Should We Continue Assessing Glomerular Filtration Rate with the Cockroft–Gault Formula in NOAC-Treated Patients? The Magnitude of the Problem
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
Patient Reclassification According to CrCl, eGFR by the CKD-EPI and CKD-EPI_noBSA Equations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellá, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration With EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jiménez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Aboyans, V.; Ageno, W.; Agnelli, G.; Alatri, A.; Bauersachs, R.; Brekelmans, M.P.A.; Büller, H.R.; Elias, A.; Farge, D.; et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Heart J. 2018, 39, 4208–4218. [Google Scholar] [CrossRef] [Green Version]
- Huisman, M.V.; Rothman, K.J.; Paquette, M.; Teutsch, C.; Diener, H.-C.; Dubner, S.J.; Halperin, J.L.; Ma, C.S.; Zint, K.; Elsaesser, A.; et al. The Changing Landscape for Stroke Prevention in AF. J. Am. Coll. Cardiol. 2017, 69, 777–785. [Google Scholar] [CrossRef]
- De Caterina, R.; Ageno, W.; Agnelli, G.; Chan, N.C.; Diener, H.-C.; Hylek, E.; Raskob, G.E.; Siegal, D.M.; Verheugt, F.W.A.; Lip, G.Y.H.; et al. The Non-Vitamin K Antagonist Oral Anticoagulants in Heart Disease: Section V—Special Situations. Thromb. Haemost. 2019, 119, 14–38. [Google Scholar] [CrossRef] [Green Version]
- Harel, Z.; Sholzberg, M.; Shah, P.S.; Pavenski, K.; Harel, S.; Wald, R.; Bell, C.M.; Perl, J. Comparisons between Novel Oral Anticoagulants and Vitamin K Antagonists in Patients with CKD. J. Am. Soc. Nephrol. 2014, 25, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Watanabe, T.; Sasaki, S.; Nagai, K.; Roden, D.M.; Aizawa, Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: The Niigata preventive medicine study. Am. Heart J. 2009, 158, 629–636. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.R.; Alonso, A.; Benjamin, E.J.; De Boer, I.H.; Deo, R.; Katz, R.; Kestenbaum, B.; Mathew, J.; Robinson-Cohen, C.; et al. eGFR and Albuminuria in Relation to Risk of Incident Atrial Fibrillation: A Meta-Analysis of the Jackson Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Noël, P.; Gregoire, F.; Capon, A.; Lehert, P. Atrial fibrillation as a risk factor for deep venous thrombosis and pulmonary emboli in stroke patients. Stroke 1991, 22, 760–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziki, M.D.A.; Lip, G.Y.H.; Bikdeli, B. Pulmonary Embolism and Atrial Fibrillation: Two Sides of the Same Coin? A Systematic Review. Semin. Thromb. Hemost. 2017, 43, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; Noordzij, M.; Rookmaaker, M.B.; Cases, A.; Couchoud, C.; Heaf, J.G.; Jarraya, F.; De Meester, J.; Groothoff, J.W.; Waldum-Grevbo, B.E.; et al. Mortality due to bleeding, myocardial infarction and stroke in dialysis patients. J. Thromb. Haemost. 2018, 16, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Brand, E.; Mesters, R.; Schäbitz, W.-R.; Fisher, M.; Pavenstädt, H.; Breithardt, G. Dilemmas in the Management of Atrial Fibrillation in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Steffel, J.; Hindricks, G. Apixaban in renal insufficiency: Successful navigation between the Scylla and Charybdis. Eur. Heart J. 2012, 33, 2766–2768. [Google Scholar] [CrossRef] [Green Version]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.; Eikelboom, J.; Joyner, C.; Diener, H.-C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Andrivet, P.; Pham, I.; Griuncelli, M.; Adnot, S. A modified Cockcroft-Gault formula taking into account the body surface area gives a more accurate estimation of the glomerular filtration rate. J. Nephrol. 2007, 20, 576–585. [Google Scholar] [PubMed]
- Stevens, P.E. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Inker, A.S. Frequently Asked Questions About GFR Estimates; The National Kidney Foundation: New York, NY, USA, 2011. [Google Scholar]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- National Kidney Foundation. Available online: https://www.kidney.org/professionals/KDOQI/gfr_calculatorCoc (accessed on 25 August 2019).
