Regular Training Increases sTWEAK and Its Decoy Receptor sCD163–Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect?
Abstract
1. Introduction
2. Participants, Material and Methods:
2.1. Study Population
2.2. Bicycle Stress Test
2.3. Lab Analysis
2.4. Statistical Analysis
- -
- Group 1: initially unathletic (initial performance < 100%), performance gain ≤ 2.9% (n = 9)
- -
- Group 2: initially unathletic (initial performance < 100%), performance gain > 2.9% (n = 32)
- -
- Group 3: initially athletic (initial performance ≥ 100%), performance gain ≤ 2.9% (n = 18)
- -
- Group 4: initially athletic (initial performance ≥ 100%), performance gain > 2.9% (n = 39)
2.5. Ethics Statement:
3. Results
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| ASAT/AST | aspartate aminotransferase |
| BMI | body mass index |
| CABG | coronary artery bypass graft |
| CD163 (sCD163) | (soluble) cluster differentiation 16 |
| CHD | chronic heart disease |
| CV | coefficient of variation |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| ELISA | enzyme-linked immunosorbent assay |
| Fn14 | fibroblast growth factor inducible molecule 14 |
| GT | glutamyl transferase |
| hsCRP | high-sensitivity C-reactive protein |
| IL | interleukin |
| MI | myocardial infarction |
| MMP | matrix metalloproteinases |
| PCI | percutaneous coronary intervention |
| proBNP | pro natriuretic peptide |
| TWEAK (sTWEAK) | (soluble) tumor necrosis factor-like weak inducer of apoptosis |
| T1DM | type-1 diabetes mellitus |
References
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Chicheportiche, Y.; Bourdon, P.R.; Xu, H.; Hsu, Y.M.; Scott, H.; Hession, C.; Garcia, I.; Browning, J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997, 272, 32401–32410. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, Y.J.; Kim, W.J.; Woo, D.K.; Lee, Y.; Kim, D.I.; Park, Y.B.; Kwon, B.S.; Park, J.E.; Lee, W.H. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ. J. 2004, 68, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colio, L.M.; Martin-Ventura, J.L.; Munoz-Garcia, B.; Moreno, J.A.; Meilhac, O.; Ortiz, A.; Egido, J. TWEAK and Fn14. New players in the pathogenesis of atherosclerosis. Front. Biosci. 2007, 12, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Bover, L.C.; Cardo-Vila, M.; Kuniyasu, A.; Sun, J.; Rangel, R.; Takeya, M.; Aggarwal, B.B.; Arap, W.; Pasqualini, R. A previously unrecognized protein-protein interaction between TWEAK and CD163: Potential biological implications. J. Immunol. 2007, 178, 8183–8194. [Google Scholar] [CrossRef]
