google Scholar] [CrossRef] [Green Version]
- Talisa, E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015, 35, 2364–2370. [Google Scholar]
- Bearse , M.A., Jr.; Adams, A.J.; Han, Y.; Schneck, M.E.; Ng, J.; Bronson-Castain, K.; Barez, S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog. Retin. Eye Res. 2006, 25, 425–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, D.S.; Chiang, P.P.; Tan, G.; Cheung, C.G.; Cheng, C.Y.; Cheung, C.Y.; Wong, T.Y.; Lamoureux, E.L. Mohammad K Ikram. Retinal ganglion cell neuronal damage in diabetes and diabetic retinopathy. Clin. Exp. Ophthalmol. 2016, 44, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.B.; Shin, Y.I.; Lee, M.W.; Kim, J.Y. Ganglion Cell–Inner Plexiform Layer Damage in Diabetic Patients: 3-Year Prospective, Longitudinal, Observational Study. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, T.S.; Barber, A.J. Retinal ganglion cells in diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Carrasco, E.; Hernández, C.; Miralles, A.; Huguet, P.; Farrés, J.; Simó, R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Ciudin, A.; Simó-Servat, O.; Hernández, C. Cognitive impairment and dementia: A new emerging complication of type 2 diabetes—The diabetologist’s perspective. Acta Diabetol. 2017, 54, 417–424. [Google Scholar] [CrossRef]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef]
- Abcouwer, S.F.; Gardner, T.W. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson-Berka, J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006, 38, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.; Biswas, S.K.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes 2009, 58, 1382–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liu, W.; Chen, S.; Backman, V.; Sheibani, N.; Sorenson, C.M.; Fawzi, A.A.; Linsenmeier, R.A.; Zhang, H.F. Visible light optical coherence tomography measures retinal oxygen metabolic response to systemic oxygenation. Light Sci. Appl. 2015, 4, e334. [Google Scholar] [CrossRef] [Green Version]
- Trick, G.L.; Burde, R.M.; Cordon, M.O.; Santiago, J.V.; Kilo, C. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology 1988, 95, 693–698. [Google Scholar] [CrossRef]
- Kurtenbach, A.; Wagner, U.; Neu, A.; Schiefer, U.; Ranke, M.B.; Zrenner, E. Brightness matching and colour discrimination in young diabetics without retinopathy. Vis. Res. 1994, 34, 115–122. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef] [Green Version]
- Vujosevic, S.; Muraca, A.; Gatti, V.; Masoero, L.; Brambilla, M.; Cannillo, B.; Villani, E.; Nucci, P.; De Cillà, S. Peripapillary microvascular and neural changes in diabetes mellitus: An OCT-angiography study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5074–5081. [Google Scholar] [CrossRef] [Green Version]
