A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder
Abstract
:1. Introduction
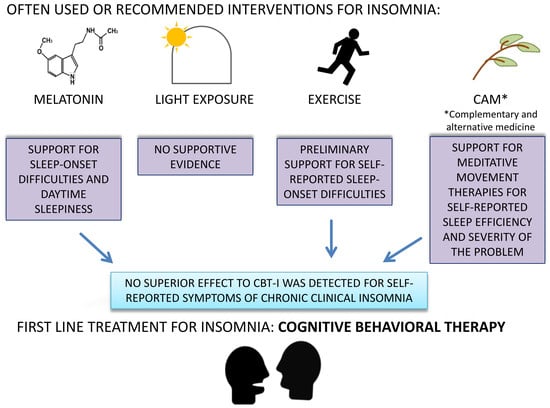
- Melatonin is generally not recommended because evidence shows low efficacy.
- Light therapy and physical activity may be useful as adjunct therapy, but recommendation is weak due to low-quality evidence.
- Complementary and alternative interventions are not recommended because of poor evidence. In the area of complementary and alternative interventions, several treatments for insomnia have been suggested, including acupuncture, acupressure, aromatherapy, reflexology, homeopathy, meditative movement therapies, moxibustion, music therapy, and yoga [40].
2. Methods
2.1. Search Procedure
2.2. Eligibility Criteria
- (1)
- Population: Individuals with insomnia disorder of all ages (including adult and pediatric populations) and of both gender with or without any mental, somatic or sleep comorbidity.
- (2)
- Intervention: Experimental interventions including one of the following administered alone (i.e., not in combination with recommended therapies for insomnia): ayurveda, chelation, diet-based therapy, energy healing therapy, exercise, folk medicine, homeopathy, hypnosis, light exposure, massage, meditation, melatonin, music therapy, natural herbs, naturopathy, qi gong, reiki, tai chi, transcranial magnetic stimulation, valerian, vitamin, and yoga.
- (3)
- Comparison: waiting list, no treatment, pharmacological and psychological (e.g., psychoeducation) placebo, standard therapy for insomnia: sleep pharmacotherapy (hypnotics: benzodiazepine (BZ) and benzodiazepine receptor agonists (BZRA) and recommended psychological treatment, i.e., CBT-I (CBT-I, sleep restriction, stimulus control).
- (4)
- Outcomes: objective and subjective standardized measures of sleep and/or insomnia.
- (5)
- Study design: Randomized controlled trial.
- (6)
- (7)
- Written in English, German, Italian, Spanish, French, Bulgarian, or Russian.
2.3. Data Extraction
2.4. Assessment of Risk of Bias
- (1)
- Selection bias. This domain refers to systematic differences between baseline characteristics and covers two parts: (1) Did the investigators use a random sequence generation process? (2) Could intervention allocations have been foreseen in advance of enrolment?
- (2)
- Performance bias. This domain judges whether participants and personnel were blinded. Because blinding therapists and patients is not desirable in some form of interventional studies (such as psychotherapy or mindfulness or yoga), performance biases for these types of studies was systematically scored as “low risk”.
- (3)
- Detection bias. This domain refers to whether outcome assessors are aware of intervention assignments.
- (4)
- Attrition bias. This domain refers to systematic differences between groups in withdrawals from a study. Amount, nature, and handling of incomplete outcome data are evaluated.
- (5)
- Reporting bias. This domain refers to selective outcome reporting. For each included clinical study a search was conducted to find registered protocols in order to check the consistency between the planned and the reported analyses.
2.5. Statistical Analyses
- (1)
- Self-reported sleep efficiency: defined as a sleep efficiency index from sleep diaries or, if this was not reported, as sleep quality perception from sleep diaries;
- (2)
- Sleep efficiency measured through physiological indices: defined as sleep efficiency index measured by polysomnography, or, if this was not reported, by actigraphy;
- (3)
- (4)
- (5)
- Self-reported sleep onset latency: defined as sleep onset latency measured through sleep diaries;
- (6)
- Sleep onset latency measured through physiological indices: defined as sleep onset latency measured by polysomnography or, if this was not reported, by actigraphy.
- (7)
- Self-reported wake time during the night: defined as wake after sleep onset latency measured through sleep diaries;
- (8)
- Wake time during the night measured through physiological indices: defined as wake after sleep onset latency measured by polysomnography or, if this was not reported, by actigraphy.
