Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic MRI Technique
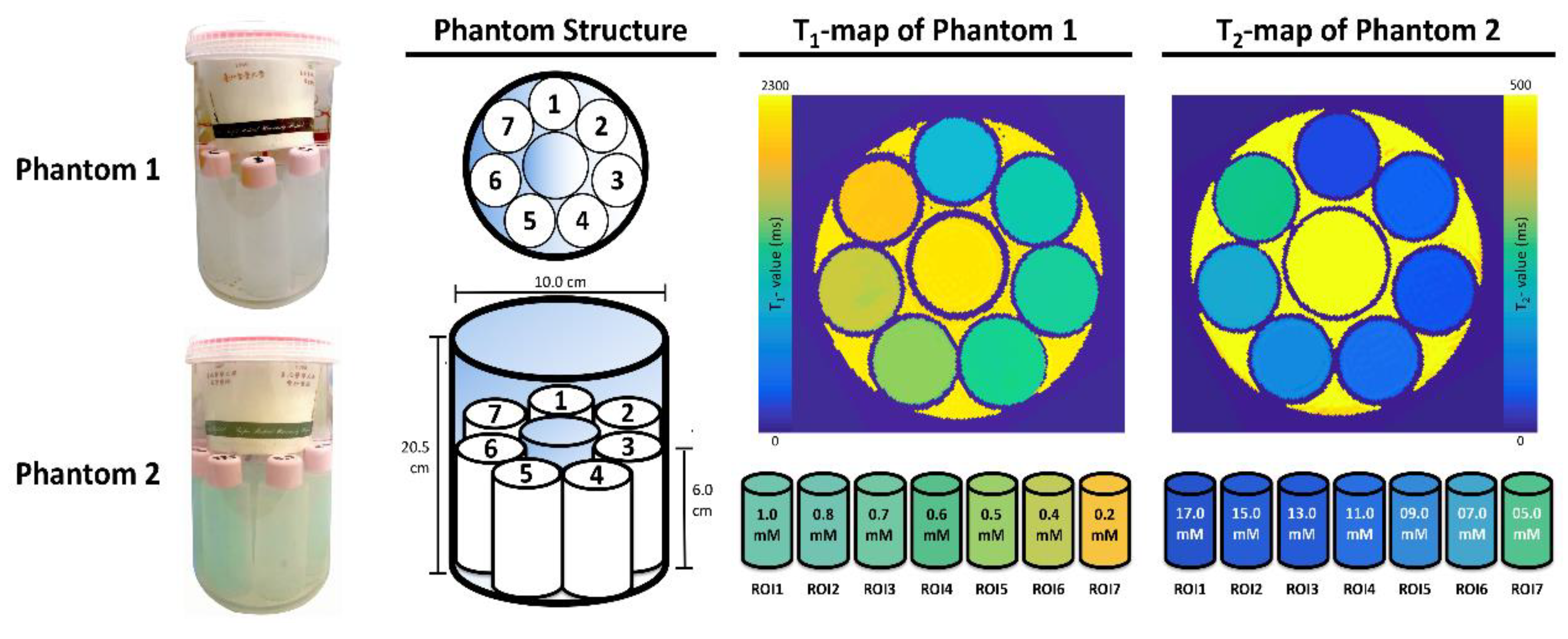
2.2. Specifically Designed Phantoms
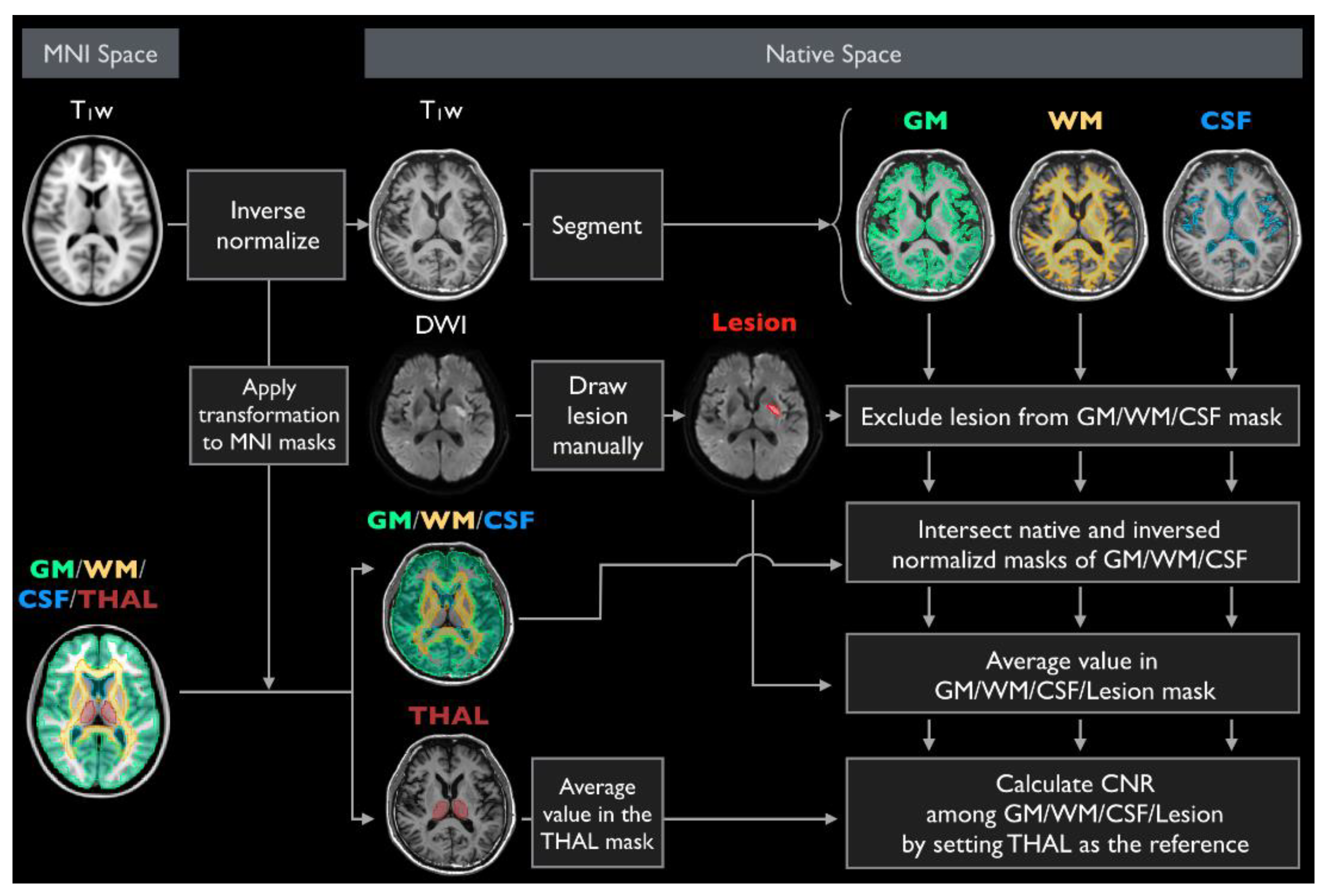
2.3. Phantom Evaluation
2.4. Participant and Diagnostic Score Evaluation
2.5. Statistical Analyses
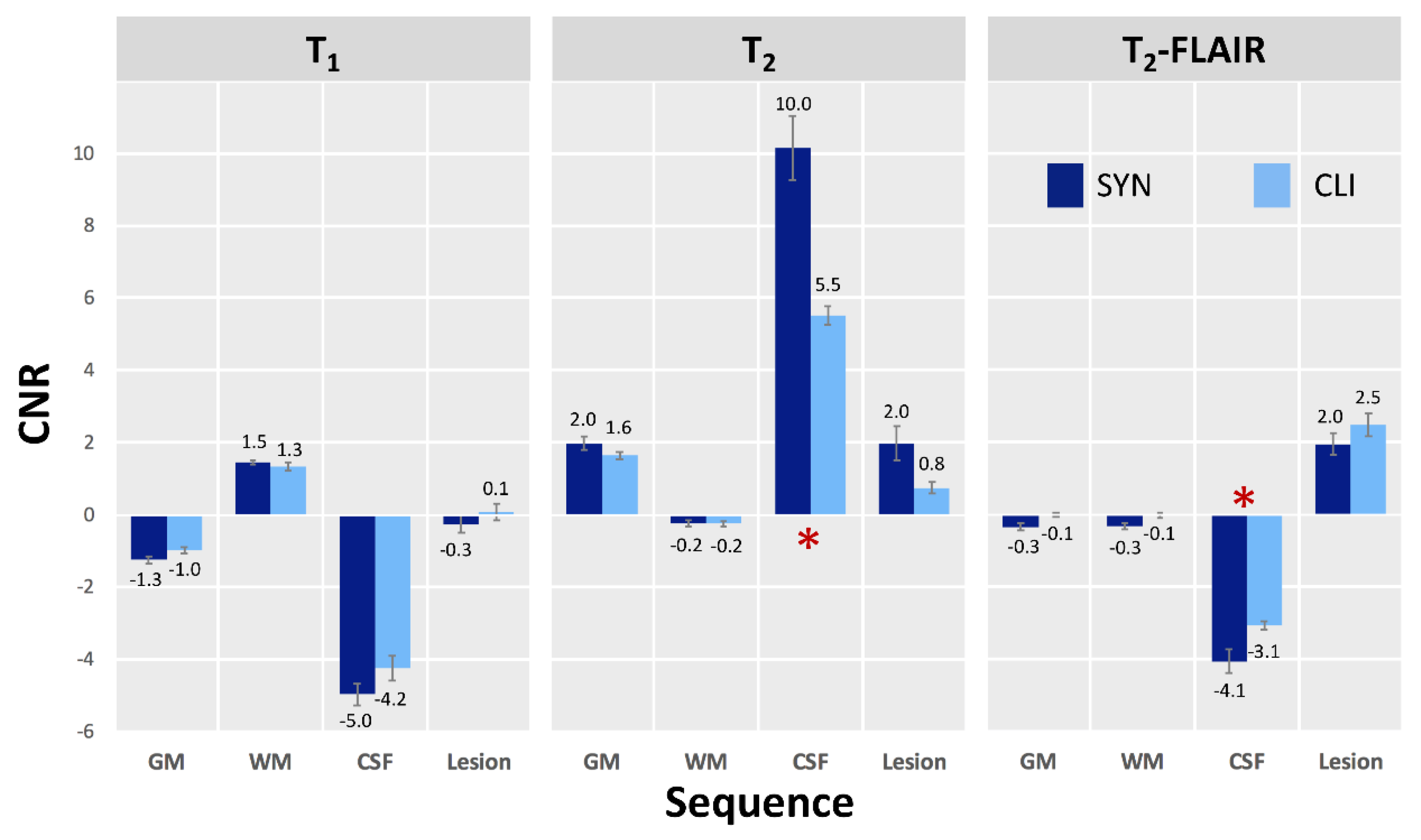
3. Results
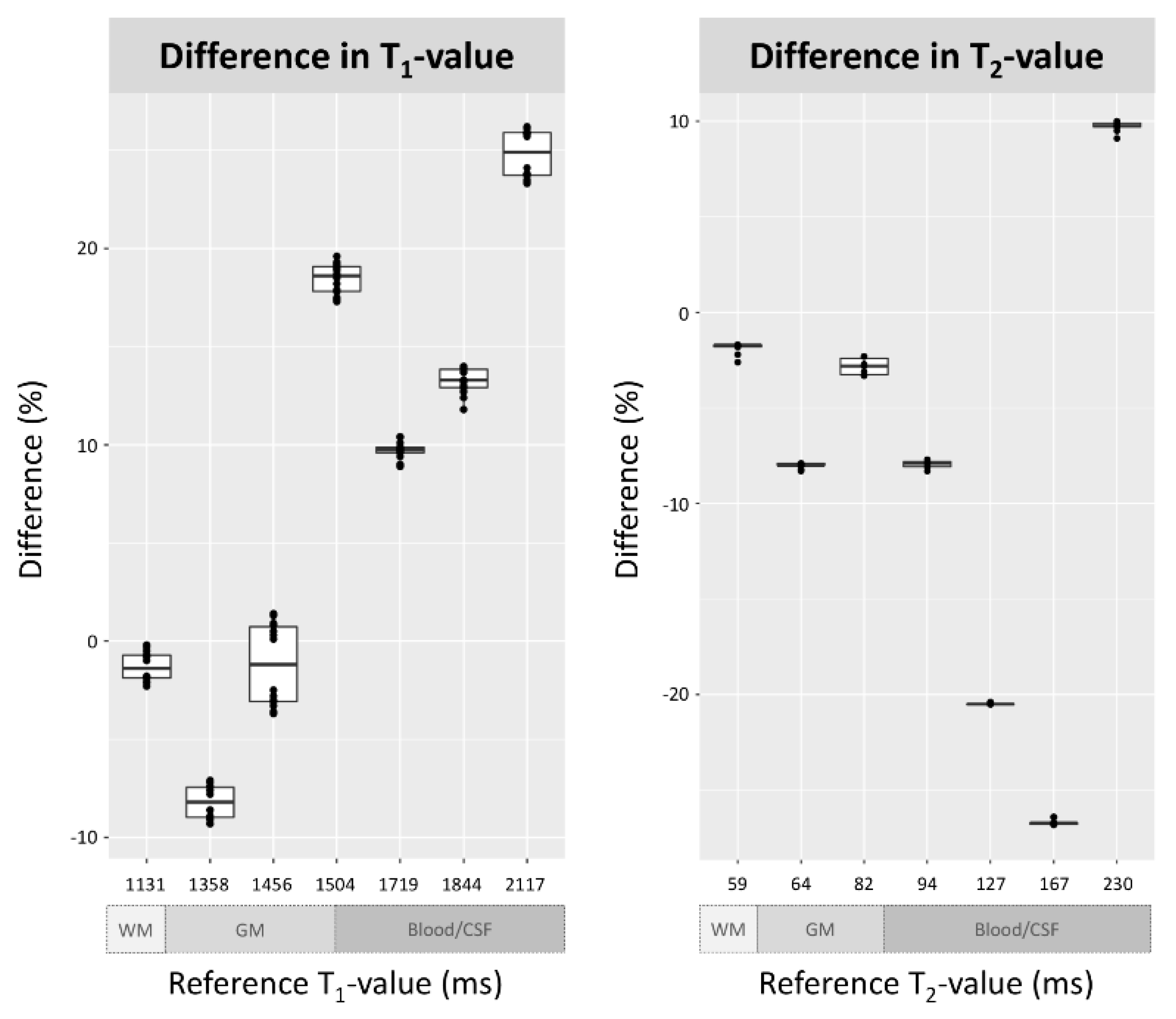
3.1. Phantom Evaluation
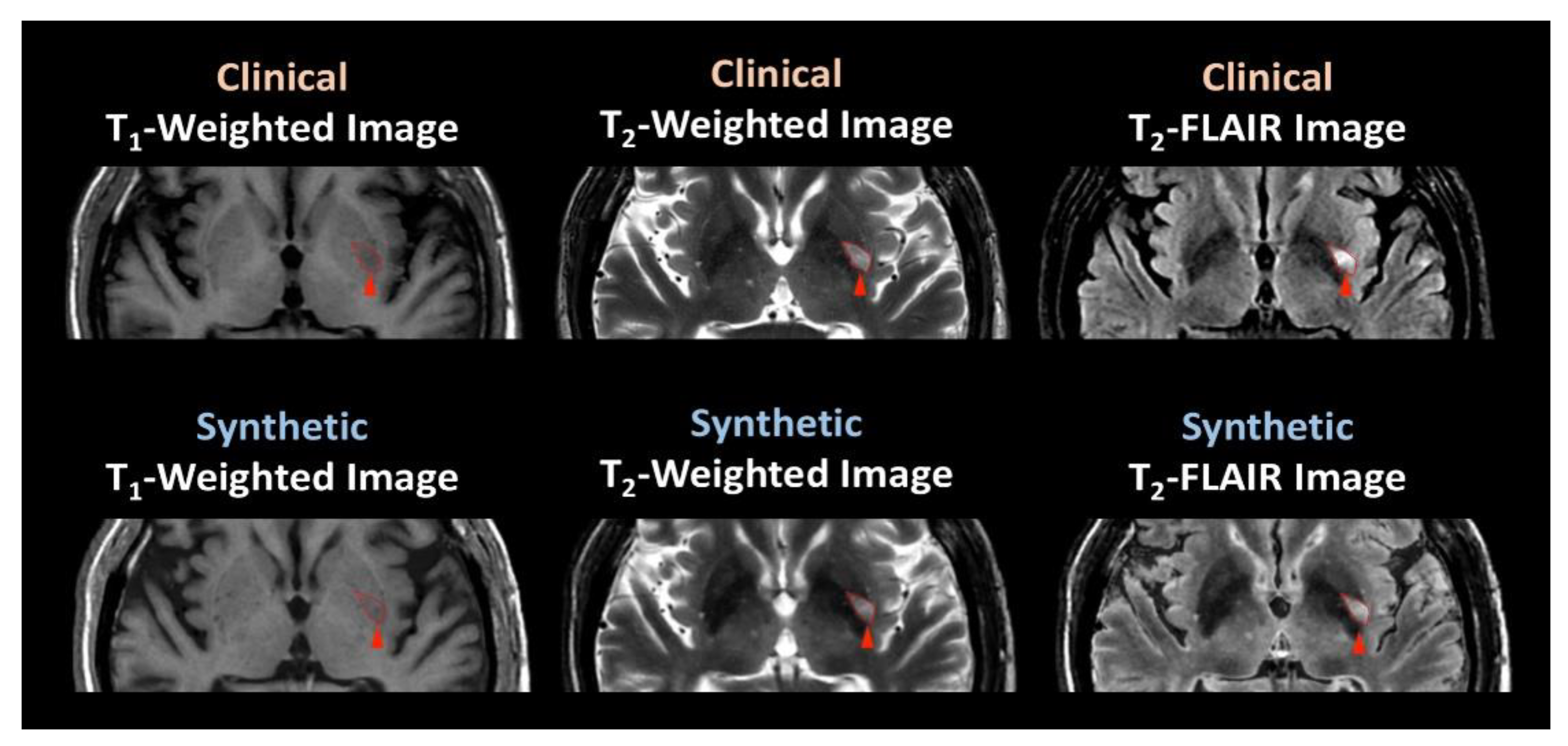
3.2. Participant Evaluation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNR | contrast-to-noise ratio |
| CSF | cerebrospinal fluid |
| FLAIR | fluid-attenuated inversion recovery |
| GM | gray matter |
| MAGiC | magnetic resonance image compilation |
| SE | spin-echo |
| TE | echo time |
| TR | repetition time |
| TI | inversion time |
| WM | white matter |
References
- Krauss, W.; Gunnarsson, M.; Andersson, T.; Thunberg, P. Accuracy and reproducibility of a quantitative magnetic resonance imaging method for concurrent measurements of tissue relaxation times and proton density. Magn. Reason. Imaging 2015, 33, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Warntjes, J.B.M.; Dahlqvist, O.; Lundberg, P. Novel method for rapid, simultaneous T1, T*2, and proton density quantification. Magn. Reason. Med. 2007, 57, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Riederer, S.J.; Suddarth, S.A.; Bobman, S.A.; Lee, J.N.; Wang, H.Z.; MacFall, J.R. Automated MR image synthesis: Feasibility studies. Radiology 1984, 153, 203–206. [Google Scholar] [CrossRef]
