Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Culture Isolation, Purification and Cultivation
2.2. DNA Isolation, 16S rDNA Amplification and Sequence Analysis
2.3. Measurement of Sulfate Consumption
2.4. Measurement of Hydrogen Sulfide Production
2.5. Determination of Biomass Concentration
2.6. Statistical Analysis
3. Results
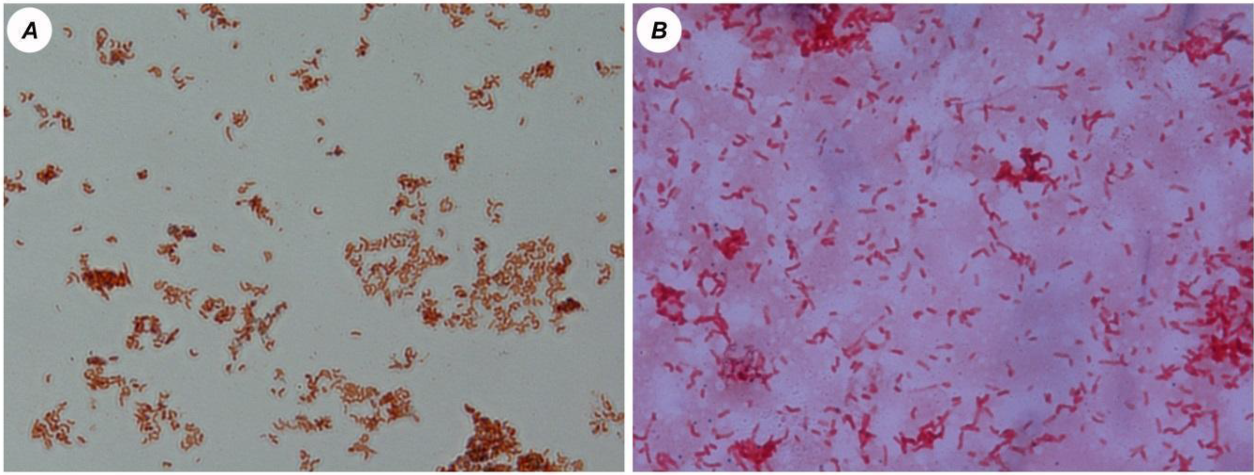
3.1. Microscopic Analysis of the Cultures
3.2. Sequence Analysis
3.3. Sulfate Consumption, H2S Production and Biomass Accumulation
3.4. Statistical and Cluster Analysis of Sulfate Reduction Parameters and Trace Elements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katz, J.A.; Itoh, J.; Fiocchi, C. Pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 1999, 15, 291. [Google Scholar] [CrossRef]
- Danese, S.; De La Motte, C.; Fiocchi, C. Platelets in inflammatory bowel disease: Clinical, pathogenic, and therapeutic implications. Am. J. Gastroenterol. 2004, 99, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4807. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Fiocchi, C. Inflammatory bowel disease: Progress and current concepts of etiopathogenesis. J. Dig. Dis. 2007, 8, 171–178. [Google Scholar] [CrossRef]
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef]
- Loubinoux, J.; Bronowicji, J.P.; Pereira, I.A. Sulphate-reducing bacteria in human feces and their association with inflammatory diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Rowan, F.E.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br. J. Surg. 2009, 96, 151–158. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Toxicity of hydrogen sulfide toward sulfate-reducing bacteria Desulfovibrio piger Vib-7. Arch. Microbiol. 2019, 201, 389–397. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small–Large Intestine Axis and its Role in IBD Development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Kotrsová, V.; Dordević, D.; Buňková, L.; Vítězová, M.; Amedei, A. Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. Biomolecules 2019, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Macfarlane, G.T.; Macfarlane, S. Intestinal Bacteria and Ulcerative Colitis. Curr. Issues Intest. Microbiol. 2003, 4, 9–20. [Google Scholar]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Growth and activities of sulphate-reducing bacteria in gut contents of health subjects and patients with ulcerative colitis. FEMS Microbiol. Ecol. 1991, 86, 103–112. [Google Scholar] [CrossRef]
- Coutinho, C.M.L.M.; Coutinho-Silva, R.; Zinkevich, V.; Pearce, C.B.; Ojcius, D.M.; Beech, I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells. Microb. Pathog. 2017, 112, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Vítězová, M.; Kos, J.; Kollár, P.; Jampilek, J. Effect of selected 8-hydroxyquinoline-2-carboxanilides on viability and sulfate metabolism of Desulfovibrio piger. J. Appl. Biomed. 2018, 16, 241–246. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kollar, P.; Suchy, P.; Parak, T.; Pauk, K.; Imramovsky, A. Activity of selected salicylamides against intestinal sulfate-reducing bacteria. Neuro Endocrinol. Lett. 2015, 36, 106–113. [Google Scholar] [PubMed]