- Moreno, J.A.; Munoz-Garcia, B.; Martin-Ventura, J.L.; Madrigal-Matute, J.; Orbe, J.; Paramo, J.A.; Ortega, L.; Egido, J.; Blanco-Colio, L.M. The CD163-expressing macrophages recognize and internalize TWEAK: Potential consequences in atherosclerosis. Atherosclerosis 2009, 207, 103–110. [Google Scholar] [CrossRef]
- Burkly, L.C.; Michaelson, J.S.; Hahm, K.; Jakubowski, A.; Zheng, T.S. TWEAKing tissue remodeling by a multifunctional cytokine: Role of TWEAK/Fn14 pathway in health and disease. Cytokine 2007, 40, 1–16. [Google Scholar] [CrossRef]
- Schukro, C.; Emich, M.; Fritzer-Szekeres, M.; Strametz-Juranek, J.; Sponder, M. Paper-based training diaries for monitoring of performance progress due to long-term physical activity. Pol. Arch. Intern. Med. 2019, 129, 679–685. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311. [Google Scholar]
- Sponder, M.; Campean, I.A.; Dalos, D.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Bergler-Klein, J.; Graf, S.; Strametz-Juranek, J. Influence of long-term physical activity on PCSK9, HDL/LDL-C and Lp(a)—A prospective observational trial. Pol. Arch. Intern. Med. 2017. [Google Scholar] [CrossRef]
- Sponder, M.; Campean, I.A.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Graf, S.; Dalos, D.; Strametz-Juranek, J. Long-term physical activity leads to a significant increase in serum sRAGE levels: A sign of decreased AGE-mediated inflammation due to physical activity? Heart Vessel. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sponder, M.; Campean, I.A.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Bergler-Klein, J.; Graf, S.; Strametz-Juranek, J. Long-term endurance training increases serum cathepsin S and decreases IL-6 and hsCRP levels. J. Sport. Sci. 2016, 1–6. [Google Scholar] [CrossRef]
- Fernandez-Laso, V.; Sastre, C.; Valdivielso, J.M.; Betriu, A.; Fernandez, E.; Egido, J.; Martin-Ventura, J.L.; Blanco-Colio, L.M. Soluble TWEAK and Major Adverse Cardiovascular Events in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 413–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jelic-Ivanovic, Z.; Bujisic, N.; Spasic, S.; Bogavac-Stanojevic, N.; Spasojevic-Kalimanovska, V.; Kotur-Stevuljevic, J. Circulating sTWEAK improves the prediction of coronary artery disease. Clin. Biochem. 2009, 42, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, E.; Rosenberg, M.; Zugck, C.; Wolf, J.; Katus, H.A.; Frey, N. Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur. J. Heart Fail. 2009, 11, 1050–1056. [Google Scholar] [CrossRef]