- Chew-Harris, J.S.C.; Chin, P.K.; Florkowski, C.M.; George, P.; Endre, Z.H. Removal of body surface area normalisation improves raw-measured glomerular filtration rate estimation by the Chronic Kidney Disease Epidemiology Collaboration equation and drug dosing in the obese. Intern. Med. J. 2015, 45, 766–773. [Google Scholar] [CrossRef]
- Lip, G.Y.; Banerjee, A.; Boriani, G.; Chiang, C.-E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Redal-Baigorri, B.; Rasmussen, K.; Heaf, J. The use of absolute values improves performance of estimation formulae: A retrospective cross sectional study. BMC Nephrol. 2013, 14, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.I.-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.J.; Lewis, J.W. A note on concordance correlation coefficient. PDA J. Pharm. Sci. Technol. 2000, 54, 23–26. [Google Scholar]
- Bland, M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Manzano, S.; Andreu-Cayuelas, J.M.; Marin, F.; Orenes-Piñero, E.; Gallego, P.; Valdés, M.; Vicente, V.; Lip, G.Y.; Roldán-Schilling, V. Comparison of Estimated Glomerular Filtration Rate Equations for Dosing New Oral Anticoagulants in Patients With Atrial Fibrillation. Rev. Esp. Cardiolog. (Engl. Ed.) 2015, 68, 497–504. [Google Scholar] [CrossRef]
- Pai, M.P. Estimating the Glomerular Filtration Rate in Obese Adult Patients for Drug Dosing. Adv. Chronic Kidney Dis. 2010, 17, e53–e62. [Google Scholar] [CrossRef]
- Schwartz, J.B. Potential Effect of Substituting Estimated Glomerular Filtration Rate for Estimated Creatinine Clearance for Dosing of Direct Oral Anticoagulants. J. Am. Geriatr. Soc. 2016, 64, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. Savaysa Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf (accessed on 15 June 2020).
- Stevens, L.A.; Nolin, T.D.; Richardson, M.M.; Feldman, H.I.; Lewis, J.B.; Rodby, R.; Townsend, R.; Okparavero, A.; Zhang, Y.L.; Schmid, C.H.; et al. Comparison of Drug Dosing Recommendations Based on Measured GFR and Kidney Function Estimating Equations. Am. J. Kidney Dis. 2009, 54, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Camm, A.J.; Fox, K.A.A. Strengths and weaknesses of ‘real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart 2018, 5, e000788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