- Kushkevych, I.V. Kinetic properties of pyruvate ferredoxin oxidoreductase of intestinal sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Pol. J. Microbiol. 2015, 64, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Barton, L.L.; Hamilton, W.A. Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Cambridge University Press: Cambridge, UK, 2010; p. 553. [Google Scholar]
- Kotrsová, V.; Kushkevych, I. Possible methods for evaluation of hydrogen sulfide toxicity against lactic acid bacteria. Biointerface Res. Appl. Chem. 2019, 9, 4066–4069. [Google Scholar]
- Pitcher, M.C.; Cummings, J.H. Hydrogen sulphide: A bacterial toxin in ulcerative colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushkevych, I.; Leščanová, O.; Dordević, D.; Jančíková, S.; Hošek, J.; Vítězová, M.; Buňková, L.; Drago, L. The sulfate-reducing microbial communities and meta-analysis of their occurrence during diseases of small-large intestine axis. J. Clin. Med. 2019, 8, 1656. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Fafula, R.; Parak, T.; Bartoš, M. Activity of Na+/K+-activated Mg2+-dependent ATP hydrolase in the cell-free extracts of the sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Acta Vet. Brno 2015, 84, 3–12. [Google Scholar] [CrossRef]
- Kushkevych, I.V. Activity and kinetic properties of phosphotransacetylase from intestinal sulfate-reducing bacteria. Acta Biochem. Pol. 2015, 62, 1037–1108. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Dordević, D.; Vítězová, M.; Kollár, P. Cross-correlation analysis of the Desulfovibrio growth parameters of intestinal species isolated from people with colitis. Biologia 2018, 73, 1137–1143. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Open Med. 2019, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut bacteria and hydrogen sulfide: The new old players in circulatory system homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kováč, J.; Vítězová, M.; Kushkevych, I. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med. 2018, 13, 344–349. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Fedrová, P.; Vochyanová, Z.; Paráková, L.; Hošek, J. Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet. Brno 2017, 86, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 1988, 65, 103–111. [Google Scholar] [CrossRef]
- Černý, M.; Vítězová, M.; Vítěz, T.; Bartoš, M.; Kushkevych, I. Variation in the distribution of hydrogen producers from the clostridiales order in biogas reactors depending on different input substrates. Energies 2018, 11, 3270. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Bartoš, M. Production of biogas: Relationship between methanogenic and sulfate-reducing microorganisms. Open Life Sci. 2017, 12, 82–91. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Kováč, J.; Kaucká, P.; Jesionek, W.; Bartoš, M.; Barton, L. A new combination of substrates: Biogas production and diversity of the methanogenic microorganisms. Open Life Sci. 2018, 13, 119–128. [Google Scholar] [CrossRef]
- Peck, H.D.; Legall, J.; Vanbeeumen, J. Biochemistry of dissimilatory sulfate reduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 443–466. [Google Scholar]
- Kushkevych, I.; Dordević, D.; Kollar, P. Analysis of physiological parameters of Desulfovibrio strains from individuals with colitis. Open Life Sci. 2018, 13, 481–488. [Google Scholar] [CrossRef]