- Ptaszynska-Kopczynska, K.; Marcinkiewicz-Siemion, M.; Lisowska, A.; Waszkiewicz, E.; Witkowski, M.; Jasiewicz, M.; Miklasz, P.; Jakim, P.; Galar, B.; Musial, W.J.; et al. Alterations of soluble TWEAK and CD163 concentrations in patients with chronic heart failure. Cytokine 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Kralisch, S.; Ziegelmeier, M.; Bachmann, A.; Seeger, J.; Lossner, U.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 2008, 199, 440–444. [Google Scholar] [CrossRef]
- Richter, B.; Rychli, K.; Hohensinner, P.J.; Berger, R.; Mortl, D.; Neuhold, S.; Zorn, G.; Huber, K.; Maurer, G.; Wojta, J.; et al. Differences in the predictive value of tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in advanced ischemic and non-ischemic heart failure. Atherosclerosis 2010, 213, 545–548. [Google Scholar] [CrossRef]
- Urbonaviciene, G.; Martin-Ventura, J.L.; Lindholt, J.S.; Urbonavicius, S.; Moreno, J.A.; Egido, J.; Blanco-Colio, L.M. Impact of soluble TWEAK and CD163/TWEAK ratio on long-term cardiovascular mortality in patients with peripheral arterial disease. Atherosclerosis 2011, 219, 892–899. [Google Scholar] [CrossRef]
- Filusch, A.; Zelniker, T.; Baumgartner, C.; Eschricht, S.; Frey, N.; Katus, H.A.; Chorianopoulos, E. Soluble TWEAK predicts hemodynamic impairment and functional capacity in patients with pulmonary arterial hypertension. Clin. Res. Cardiol. 2011, 100, 879–885. [Google Scholar] [CrossRef]
- Moller, H.J. Soluble CD163. Scand. J. Clin. Lab. Investig. 2012, 72, 1–13. [Google Scholar] [CrossRef]
- Zhi, Y.; Gao, P.; Xin, X.; Li, W.; Ji, L.; Zhang, L.; Zhang, X.; Zhang, J. Clinical significance of sCD163 and its possible role in asthma (Review). Mol. Med. Rep. 2017, 15, 2931–2939. [Google Scholar] [CrossRef]
- Kneidl, J.; Mysore, V.; Geraci, J.; Tuchscherr, L.; Loffler, B.; Holzinger, D.; Roth, J.; Barczyk-Kahlert, K. Soluble CD163 masks fibronectin-binding protein A-mediated inflammatory activation of Staphylococcus aureus infected monocytes. Cell. Microbiol. 2017, 16, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; van der Zanden, L.; Ottenhoff, T.H. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J. Leukoc. Biol. 2006, 79, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Ritter, M.; Orso, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar] [CrossRef]