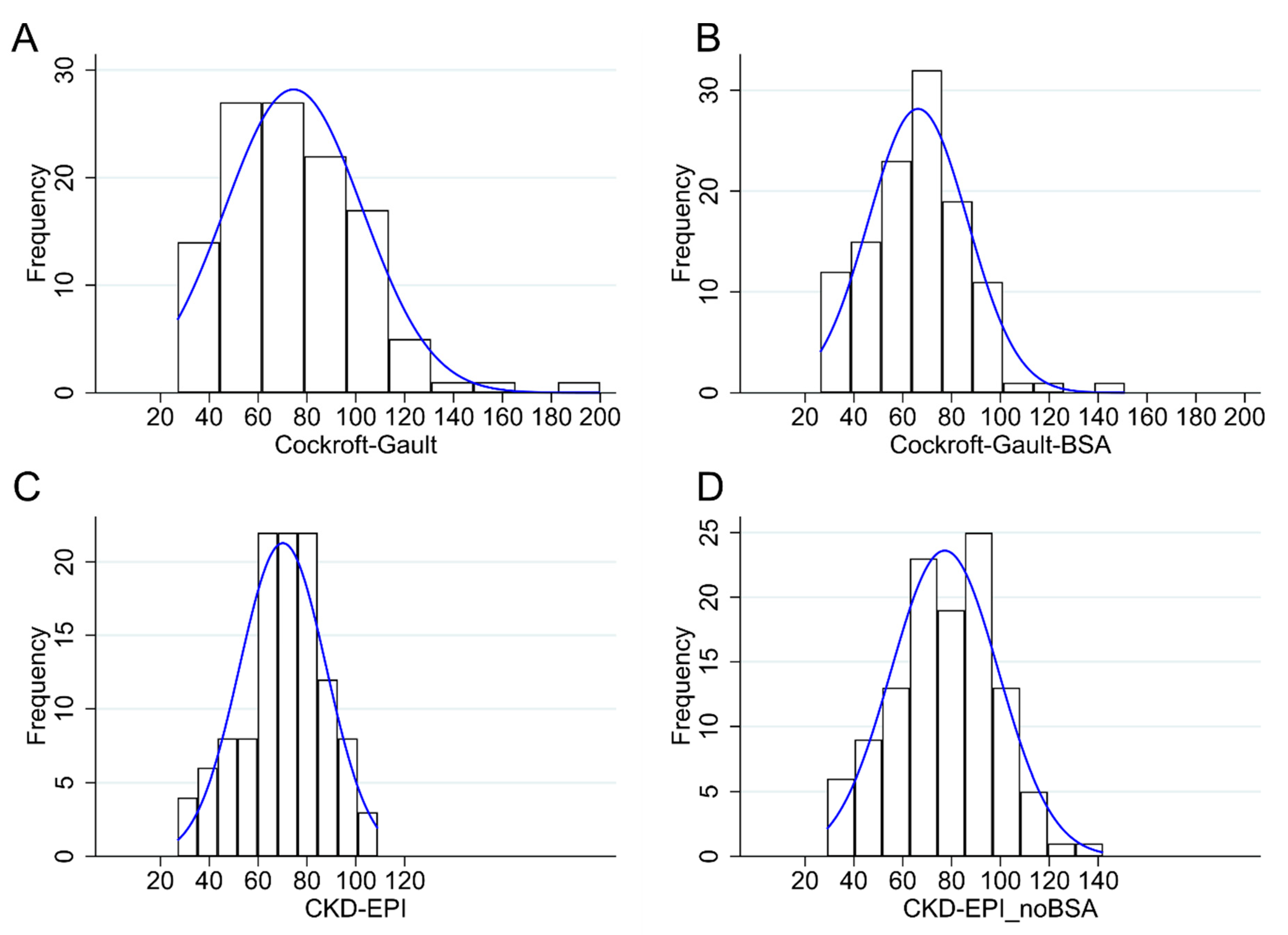



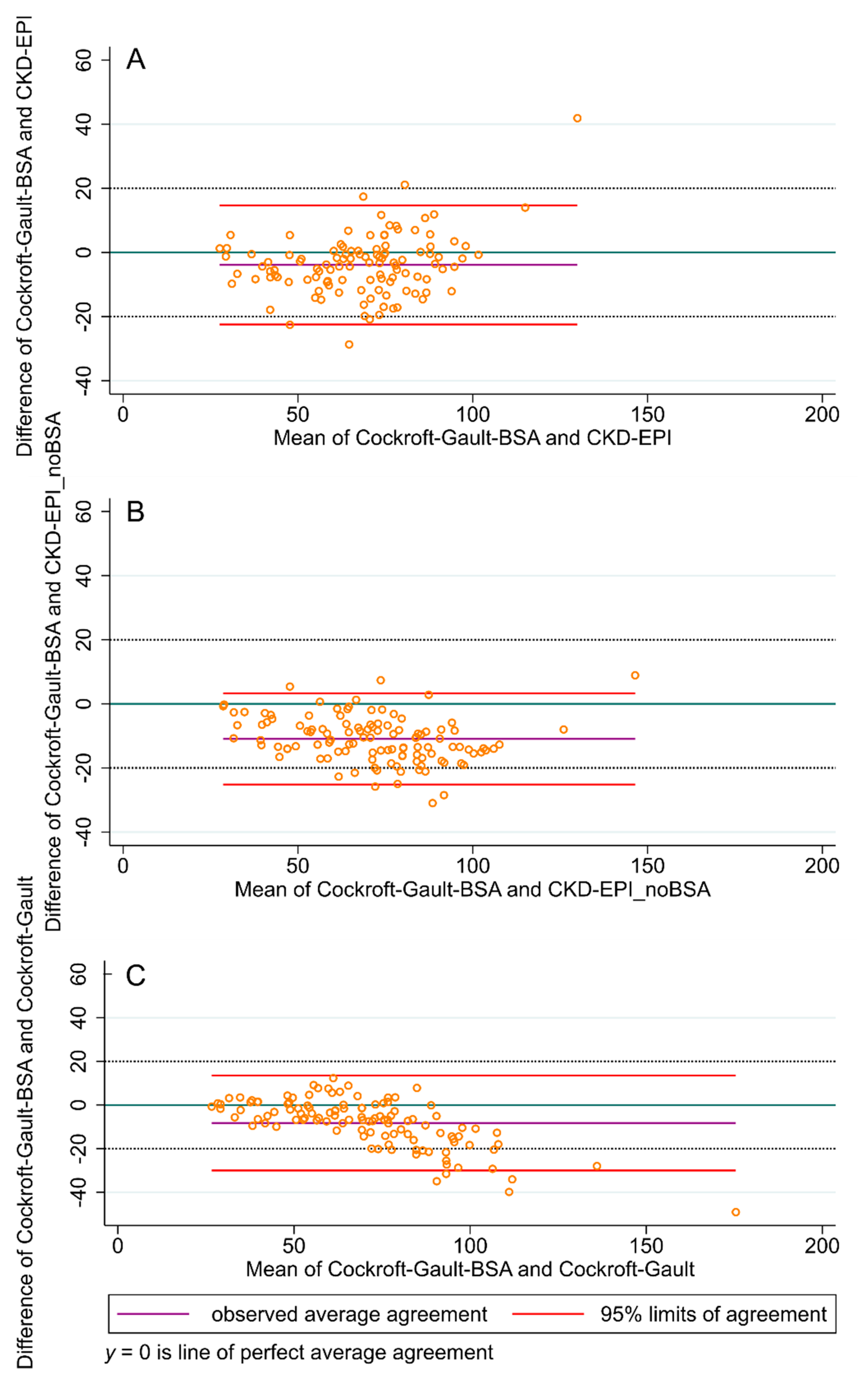
| Range | Mean (Standard Deviation) | Median (Interquartile Range) | |
|---|---|---|---|
| Age (years) | 43.7–95.1 | 69 (12.1) | 69.9 (57.7–78.8) |
| Weight (kg) | 45–124 | 78.1 (16.8) | 75 (66–89) |
| Height (cm) | 154–190 | 171.1 (8.8) | 172 (164–178) |
| Body Mass Index (kg/cm2) | 17.6–37.9 | 26.5 (4.5) | 25.4 (23.7–28.7) |
| Creatinine (mg/dL) | 0.5–2.3 | 1.1 (0.3) | 1 (0.9–1.2) |
| GFR (Cockcroft–Gault) (mL/min) | 29–142 | 77.2 (22) | 75 (54–92) |
| GFR (CKD-EPI) (mL/min/1.73 m2) | 27–109 | 70.1 (17.7) | 72 (60–82) |
| GFR (CKD-EPI_noBSA) (mL/min) | 27–200 | 74.5 (28.1) | 79 (63–92) |
| GFR (Cockcroft–Gault-BSA) (1.73 mL/min) | 26–151 | 66.3 (20.3) | 67 (53–78) |
| CKD-EPI | % Reclassified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cockcroft–Gault | Hyper Normal | Normal | Mild or Moderately Depressed | Severely Depressed | End Stage | Total | To Higher GFR | To Lower GFR | Total |
| Hyper Normal | 6 (23.08) | 20 (76.92) | 0 (0) | 0 (0) | 0 (0) | 26 (100) | 76.9 | 76.9 | |
| Normal | 1 (1.47) | 64 (94.12) | 3 (4.41) | 0 (0) | 0 (0) | 68 (100) | 1.5 | 4.4 | 5.9 |
| Mild or Moderately Depressed | 0 (0) | 7 (38.89) | 8 (44.44) | 3 (16.67) | 0 (0) | 18 (100) | 38.9 | 16.7 | 55.6 |
| Severely Depressed | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 3 (100) | 100 | 100 | |
| End Stage | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Total | 7 (6.09) | 91 (79.13) | 14 (12.17) | 3 (2.61) | 0 (0) | 115 (100) | |||
| CKD-EPI_noBSA | % Reclassified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cockcroft–Gault | Hyper Normal | Normal | Mild or Moderately Depressed | Severely Depressed | End Stage | Total | To Higher GFR | To Lower GFR | Total |
| Hyper Normal | 18 (69.23) | 8 (30.77) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 26 (100) | 30.8 | 30.8 | |