- Mase, T.; Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Yoshida, A. Radial peripapillary capillary network visualized using wide-field montage optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.B.; Lee, M.W.; Park, J.H.; Kim, K.; Jo, Y.J.; Kim, J.Y. Changes in ganglion cell–inner plexiform layer thickness and retinal microvasculature in hypertension: An optical coherence tomography angiography study. Am. J. Ophthalmol. 2019, 199, 167–176. [Google Scholar] [CrossRef]
- Leung, C.K.; Chan, W.M.; Yung, W.H.; Ng, A.C.K.; Woo, J.; Tsang, M.K.; Tse, R.K.K. Comparison of macular and peripapillary measurements for the detection of glaucoma: An optical coherence tomography study. Ophthalmology 2005, 112, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Henkind, P. Symposium on glaucoma: Joint meeting with the national society for the prevention of blindness: New observations on the radial peripapillary capillaries. Investig. Ophthalmol. Vis. Sci. 1967, 6, 103–108. [Google Scholar]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Kergoat, H.; Hérard, M.E.; Lemay, M. RGC sensitivity to mild systemic hypoxia. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5423–5427. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence angiography. Retina 2015, 35, 2163. [Google Scholar] [CrossRef]
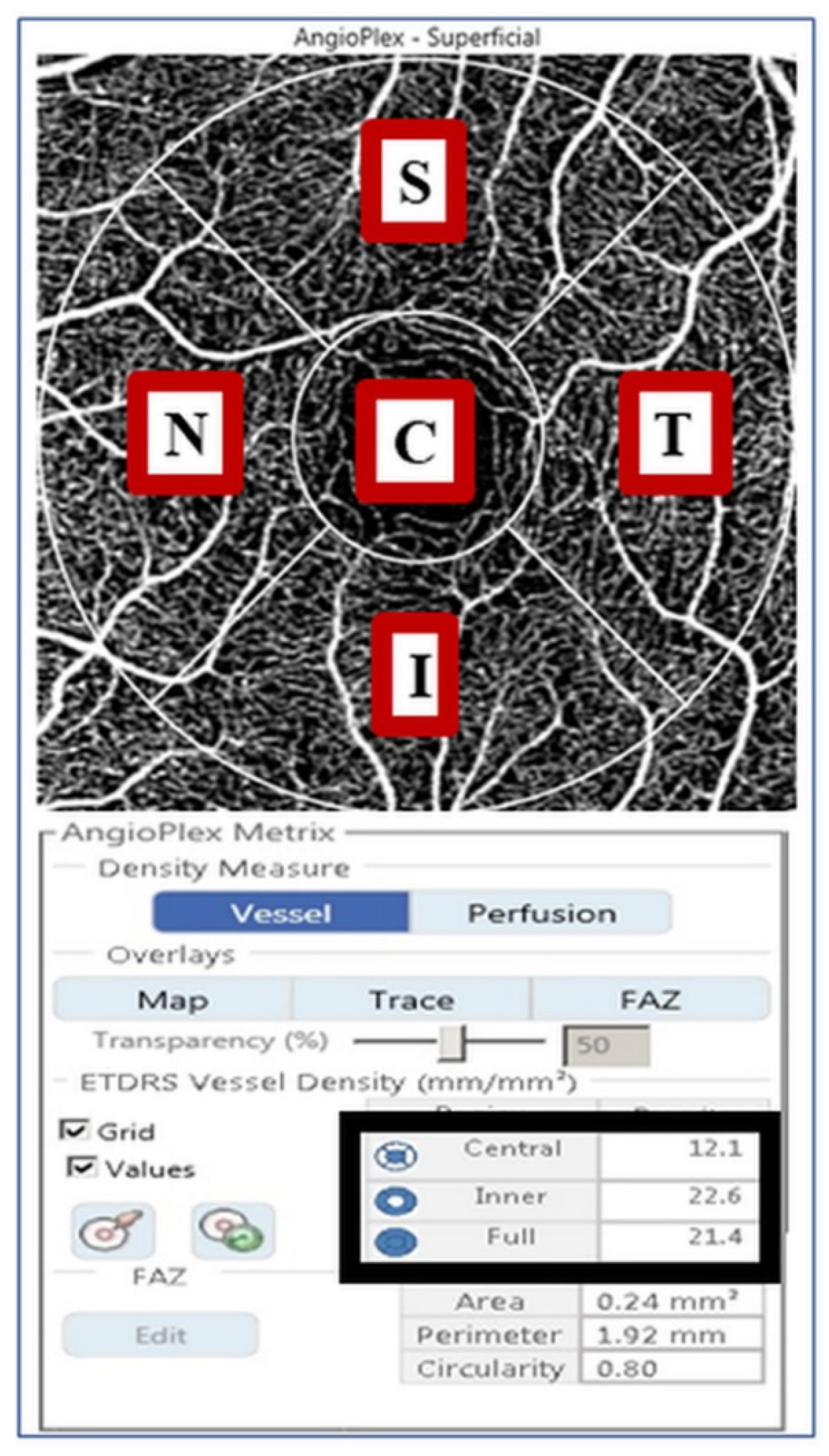

| Normal Controls (n = 97) | DM Group 1 (n = 92) | DM Group 2 (n = 57) | p-Value | |
|---|---|---|---|---|
| Age (mean ± SE, years) | 63.82 ± 0.44 | 62.43 ± 1.09 | 64.12 ± 1.11 | 0.356 |
| Sex (male, %) | 44 (45.4%) | 35 (38.0%) | 28 (49.1%) | 0.371 |
| Laterality (right, %) | 55 (56.7%) | 46 (50.0%) | 28 (49.1%) | 0.555 |
| BCVA (mean ± SE, logMAR) | −0.019 ± 0.006 | 0.002 ± 0.007 | 0.003 ± 0.007 | 0.027 |
| SE (mean ± SE, diopters) | 0.06 ± 0.11 | −0.36 ± 0.14 | 0.07 ± 0.18 | 0.060 |
| IOP (mean ± SE, mmHg) | 15.64 ± 0.27 | 15.96 ± 0.32 | 15.47 ± 0.36 | 0.600 |
| Axial length (mean ± SE, mm) | 23.57 ± 0.07 | 23.72 ± 0.08 | 23.51 ± 0.11 | 0.196 |
| DM duration (mean ± SE, years) | 0 | 3.51 ± 0.29 | 14.61 ± 0.57 | <0.001 |
| HbA1C (mean ± SE, %) | N/A | 6.90 ± 0.10 | 7.04 ± 0.12 | 0.397 |
| CMT (mean ± SE, μm) | 250.22 ± 1.99 | 246.04 ± 1.93 | 248.49 ± 2.44 | 0.317 |
| pRNFL thickness (mean ± SE, μm) | 96.71 ± 0.88 | 94.82 ± 1.07 | 92.49 ± 1.34 | 0.032 |
| Control | DM Group 1 | DM Group 2 | p-Value | |
|---|---|---|---|---|
| Average | 84.58 ± 0.89 | 83.49 ± 0.70 | 79.04 ± 0.96 | <0.001 |