- Tanenbaum, L.N.; Tsiouris, A.J.; Johnson, A.N.; Naidich, T.P.; de Lano, M.C.; Melhem, E.R.; Quarterman, P.; Parameswaran, S.X.; Shankaranarayanan, A.; Goyen, M.; et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. AJNR Am. J. Neuroradiol. 2017, 38, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Granberg, T.; Uppman, M.; Hashim, F.; Cananau, C.; Nordin, L.E.; Shams, S.; Berglund, J.; Forslin, Y.; Aspelin, P.; Fredrikson, S.; et al. Clinical Feasibility of Synthetic MRI in Multiple Sclerosis: A Diagnostic and Volumetric Validation Study. AJNR Am. J. Neuroradiol. 2016, 37, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Blystad, I.; Warntjes, J.B.M.; Smedby, O.; Landtblom, A.-M.; Lundberg, P.; Larsson, E.-M. Synthetic MRI of the brain in a clinical setting. Acta Radiol. 2012, 53, 1158–1163. [Google Scholar] [CrossRef]
- Warntjes, J.B.M.; Leinhard, O.D.; West, J.; Lundberg, P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn. Reason. Med. 2008, 60, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J.; McGarry, B.L.; Knight, M.J.; Jokivarsi, K.T.; Gröhn, O.H.J.; Kauppinen, R.A. Timing the ischaemic stroke by 1H-MRI: Improved accuracy using absolute relaxation times over signal intensities. NeuroReport 2014, 25, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Siemonsen, S.; Mouridsen, K.; Holst, B.; Ries, T.; Finsterbusch, J.; Thomalla, G.; Ostergaard, L.; Fiehler, J. Quantitative T2 values predict time from symptom onset in acute stroke patients. Stroke 2009, 40, 1612–1616. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, K.; Saritaş, E.Ü. Aqueous paramagnetic solutions for MRI phantoms at 3 T: A detailed study on relaxivities. Turk. J. Elect. Engineer. Comput. Sci. 2017, 25, 2108–2121. [Google Scholar] [CrossRef]
- Lu, H.; Clingman, C.; Golay, X.; van Zijl, P.C.M. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn. Reason. Med. 2004, 52, 679–682. [Google Scholar] [CrossRef]
- Ethofer, T.; Mader, I.; Seeger, U.; Helms, G.; Erb, M.; Grodd, W.; Ludolph, A.; Klose, U. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 Tesla. Magn. Reason. Med. 2003, 50, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Greenman, R.L.; Shirosky, J.E.; Mulkern, R.V.; Rofsky, N.M. Double inversion black-blood fast spin-echo imaging of the human heart: A comparison between 1.5T and 3.0T. J. Magn. Reason. Imaging 2003, 17, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Gelman, N.; Gorell, J.M.; Barker, P.B.; Savage, R.M.; Spickler, E.M.; Windham, J.P.; Knight, R.A. MR imaging of human brain at 3.0 T: Preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 1999, 210, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Barral, J.K.; Gudmundson, E.; Stikov, N.; Etezadi-Amoli, M.; Stoica, P.; Nishimura, D.G. A robust methodology for in vivo T1 mapping. Magn. Reason. Med. 2010, 64, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Schellinger, P.D.; Jansen, O.; Fiebach, J.B.; Hacke, W.; Sartor, K. A standardized MRI stroke protocol: Comparison with CT in hyperacute intracerebral hemorrhage. Stroke 1999, 30, 765–768. [Google Scholar] [CrossRef] [Green Version]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L. Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.R.; Collins, D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 2009, 47 (Suppl. 1), S102. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 2015, 122, 1–5. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- McGarry, B.L.; Jokivarsi, K.T.; Knight, M.J.; Gröhn, O.H.J.; Kauppinen, R.A. A Magnetic Resonance Imaging Protocol for Stroke Onset Time Estimation in Permanent Cerebral Ischemia. J. Vis. Exp. 2017, 16, e55277. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Otsuka, Y.; Hori, M.; Tachibana, Y.; Yokoyama, K.; Fujita, S.; Andica, C.; Kamagata, K.; Irie, R.; Koshino, S.; et al. Improving the Quality of Synthetic FLAIR Images with Deep Learning Using a Conditional Generative Adversarial Network for Pixel-by-Pixel Image Translation. AJNR Am. J. Neuroradiol. 2019, 40, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, K.; Nam, Y.; Gho, S.-M.; Jang, J.; Lee, H.-J.; Cha, J.; Baek, H.J.; Jiyong, P.; Kim, D.H. Data-driven synthetic MRI FLAIR artifact correction via deep neural network. J. Magn. Reason. Imaging 2019, 50, 1413–1423. [Google Scholar] [CrossRef]