| Normal | 4 (5.88) | 63 (92.65) | 1 (1.47) | 0 (0.0) | 0 (0.0) | 68 (100) | 5.9 | 1.5 | 7.4 |
| Mild or Moderately Depressed | 0 (0.0) | 8 (44.44) | 9 (50.00) | 1 (5.56) | 0 (0.0) | 18 (100) | 44.4 | 5.6 | 50.0 |
| Severely Depressed | 0 (0.0) | 0 (0.0) | 2 (66.67) | 1 (33.33) | 0 (0.0) | 3 (100) | 66.7 | 66.7 | |
| End Stage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 115 (100) | |||
| Total | 22 (19.13) | 79 (68.70) | 12 (10.43) | 2 (1.74) | 115 (100) | ||||
| CKD-EPI | % Reclassified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cockcroft–Gault-BSA | Hyper Normal | Normal | Mild or Moderately Depressed | Severely Depressed | End Stage | Total | To Higher GFR | To Lower GFR | Total |
| Hyper Normal | 5 (83.33) | 1 (16.67) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (100) | 16.7 | 16.7 | |
| Normal | 2 (2.38) | 81 (96.43) | 1 (1.19) | 0 (0.0) | 0 (0.0) | 84 (100) | 2.4 | 1.2 | 3.6 |
| Mild or Moderately Depressed | 0 (0.0) | 9 (42.86) | 10 (47.62) | 2 (9.52) | 0 (0.0) | 21 (100) | 42.9 | 9.5 | 52.4 |
| Severely Depressed | 0 (0.0) | 0 (0.0) | 3 (75.00) | 1 (25.00) | 0 (0.0) | 4 (100) | 75.0 | 75.0 | |
| End Stage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (100) | |||
| Total | 7 (6.09) | 91 (79.13) | 14 (12.17) | 3 (2.61) | 0 (0.0) | 115 (100) | |||
| CKD-EPI_noBSA | % Reclassified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cockcroft–Gault-BSA | Hyper Normal | Normal | Mild or Moderately Depressed | Severely Depressed | End Stage | Total | To Higher GFR | To Lower GFR | Total |
| Hyper Normal | 6 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (100) | 0 | 0 | |
| Normal | 16 (19.05) | 67 (79.76) | 1 (1.19) | 0 (0.0) | 0 (0.0) | 84 (100) | 19.0 | 1.2 | 20.2 |
| Mild or Moderately Depressed | 0 (0.0) | 12 (57.14) | 9 (42.86) | 0 (0.0) | 0 (0.0) | 21 (100) | 57.1 | 0 | 57.1 |
| Severely Depressed | 0 (0.0) | 0 (0.0) | 2 (50.00) | 2 (50.00) | 0 (0.0) | 4 (100) | 50.0 | 0 | 50.0 |
| End Stage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (100) | |||