- Gaini, S.; Pedersen, S.S.; Koldkaer, O.G.; Pedersen, C.; Moestrup, S.K.; Moller, H.J. New immunological serum markers in bacteraemia: Anti-inflammatory soluble CD163, but not proinflammatory high mobility group-box 1 protein, is related to prognosis. Clin. Exp. Immunol. 2008, 151, 423–431. [Google Scholar] [CrossRef]
- Batista, M.L., Jr.; Santos, R.V.; Cunha, L.M.; Mattos, K.; Oliveira, E.M.; Seelaender, M.C.; Costa Rosa, L.F. Changes in the pro-inflammatory cytokine production and peritoneal macrophage function in rats with chronic heart failure. Cytokine 2006, 34, 284–290. [Google Scholar] [CrossRef]
- Moller, H.J.; Peterslund, N.A.; Graversen, J.H.; Moestrup, S.K. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 2002, 99, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Llaurado, G.; Gonzalez-Clemente, J.M.; Maymo-Masip, E.; Subias, D.; Vendrell, J.; Chacon, M.R. Serum levels of TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus: Relationship with cardiovascular risk factors. a case-control study. PLoS ONE 2012, 7, e43919. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Dejouvencel, T.; Labreuche, J.; Smadja, D.M.; Dussiot, M.; Martin-Ventura, J.L.; Egido, J.; Gaussem, P.; Emmerich, J.; Michel, J.B.; et al. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1253–1262. [Google Scholar] [CrossRef]
| Parameter | Group 1 Unathletic Gain ≤ 2.9% (n = 9) | Group 2 Unathletic Gain > 2.9% (n = 32) | Group 3 Athletic Gain ≤ 2.9% (n = 18) | Group 4 Athletic Gain > 2.9% (n = 39) | Total Population |
|---|---|---|---|---|---|
| Age (years) | 50.3 ± 6.1 | 48.6 ± 7.9 | 50.4 ± 6.5 | 49.1 ± 6.0 | 49.3 ± 6.7 |
| BMI (kg/m2) | 27.8 ± 4.2 | 28.5 ± 5.2 | 27.2 ± 3.8 | 26.8 ± 3.3 | 27.5 ± 4.2 |
| Body fat (%) | 33.9 ± 3.3 | 31.6 ± 6.7 | 26.8 ± 9.1 | 27.8 ± 11.8 | 29.4 ± 9.5 |
| Body muscle (%) | 32.4 ± 3.3 | 33.9 ± 4.1 | 34.3 ± 3.8 | 36.1 ± 4.0 | 34.7 ± 4.1 |
| Body water (%) | 48.6 ± 2.4 | 50.3 ± 4.9 | 53.8 ± 6.7 | 54.2 ± 5.9 | 52.3 ± 5.9 |
| Performance baseline (%) | 87.4 ± 9.9 | 88.8 ± 7.1 | 122.0 ± 16.8 | 116.0 ± 15.9 | 105.6 ± 19.7 |
| Performance study end (%) | 87.0 ± 9.1 | 101.0 ± 10.0 | 118.2 ± 18.0 | 128.2 ± 15.6 | 113.7 ± 20.0 |
| Performance gain (%) | −2.7 ± 4.3 | 12.2 ± 7.1 | −3.8 ± 4.9 | 12.1 ± 5.6 | 7.8 ± 9.1 |
| Packyears | 22.4 ± 21.4 | 18.9 ± 15.8 | 12.2 ± 9.2 | 16.3 ± 14.6 | 17.1 ± 14.9 |
| Alcohol intake (units/week) | 0.7 ± 1.0 | 2.8 ± 3.2 | 3.4 ± 4.0 | 3.2 ± 4.4 | 2.9 ± 3.8 |