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Network Meta-Analysis
3.5. Self-Reported Sleep Efficiency
3.6. Sleep Efficiency Measured Through Physiological Indices
3.7. Subjective Severity of the Sleep Problem
3.8. Daytime Sleepiness
3.9. Self-Reported Sleep Onset Latency
3.10. Sleep Onset Latency Measured Through Physiological Indices
3.11. Self-Reported Wake During the Night
3.12. Wake during the Night Measured Through Physiological Indices
4. Discussion
5. Clinical Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis. Prim. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.D.; Espie, C.A.; Morgan, K. “… Not just a minor thing, it is something major, which stops you from functioning daily”: Quality of life and daytime functioning in insomnia. Behav. Sleep Med. 2010, 8, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Grippe, T.; Bayon, V. Societal costs of insomnia. Sleep Med. Rev. 2010, 14, 379–389. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Zhang, B.; Wing, Y.-K. Sex differences in insomnia: A meta-analysis. Sleep 2006, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Roth, T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J. Psychosom. Res. 2002, 53, 547–554. [Google Scholar] [CrossRef]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Vasquez, M.M.; Halonen, M.; Bootzin, R.; Quan, S.F.; Martinez, F.D.; Guerra, S. Persistent insomnia is associated with mortality risk. Am. J. Med. 2014, 128, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Strand, L.B.; Vatten, L.J.; Janszky, I.; Bjørngaard, J.H. Insomnia symptoms and risk for unintentional fatal injuries—The HUNT study. Sleep 2014, 37, 1777–1786. [Google Scholar] [CrossRef] [Green Version]
- Laugsand, L.E.; Vatten, L.; Platou, C.; Janszky, I. Insomnia and the risk of acute myocardial infarction. Circulation 2011, 124, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Palagini, L.; Bruno, R.M.; Gemignani, A.; Baglioni, C.; Ghiadoni, L.; Riemann, D. Sleep loss and hypertension: A systematic review. Curr. Pharm. Des. 2013, 19, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Marcelli, E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc. Sci. Med. 2010, 71, 1027–1036. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; A Miller, M. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef]
- Faraut, B.; Touchette, E.; Gamble, H.; Royant-Parola, S.; Safar, M.E.; Varsat, B.; Leger, D. Short sleep duration and increased risk of hypertension. J. Hypertens. 2012, 30, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Z.; Xu, C.; Rota, M.; Cai, H.; Zhang, C.; Shi, M.-J.; Yuan, R.-X.; Weng, H.; Meng, X.-Y.; Kwong, J.S.W.; et al. Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med. Rev. 2017, 32, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.A.; Ebben, M.R. The cost of insomnia and the benefit of increased access to evidence-based treatment. Sleep Med. Clin. 2017, 12, 39–46. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Shaya, F.T.; Scharf, S.M. Health economics of insomnia treatments: The return on investment for a good night’s sleep. Sleep Med. Rev. 2016, 30, 72–82. [Google Scholar] [CrossRef]
- Ozminkowski, R.J.; Wang, S.; Walsh, J.K. The direct and indirect costs ofuntreated insomnia in adults in the United States. Sleep 2007, 30, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, C.; Altena, E.; Bjorvatn, B.; Blom, K.; Bothelius, K.; Devoto, A.; Espie, C.A.; Frase, L.; Gavriloff, D.; Tuuliki, H.; et al. The european academy for cognitive behavioural therapy for insomnia: An initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J. Sleep Res. 2019, 29, e12967. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Management of chronic insomnia disorder in adults: A clinical practice guideline from the american college of physicians. Ann. Intern. Med. 2016, 165, 125. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.L.; Bjorvatn, B.; Groselj, L.D.; Ellis, J.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Lack, L.; Dawson, D. Sleep-onset insomniacs have delayed temperature rhythms. Sleep 1990, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscemi, N.; Vandermeer, B.; Pandya, R.; Hooton, N.; Tjosvold, L.; Hartling, L. Melatonin for treatment of sleep disorders. Evid. Rep. Technol. Assess. (Summ) 2004, 108. [Google Scholar] [CrossRef]
- Hajak, G.; Lemme, K.; Zisapel, N. Lasting treatment effects in a postmarketing surveillance study of prolonged-release melatonin. Int. Clin. Psychopharmacol. 2014, 30, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Shechter, A.; Lespérance, P.; Kin, K.N.Y.; Boivin, D.B. Pilot investigation of the circadian plasma melatonin rhythm across the menstrual cycle in a small group of women with premenstrual dysphoric disorder. PLoS ONE 2012, 7, e51929. [Google Scholar] [CrossRef] [Green Version]
- Chesson, A.L.; Anderson, W.M.; Littner, M.; Davila, D.; Hartse, K.; Johnson, S.; Wise, M.; Rafecas, J. Practice parameters for the nonpharmacologic treatment of chronic insomnia. Sleep 1999, 22, 1128–1133. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.-M.; Santhi, N.; Hilaire, M.S.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human responses to bright light of different durations. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Eastman, C. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol. Int. 2009, 26, 709–725. [Google Scholar] [CrossRef]