- Krzyzak, A.; Klodowski, K.; Raszewski, Z. Anisotropic Phantoms in Magnetic Resonance Imaging. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 414–417. [Google Scholar]
- Kłodowski, K.; Krzyżak, A.T. Innovative anisotropic phantoms for calibration of diffusion tensor imaging sequences. Magn. Reason. Imaging 2016, 34, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Fieremans, E.; Lee, H.-H. Physical and numerical phantoms for the validation of brain microstructural MRI: A cookbook. Neuroimage 2018, 182, 39–61. [Google Scholar] [CrossRef]
- Mazur, W.; Krzyzak, A.T.; Raszewski, Z. Towards the precise microstructural mapping. Testing new anisotropic phantoms with layered and capillary geometries. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2835–2839. [Google Scholar]
- De Souza, E.M.; Costa, E.T.; Castellano, G. Investigation of anisotropic fishing line-based phantom as tool in quality control of diffusion tensor imaging. Radiol. Phys. Technol. 2019, 12, 161–171. [Google Scholar] [CrossRef]
| Patient (n = 18) | Age (Year) | Sex | Lesion Location(s) |
|---|---|---|---|
| Patient 01 | 64 | Male | Left striatum |
| Patient 02 | 44 | Female | Left striatum |
| Patient 03 | 64 | Male | Left cerebellum |
| Patient 04 | 66 | Male | Left striatum |
| Patient 05 | 63 | Female | Right temporal and frontal lobes |
| Patient 06 | 50 | Male | Left parietal lobe |
| Patient 07 | 66 | Male | Left frontal lobe |
| Patient 08 | 55 | Male | Left striatum |