| Male sex (%) | 44.4 | 53.1 | 61.1 | 71.8 | 61.2 |
| Active smoking (%) | 55.6 | 25.0 | 16.7 | 10.3 | 20.4 |
| Positive cardiac history (%) | 11.1 | 15.6 | 5.6 | 23.1 | 16.3 |
| Diabetes mellitus (%) | 11.1 | 3.1 | 5.6 | 0 | 3.1 |
| Hypertension (%) | 33.3 | 43.8 | 33.3 | 23.1 | 32.7 |
| Dyslipidemia (%) | 33.3 | 25.0 | 38.9 | 28.2 | 29.6 |
| Overweight (%) | 66.8 | 68.8 | 66.7 | 63.2 | 65.9 |
| Positive family history (%) | 66.8 | 43.8 | 50.0 | 38.5 | 44.9 |
| Erythrocytes (T/L) | 4.6 ± 0.4 | 4.8 ± 0.5 | 4.6 ± 0.4 | 4.7 ± 0.4 | 4.7 ± 0.4 |
| Haemoglobin (g/dL) | 13.3 ± 1.5 | 14.2 ± 1.5 | 13.8 ± 1.0 | 14.2 ± 1.2 | 14.0 ± 1.3 |
| Sodium (mmol/L) | 141 ± 2 | 141 ± 2 | 141 ± 2 | 142 ± 2 | 141 ± 1.7 |
| Potassium (mmol/L) | 4.2 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.3 | 4.2 ± 0.2 | 4.2 ± 0.3 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 |
| ASAT (U/L) | 23 ± 4 | 26 ± 10 | 27 ± 7 | 24 ± 5 | 25 ± 7 |
| Triglycerides (mg/dL) | 154 ± 86 | 149 ± 100 | 111 ± 72 | 119 ± 62 | 131 ± 81 |
| Cholesterol (mg/dL) | 209 ± 54 | 200 ± 37 | 196 ± 29 | 201 ± 39 | 200 ± 38 |
| HDL-cholesterol (mg/dL) | 52 ± 19 | 56 ± 22 | 62 ± 12 | 60 ± 15 | 59 ± 17 |
| LDL-cholesterol (mg/dL) | 126 ± 50 | 117 ± 32 | 112 ± 29 | 116 ± 35 | 117 ± 34 |
| HbA1c (rel.%) | 5.5 ± 0.4 | 5.4 ± 0.8 | 5.5 ± 0.9 | 5.2 ± 0.3 | 5.3 ± 0.6 |
| proBNP (pg/mL) | 39 ± 27 | 59 ± 54 | 50 ± 35 | 32 ± 21 | 45 ± 39 |
| Regression Coefficient B | Standard Error | β | T | Significance | ||
|---|---|---|---|---|---|---|
| sTWEAK | Constant | −474.796 | 233.276 | −2.035 | 0.045 | |
| erythrocytes | 144.935 | 49.338 | 0.289 | 2.938 | 0.004 | |
| sCD163 | Constant | 23.408 | 199.495 | 0.177 | 0.907 | |
| erythrocytes | 195.298 | 68.091 | 0.454 | 2.868 | 0.005 | |
| hematocrit | −23.627 | 9.282 | −0.419 | −2.545 | 0.013 | |
| ASAT | 10.772 | 2.334 | 0.428 | 4.615 | <0.001 | |
| lipoprotein (a) | 1.018 | 0.287 | 0.314 | 3.547 | 0.001 |
| Parameter | Group 1 Unathletic Gain ≤ 2.9% (n = 9) | Group 2 Unathletic Gain > 2.9% (n = 32) | Group 3 Athletic Gain ≤ 2.9% (n = 18) | Group 4 Athletic Gain > 2.9% (n = 39) | Total Population |
|---|---|---|---|---|---|
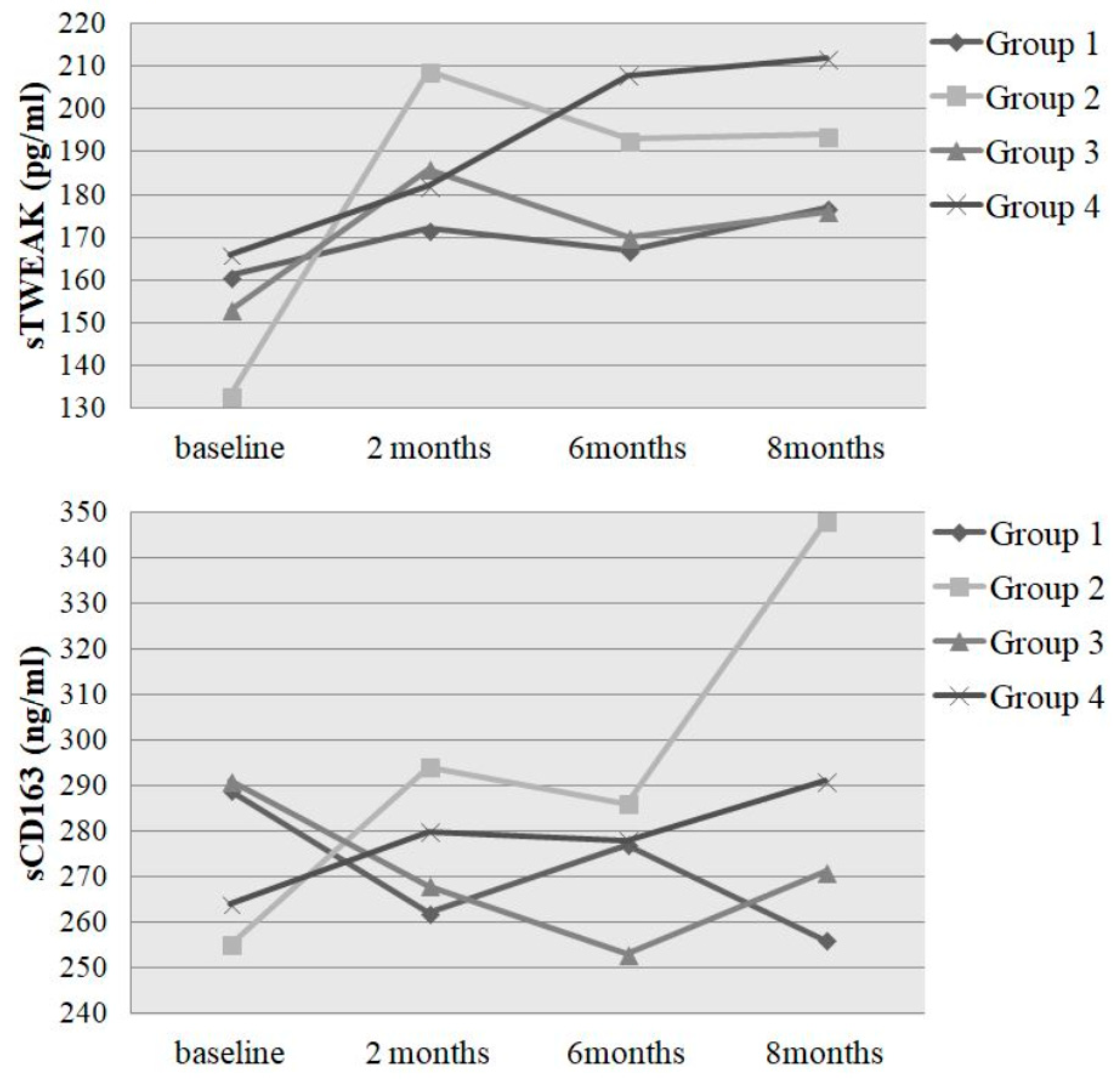
| TWEAK baseline (pg/mL) | 161 (105/213) | 133 (94/216) | 153 (96/209) | 166 (123/214) | 155 (104/213) |
| TWEAK 2 months | 172 (132/270) | 209 (111/309) | 186 (145/274) | 182 (133/274) | 182 (135/271) |
| TWEAK 6 months | 167 (122/215) | 193 (148/255) | 170 (122/267) | 208 (132/288) | 193 (133/253) |