| Total | 22 (19.13) | 79 (68.70) | 12 (10.43) | 2 (1.74) | 0 (0.0) | 115 (100) | |||
| Cockcroft–Gault | % Reclassified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cockcroft–Gault-BSA | Hyper Normal | Normal | Mild or Moderately Depressed | Severely Depressed | End Stage | Total | To Higher GFR | To Lower GFR | Total |
| Hyper Normal | 6 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (100) | 0 | 0 | |
| Normal | 20 (23.81) | 62 (73.81) | 2 (2.38) | 0 (0.0) | 0 (0.0) | 84 (100) | 23.8% | 2.4% | 26.2% |
| Mild or Moderately Depressed | 0 (0.0) | 6 (28.57) | 15 (71.43) | 0 (0.0) | 0 (0.0) | 21 (100) | 28.6% | 0 | 28.6% |
| Severely Depressed | 0 (0.0) | 0 (0.0) | 1 (25.00) | 3 (75.00) | 0 (0.0) | 4 (100) | 25.0% | 25.0% | |
| End Stage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (100) | |||
| Total | 26 (22.61) | 68 (59.13) | 18 (15.65) | 3 (2.61) | 0 (0.0) | 115 (100) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cemin, R.; Foco, L.; Zoccali, C.; De Caterina, R. Should We Continue Assessing Glomerular Filtration Rate with the Cockroft–Gault Formula in NOAC-Treated Patients? The Magnitude of the Problem. J. Clin. Med. 2020, 9, 1893. https://doi.org/10.3390/jcm9061893
Cemin R, Foco L, Zoccali C, De Caterina R. Should We Continue Assessing Glomerular Filtration Rate with the Cockroft–Gault Formula in NOAC-Treated Patients? The Magnitude of the Problem. Journal of Clinical Medicine. 2020; 9(6):1893. https://doi.org/10.3390/jcm9061893
Chicago/Turabian StyleCemin, Roberto, Luisa Foco, Carmine Zoccali, and Raffaele De Caterina. 2020. "Should We Continue Assessing Glomerular Filtration Rate with the Cockroft–Gault Formula in NOAC-Treated Patients? The Magnitude of the Problem" Journal of Clinical Medicine 9, no. 6: 1893. https://doi.org/10.3390/jcm9061893
APA StyleCemin, R., Foco, L., Zoccali, C., & De Caterina, R. (2020). Should We Continue Assessing Glomerular Filtration Rate with the Cockroft–Gault Formula in NOAC-Treated Patients? The Magnitude of the Problem. Journal of Clinical Medicine, 9(6), 1893. https://doi.org/10.3390/jcm9061893