| Patient 09 | 56 | Male | Left parietal lobe |
| Patient 10 | 56 | Female | Left insula |
| Patient 11 | 58 | Female | Right parietal lobe |
| Patient 12 | 69 | Male | Left striatum |
| Patient 13 | 55 | Male | Right striatum |
| Patient 14 | 62 | Male | Left cerebellum |
| Patient 15 | 57 | Male | Left parietal lobe and right striatum |
| Patient 16 | 57 | Male | Left striatum |
| Patient 17 | 66 | Male | Right striatum |
| Patient 18 | 64 | Male | Right parietal lobe |
| T1 Value | |||||||||||||||
| CuSO4 Concentration | Reference Value | Quantitative Value, Synthetic Scan, Day 1 | Quantitative Value, Synthetic Scan, Day 2 | ||||||||||||
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 1–6 | 1–7 | 2–1 | 2–2 | 2–3 | 2–4 | 2–5 | 2–6 | 2–7 | ||
| 1.0 mM | 1131.5 | 1123.1 | 1128.8 | 1122.6 | 1127.7 | 1122.2 | 1119.9 | 1126.2 | 1110.5 | 1111.0 | 1111.1 | 1105.6 | 1107.5 | 1111.2 | 1107.8 |
| 0.8 mM | 1357.8 | 1254.1 | 1254.9 | 1257.5 | 1260.1 | 1252.1 | 1262.0 | 1257.9 | 1240.8 | 1241.3 | 1231.0 | 1231.7 | 1236.6 | 1235.1 | 1233.9 |
| 0.7 mM | 1456.4 | 1463.0 | 1474.8 | 1476.3 | 1470.0 | 1460.8 | 1468.6 | 1457.3 | 1419.8 | 1416.0 | 1411.2 | 1402.4 | 1412.8 | 1407.9 | 1404.4 |
| 0.6 mM | 1503.7 | 1785.4 | 1798.6 | 1794.5 | 1790.5 | 1787.7 | 1781.8 | 1793.0 | 1789.3 | 1776.9 | 1767.5 | 1764.9 | 1771.9 | 1772.5 | 1764.5 |
| 0.5 mM | 1719.0 | 1883.3 | 1892.6 | 1889.5 | 1885.2 | 1879.7 | 1874.3 | 1898.2 | 1887.6 | 1884.6 | 1872.3 | 1887.2 | 1897.6 | 1888.2 | 1885.8 |