| TWEAK 8 months | 177 (123/193) | 200 (152/286) | 176 (140/212) | 212 (151/274) | 196 (148/230) |
| Chi2 | 0.333 | 9.267 | 6.035 | 3.833 | 15.971 |
| p-value (Friedman Test) | 0.954 | 0.026 | 0.110 | 0.280 | 0.001 |
| p-value (Wilcoxon Test) | 0.953 | 0.002 | 0.744 | 0.031 | 0.001 |
| Change in % (baseline–end) | +9.9 | +50.4 | +15.0 | +27.7 | +26.5 |
| CD163 baseline (ng/mL) | 289 (249/354) | 255 (200/403) | 291 (206/351) | 247 (206/325) | 264 (208/332) |
| CD163 2 months | 262 (234/323) | 294 (221/437) | 268 (236/391) | 276 (227/353) | 280 (228/356) |
| CD163 6 months | 277 (261/370) | 286 (221/419) | 253 (195/390) | 288 (225/358) | 278 (232/383) |
| CD163 8 months | 256 (221/331) | 348 (273/396) | 271 (247/359) | 288 (243/394) | 291 (247/384) |
| Chi2 | 5.000 | 4.644 | 1.800 | 7.560 | 8.032 |
| p-value (Friedman Test) | 0.172 | 0.200 | 0.615 | 0.056 | 0.045 |
| p-value (Wilcoxon Test) | 0.374 | 0.035 | 0.913 | 0.025 | 0.016 |
| Change in % (baseline–end) | −11.4 | +36.5 | −6.9 | +16.6 | +10.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönbauer, R.; Lichtenauer, M.; Paar, V.; Emich, M.; Fritzer-Szekeres, M.; Schukro, C.; Strametz-Juranek, J.; Sponder, M. Regular Training Increases sTWEAK and Its Decoy Receptor sCD163–Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect? J. Clin. Med. 2020, 9, 1899. https://doi.org/10.3390/jcm9061899
Schönbauer R, Lichtenauer M, Paar V, Emich M, Fritzer-Szekeres M, Schukro C, Strametz-Juranek J, Sponder M. Regular Training Increases sTWEAK and Its Decoy Receptor sCD163–Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect? Journal of Clinical Medicine. 2020; 9(6):1899. https://doi.org/10.3390/jcm9061899
Chicago/Turabian StyleSchönbauer, Robert, Michael Lichtenauer, Vera Paar, Michael Emich, Monika Fritzer-Szekeres, Christoph Schukro, Jeanette Strametz-Juranek, and Michael Sponder. 2020. "Regular Training Increases sTWEAK and Its Decoy Receptor sCD163–Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect?" Journal of Clinical Medicine 9, no. 6: 1899. https://doi.org/10.3390/jcm9061899
APA StyleSchönbauer, R., Lichtenauer, M., Paar, V., Emich, M., Fritzer-Szekeres, M., Schukro, C., Strametz-Juranek, J., & Sponder, M. (2020). Regular Training Increases sTWEAK and Its Decoy Receptor sCD163–Does Training Trigger the sTWEAK/sCD163-Axis to Induce an Anti-Inflammatory Effect? Journal of Clinical Medicine, 9(6), 1899. https://doi.org/10.3390/jcm9061899