- Loubinoux, J.; Valente, F.M.A.; Pereira, I.A.C. Reclassification of the only species of the genus Desulfomonas, Desulfomonas pigra, as Desulfovibrio piger comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1305–1308. [Google Scholar] [PubMed] [Green Version]
- Postgate, J.R. The Sulfate-Reducing Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984; p. 208. [Google Scholar]
- Abdulina, D.; Kováč, J.; Iutynska, G.; Kushkevych, I. ATP sulfurylase activity of sulfate-reducing bacteria from various ecotopes. 3Biotech 2020, 10, 55. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kollar, P.; Ferreira, A.L.; Palma, D.; Duarte, A.; Lopes, M.M.; Bartos, M.; Pauk, K.; Imramovsky, A.; Jampilek, J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016, 14, 125–130. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kos, J.; Kollar, P.; Kralova, K.; Jampilek, J. Activity of ring-substituted 8-hydroxyquinoline-2-carboxanilides against intestinal sulfate-reducing bacteria Desulfovibrio piger. Med. Chem. Res. 2018, 27, 278–284. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2020, 25, 1–15. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Moore, W.E.; Johnson, J.L.; Holdeman, L.V. Emendation of Bacteroidaceae and Butyrivibrio and descriptions of Desulfomonas gen. nov. and ten new species of the genera Desulfomonas, Butyrivibrio, Eubacterium, Clostridium and Ruminococcus. Int. J. Syst. Bact. 1976, 26, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M. Volume two: The proteobacteria, part C: The alpha-, beta-, delta-, and epsilonproteobacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; The United States of America Springer: Boston, MA, USA, 2005; p. 1388. [Google Scholar]
- Kováč, J.; Kushkevych, I. New modification of cultivation medium for isolation and growth of intestinal sulfate-reducing bacteria. In Proceedings of the International PhD Students Conference Mendel Net, Brno, Czech Republic, 6–7 November 2019; pp. 702–707. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Persing, D.H. Molecular Microbiology: Diagnostic Principles and Practice, 2nd ed.; ASM Press: Washington, DC, USA, 2011; p. 960. [Google Scholar]
- Kolmert, A.; Wikstrom, P.; Hallberg, K.B. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods 2000, 41, 179–184. [Google Scholar] [CrossRef]
- Sugiyama, M. Reagent Composition for Measuring Hydrogen Sulfide and Method for Measuring Hydrogen. U.S. Patent 6,340,596, 22 January 2002. [Google Scholar]
- Bailey, N.T.J. Statistical Methods in Biology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Florin, T.H.; Neale, G.; Goretski, S. Sulfate in food and beverages. J. Food Compos. Anal. 1993, 6, 140–151. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kováč, J.; Vítězová, M.; Vítěz, T.; Bartoš, M. The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch. Microbiol. 2018, 200, 945–950. [Google Scholar] [CrossRef]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Blachier, F.; Davila, A.M.; Mimoun, S. Luminal sulfide and large intestine mucosa: Friend or foe? Amino Acids 2010, 39, 335–347. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef] [Green Version]