| 0.4 mM | 1843.8 | 2071.6 | 2088.3 | 2086.3 | 2082.1 | 2077.3 | 2061.3 | 2084.3 | 2099.5 | 2095.6 | 2088.2 | 2096.7 | 2101.7 | 2099.8 | 2100.5 |
| 0.2 mM | 2117.2 | 2612.3 | 2621.5 | 2620.8 | 2620.0 | 2614.7 | 2610.0 | 2628.2 | 2663.2 | 2665.0 | 2662.4 | 2665.8 | 2669.1 | 2666.3 | 2671.7 |
| T2 Value | |||||||||||||||
| CuSO4 Concentration | Reference Value | Quantitative Value, Synthetic Scan, Day 1 | Quantitative Value, Synthetic Scan, Day 2 | ||||||||||||
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 1–6 | 1–7 | 2–1 | 2–2 | 2–3 | 2–4 | 2–5 | 2–6 | 2–7 | ||
| 20.0 mM | 58.5 | 57.4 | 57.5 | 57.5 | 57.5 | 57.4 | 57.4 | 56.9 | 57.4 | 57.5 | 57.5 | 57.5 | 57.4 | 57.4 | 57.2 |
| 15.0 mM | 64.0 | 58.9 | 58.9 | 58.9 | 58.9 | 58.9 | 58.9 | 58.7 | 58.9 | 58.9 | 58.9 | 58.9 | 58.9 | 58.9 | 58.7 |
| 13.0 mM | 82.4 | 80.5 | 80.6 | 80.1 | 80.1 | 79.7 | 79.7 | 80.2 | 80.5 | 80.6 | 80.1 | 80.1 | 79.7 | 79.7 | 79.9 |
| 11.0 mM | 94.0 | 86.4 | 86.6 | 86.6 | 86.2 | 86.7 | 86.7 | 86.8 | 86.4 | 86.6 | 86.6 | 86.2 | 86.7 | 86.7 | 86.5 |
| 9.0 mM | 126.7 | 100.8 | 100.7 | 100.7 | 100.7 | 100.8 | 100.8 | 100.8 | 100.8 | 100.7 | 100.7 | 100.7 | 100.8 | 100.8 | 100.7 |
| 7.0 mM | 166.9 | 122.9 | 122.4 | 122.4 | 122.4 | 122.2 | 122.2 | 122.3 | 122.9 | 122.4 | 122.4 | 122.4 | 122.2 | 122.2 | 122.4 |
| 5.0 mM | 230.3 | 253.3 | 252.7 | 253.2 | 252.2 | 252.8 | 252.8 | 253.1 | 253.2 | 252.5 | 253.1 | 252.1 | 252.7 | 252.7 | 251.3 |