| Minimum | 79.61 ± 0.91 | 80.03 ± 0.94 | 73.39 ± 1.70 | <0.001 |
| Sector | ||||
| Superior | 83.89 ± 0.82 | 84.39 ± 0.71 | 78.98 ± 1.11 | <0.001 |
| Superotemporal | 82.13 ± 1.03 | 81.91 ± 0.78 | 78.81 ± 1.03 | 0.014 |
| Inferotemporal | 82.89 ± 0.75 | 83.05 ± 0.78 | 79.54 ± 0.99 | 0.008 |
| Inferior | 80.88 ± 0.76 | 81.13 ± 0.73 | 77.26 ± 0.88 | 0.001 |
| Inferonasal | 83.27 ± 0.79 | 83.61 ± 0.79 | 79.07 ± 1.08 | 0.001 |
| Superonasal | 85.73 ± 0.82 | 86.12 ± 0.82 | 80.46 ± 1.45 | <0.001 |
| Control | DM Group 1 | DM Group 2 | p-Value | |
|---|---|---|---|---|
| Full area | 20.32 ± 0.15 | 19.46 ± 0.17 | 18.46 ± 0.23 | <0.001 |
| Inner area | 21.70 ± 0.14 | 20.77 ± 0.17 | 19.78 ± 0.24 | <0.001 |
| Central area | 9.41 ± 0.28 | 9.34 ± 0.30 | 8.27 ± 0.31 | 0.027 |
| Sector | ||||
| Superior | 21.58 ± 0.19 | 20.65 ± 0.22 | 19.72 ± 0.30 | <0.001 |
| Temporal | 21.62 ± 0.14 | 20.76 ± 0.19 | 20.05 ± 0.23 | <0.001 |
| Inferior | 21.74 ± 0.15 | 20.75 ± 0.22 | 19.89 ± 0.30 | <0.001 |
| Nasal | 21.83 ± 0.22 | 20.91 ± 0.21 | 19.49 ± 0.39 | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B (95% CI) | p-Values | B (95% CI) | p-Values | |
| Age | −0.01 (−0.03–0.03) | 0.997 | ||
| Sex | −0.33 (−0.77–0.11) | 0.138 | ||
| DM duration | −0.07 (−0.11–−0.03) | 0.002 | −0.05 (−0.09–−0.01) | 0.037 |
| BCVA | −5.13 (−9.69–−0.57) | 0.028 | −5.39 (−9.62–−1.15) | 0.013 |
| SE | 0.02 (−0.19–0.23) | 0.825 | ||
| IOP | 0.04 (−0.06–0.14) | 0.442 | ||
| Axial length | −0.03 (−0.39–0.33) | 0.866 | ||
| HbA1C | −0.27 (−0.56–0.02) | 0.069 | −0.06 (−0.35–0.23) | 0.666 |
| CMT | 0.01 (−0.02–0.02) | 0.989 | ||
| pRNFL | 0.02 (−0.01–0.05) | 0.096 | −0.02 (−0.05–0.01) | 0.226 |
| GC-IPL | 0.08 (0.05–0.12) | <0.001 | 0.07 (0.04–0.11) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-W.; Lee, W.-H.; Ryu, C.-K.; Kim, T.-Y.; Lim, H.-B.; Lee, Y.-H.; Kim, J.-Y. Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 1849. https://doi.org/10.3390/jcm9061849
Lee M-W, Lee W-H, Ryu C-K, Kim T-Y, Lim H-B, Lee Y-H, Kim J-Y. Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine. 2020; 9(6):1849. https://doi.org/10.3390/jcm9061849
Chicago/Turabian StyleLee, Min-Woo, Woo-Hyuk Lee, Cheon-Kuk Ryu, Tae-Yeon Kim, Hyung-Bin Lim, Young-Hoon Lee, and Jung-Yeul Kim. 2020. "Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study" Journal of Clinical Medicine 9, no. 6: 1849. https://doi.org/10.3390/jcm9061849
APA StyleLee, M.-W., Lee, W.-H., Ryu, C.-K., Kim, T.-Y., Lim, H.-B., Lee, Y.-H., & Kim, J.-Y. (2020). Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine, 9(6), 1849. https://doi.org/10.3390/jcm9061849