| Sample Name | Sex | Diagnosis | Age | Weight (kg) | Year of Diagnosis | State of the Disease * |
|---|---|---|---|---|---|---|
| M-01 | Female | CD | 21 | 57 | 2011 | Short-term remission |
| M-02 | Male | CD | 34 | 104 | 2013 | Short-term remission |
| M-03 | Female | UC | 66 | 78 | 2012 | Short-term remission |
| M-04 | Female | UC | 41 | 53 | 2005 | Disease flare |
| M-05 | Male | UC | 35 | 92 | 2018 | Short-term remission |
| M-06 | Female | UC | 39 | 120 | 2013 | Disease flare |
| M-07 | Male | UC | 44 | 61 | 2014 | Long-term remission |
| M-08 | Female | UC | 20 | 70 | 2003 | Short-term remission |
| M-09 | Male | CD | 24 | 73 | 2006 | Long-term remission |
| M-10 | Male | CD | 34 | 70 | 2018 | Disease flare |
| Z-8 | Male | Control | 46 | 108 | – | Healthy |
| Z-9 | Male | Control | 53 | 72 | – | Healthy |
| Z-11 | Male | Control | 42 | 95 | – | Healthy |
| Z-12 | Male | Control | 26 | 78 | – | Healthy |
| Sample Name | Azathioprine | Mesalazine | Glucocorticoids | Biological Treatment | Omeprazole |
|---|---|---|---|---|---|
| M-01 | Imuran 50 mg | – | – | – | – |
| M-02 | – | Pentasa 500 mg | – | – | – |
| M-03 | – | Pentasa 1 g | Cortiment 9 mg | – | Helic 10 mg |
| M-04 | Imuran 50 mg | Pentasa 1 g | Prednison 5 mg | – | – |
| M-05 | – | Pentasa 1 g | Cortiment 9 mg | – | – |
| M-06 | – | Asacol 400 mg | Cortiment 9 mg | Entivio 300 mg | – |
| M-07 | – | Pentasa 1 g | – | – | – |
| M-08 | Imasup 50 mg | Salofalk 3 g | – | Remsima 400 mg | – |
| M-09 | – | Pentasa 1 g | – | – | – |
| M-10 | – | Pentasa 1 g | Budenofalk 3 mg | – | – |
| Identified Bacteria and Accession in GenBank | Amplicon Length (bp) | % Identity | Reference Strains of Desulfovibrio Genus | Reference Accession in GenBank |
|---|---|---|---|---|
| Desulfovibrio vulgaris M6_1 MT027815 | 745 | 99.06 | D. vulgaris DSM 644 | NR_112657.1 |
| D. vulgaris RCH1 | CP002297.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M6_2 MT027923 | 740 | 98.65 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M6_3 MT027925 | 279 | 97.47 | D. vulgaris PhM31 | KC013878.1 |
| D. vulgaris PhM22 | KC013874.1 | |||
| D. vulgaris Hildenborough | NR_074446.1 | |||
| Desulfovibrio vulgaris M1_1 MT027899 | 288 | 93.57 | D. vulgaris PhM31 | KC013878.1 KC013874.1 CP002297.1 |
| D. vulgaris PhM22 | ||||
| D. vulgaris RCH1 | ||||
| Desulfovibrio vulgaris M1_2 MT027900 | 575 | 99.30 | D. vulgaris Hildenborough | NR_074446.1 CP002297.1 CP000527.1 |
| D. vulgaris RCH1 | ||||
| D. vulgaris DP4 | ||||
| Desulfovibrio vulgaris M3_1 MT093732 | 391 | 99.22 | D. vulgaris Hildenborough | NR_074446.1 |
| D. vulgaris DP4 | CP000527.1 | |||
| D. vulgaris RCH1 | CP002297.1 | |||
| Desulfovibrio vulgaris M3_2 MT093731 | 429 | 99.30 | D. vulgaris Hildenborough | NR_074446.1 |
| D. vulgaris RCH1 | CP002297.1 | |||
| D. vulgaris DSM 644 | NR_112657.1 | |||
| Desulfovibrio vulgaris M4_3 MT093736 | 748 | 99.87 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DSM 644 | NR_041855.1 | |||
| Desulfovibrio vulgaris M5_1 MT093737 | 568 | 98.07 | D. vulgaris PhM31 | KC013878.1 |
| D. vulgaris PhM22 | KC013874.1 | |||
| D. vulgaris Hildenborough | NR_074446.1 | |||