| Question | T1-Weighted Image | T2-Weighted Image | T2-FLAIR Image | |||
|---|---|---|---|---|---|---|
| SYN | CLI | SYN | CLI | SYN | CLI | |
| What is the image quality for diagnostic use? a | 8.06 ** (0.79) | 9.42 (0.28) | 8.53 * (1.00) | 9.42 (0.84) | 7.89 ** (1.94) | 9.36 (0.49) |
| Are these images acceptable for diagnostic use? b | 0.97 (0.17) | 0.97 (0.17) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-W.; Hsu, A.-L.; Huang, C.-W.C.; Yang, S.-H.; Lin, C.-Y.; Shieh, C.-C.; Chan, W.P. Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method. J. Clin. Med. 2020, 9, 1857. https://doi.org/10.3390/jcm9061857
Li C-W, Hsu A-L, Huang C-WC, Yang S-H, Lin C-Y, Shieh C-C, Chan WP. Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method. Journal of Clinical Medicine. 2020; 9(6):1857. https://doi.org/10.3390/jcm9061857
Chicago/Turabian StyleLi, Chia-Wei, Ai-Ling Hsu, Chi-Wen C. Huang, Shih-Hung Yang, Chien-Yuan Lin, Charng-Chyi Shieh, and Wing P. Chan. 2020. "Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method" Journal of Clinical Medicine 9, no. 6: 1857. https://doi.org/10.3390/jcm9061857
APA StyleLi, C. -W., Hsu, A. -L., Huang, C. -W. C., Yang, S. -H., Lin, C. -Y., Shieh, C. -C., & Chan, W. P. (2020). Reliability of Synthetic Brain MRI for Assessment of Ischemic Stroke with Phantom Validation of a Relaxation Time Determination Method. Journal of Clinical Medicine, 9(6), 1857. https://doi.org/10.3390/jcm9061857