| Desulfovibrio vulgaris M5_2 MT093788 | 745 | 99.46 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M7_1 MT093774 | 751 | 99.60 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M7_2 MT093772 | 762 | 99.47 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris Hildenborough | AE017285.1 | |||
| Desulfovibrio vulgaris M7_3 MT093800 | 766 | 99.61 | D. vulgaris DP4 | CP000527.1 |
| 99.35 | D. vulgaris RCH1 | CP002297.1 | ||
| D. vulgaris DSM 644 | NR_112657.1 | |||
| Desulfovibrio vulgaris M8 MT093799 | 762 | 99.34 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M9_1 MT093798 | 768 | 99.60 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M9_2 MT093820 | 315 | 98.10 | D. vulgaris PhM31 | KC013878.1 |
| D. vulgaris PhM22 | KC013874.1 | |||
| D. vulgaris Hildenborough | NR_074446.1 | |||
| Desulfovibrio vulgaris M9_3 MT093819 | 764 | 99.73 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris M10_1 MT093826 | 770 | 99.09 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris Z8_2 MT093823 | 768 | 99.48 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris Z9_2 MT093825 | 629 | 99.05 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris Z9_3 MT093830 | 448 | 99.33 | D. vulgaris RCH1 | CP002297.1 |
| D. vulgaris DSM 644 | NR_112657.1 | |||
| D. vulgaris DP4 | CP000527.1 | |||
| Desulfovibrio vulgaris Z11_1 MT093829 | 751 | 99.46 | D. vulgaris DP4 | CP000527.1 |
| D. vulgaris. vulgaris | DQ826728.1 | |||
| 99.20 | D. vulgaris RCH1 | CP002297.1 | ||
| Desulfovibrio vulgaris Z12_1 MT093831 | 463 | 99.13 | D. vulgaris Hildenborough | NR_074446.1 |
| D. vulgaris RCH1 | CP002297.1 | |||
| D. vulgaris DSM 644 | NR_112657.1 |
| Parameters | Healthy People | Patients with IBD | ||||
|---|---|---|---|---|---|---|
| 5 h | 24 h | 48 h | 5 h | 24 h | 48 h | |
| Sulfate | 23.23 ± 5.85 a* | 5.61 ± 0.99 b | 3.05 ± 0.32 d | 25.03 ± 5.34 a | 6.26 ± 1.72 bcd | 3.10 ± 0.33 cd |
| H2S | 1.62 ± 0.38 a | 30.58 ± 3.63 b | 24.48 ± 2.15 b | 2.38 ± 1.47 a | 32.14 ± 5.47 b | 25.58 ± 1.42 b |
| Biomass | 4.35 ± 1.07 a | 12.11 ± 1.35 bc | 15.04 ± 1.37 d | 4.02 ± 1.17 a | 11.55 ± 1.96 c | 14.32 ± 0.82 bd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushkevych, I.; Castro Sangrador, J.; Dordević, D.; Rozehnalová, M.; Černý, M.; Fafula, R.; Vítězová, M.; Rittmann, S.K.-M.R. Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People. J. Clin. Med. 2020, 9, 1920. https://doi.org/10.3390/jcm9061920
Kushkevych I, Castro Sangrador J, Dordević D, Rozehnalová M, Černý M, Fafula R, Vítězová M, Rittmann SK-MR. Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People. Journal of Clinical Medicine. 2020; 9(6):1920. https://doi.org/10.3390/jcm9061920
Chicago/Turabian StyleKushkevych, Ivan, Jorge Castro Sangrador, Dani Dordević, Monika Rozehnalová, Martin Černý, Roman Fafula, Monika Vítězová, and Simon K.-M. R. Rittmann. 2020. "Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People" Journal of Clinical Medicine 9, no. 6: 1920. https://doi.org/10.3390/jcm9061920
APA StyleKushkevych, I., Castro Sangrador, J., Dordević, D., Rozehnalová, M., Černý, M., Fafula, R., Vítězová, M., & Rittmann, S. K.-M. R. (2020). Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People. Journal of Clinical Medicine, 9(6), 1920. https://doi.org/10.3390/jcm9061920