- Orrow, G.; Kinmonth, A.-L.; Sanderson, S.; Sutton, S. Effectiveness of physical activity promotion based in primary care: Systematic review and meta-analysis of randomised controlled trials. BMJ 2012, 344, e1389. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.W.; Parra, D.C.; Sarmiento, O.L.; Andersen, L.B.; Owen, N.; Goenka, S.; Montes, F.; Brownson, R.C.; Lancet Physical Activity Series Working Group. Evidence-based intervention in physical activity: Lessons from around the world. Lancet 2012, 380, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Wells, R.E.; Smith, M.T.; McCarthy, E.P. Use of relaxation techniques and complementary and alternative medicine by american adults with insomnia symptoms: Results from a national survey. J. Clin. Sleep Med. 2012, 8, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.M.; Powell-Griner, E.; McFann, K.; Nahin, R. Complementary and alternative medicine use among adults: United States, 2002. In Seminars in Integrative Medicine; WB Saunders: Philadelphia, PA, USA, 2004; Volume 2, pp. 54–71. [Google Scholar]
- Wieland, S.; Manheimer, E.; Berman, B.M. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Altern. Ther. Heal. Med. 2011, 17, 50–59. [Google Scholar]
- Sarris, J.; Byrne, G.J. A systematic review of insomnia and complementary medicine. Sleep Med. Rev. 2011, 15, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, D.K.L.; Yeung, J.; Chung, K.F.; Wong, V. Acupuncture for insomnia (Cochrane Review). Cochrane Libr. 2007. [Google Scholar] [CrossRef]
- Cheuk, D.K.L.; Yeung, W.-F.; Chung, K.F.; Wong, V. Acupuncture for insomnia. Cochrane Database Syst. Rev. 2012, CD005472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, D.; Perlis, M.L. The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med. Rev. 2009, 13, 205–214. [Google Scholar] [CrossRef]
- Harvey, A.G. A cognitive theory and therapy for chronic insomnia. J. Cogn. Psychother. 2005, 19, 41–59. [Google Scholar] [CrossRef]
- Rücker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 2012, 3, 312–324. [Google Scholar] [CrossRef]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 2012, 3, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Bafeta, A.; Trinquart, L.; Seror, R.; Ravaud, P. Reporting of results from network meta-analyses: Methodological systematic review. BMJ 2014, 348, g1741. [Google Scholar] [CrossRef] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- American Academy of Sleep Medicine. International classification of sleep disorders. In Diagnostic and Coding Manual; American Academy of Sleep Medicine: Westchester, NY, USA, 2005; pp. 51–55. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Johns, M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Owens, J.A.; Spirito, A.; McGuinn, M. The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep New York 2000, 23, 1043–1052. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. Neuroimaging 1989, 28, 193–213. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rü>cker, G.; Krahn, U.; König, J.; Efthimiou, O.; Schwarzer, G. Netmeta: Network Meta-Analysis Using Frequentist Methods. [Computer Software]. 2020. Available online: https://github.com/guido-s/netmeta or http://meta-analysis-with-r.org (accessed on 11 June 2020).
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer: New York, NY, USA, 2015; Volume 4724. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- König, J.; Krahn, U.; Binder, H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat. Med. 2013, 32, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Sebastiani, T.; Panunzi, S.; Valente, D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: A randomized placebo-controlled trial. J. Sleep Res. 2012, 21, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P.; Gamble, C.; Jones, A.P.; Wiggs, L.; Williamson, P.R.; Sutcliffe, A.; Montgomery, P.; Whitehouse, W.; Choonara, I.; Allport, T.; et al. Melatonin for sleep problems in children with neurodevelopmental disorders: Randomised double masked placebo controlled trial. BMJ 2012, 345, e6664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gringras, P.; Nir, T.; Breddy, J.; Frydman-Marom, A.; Findling, R.L. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthringer, R.; Muzet, M.; Zisapel, N.; Staner, L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int. Clin. Psychopharmacol. 2009, 24, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Wang-Weigand, G.; Roth-Schechter, B.; Lehmann, R.; Staner, C.; Partinen, M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep 2009, 32, 351–360. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F.; Antoniello, N.; Manni, R.; Klersy, C. The effect of melatonin, magnesium, and Zinc on primary insomnia in long-term care facility residents in Italy: A double-blind, placebo-controlled clinical trial. J. Am. Geriatr. Soc. 2011, 59, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Wright, K.P.; Walsh, J. Effect of tiagabine on sleep in elderly subjects with primary insomnia: A randomized, double-blind, placebo-controlled study. Sleep 2006, 29, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Smits, M.G.; Nagtegaal, E.E.; Van Der Heijden, J.; Coenen, A.M.; Kerkhof, G.A. Melatonin for chronic sleep onset insomnia in children: A randomized placebo-controlled trial. J. Child. Neurol. 2001, 16, 86–92. [Google Scholar] [CrossRef]
- Van Geijlswijk, I.M.; Van Der Heijden, K.B.; Egberts, T.; Korzilius, H.P.L.M.; Smits, M.G. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: An RCT. Psychopharmacology 2010, 212, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Wade, A.G.; Ford, I.; Crawford, G.; McMahon, A.D.; Nir, T.; Laudon, M.; Zisapel, N. Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: Quality of sleep and next-day alertness outcomes. Curr. Med. Res. Opin. 2007, 23, 2597–2605. [Google Scholar] [CrossRef]
- Wang-Weigand, S.; Watissée, M.; Roth, T. Use of a post-sleep questionnaire-interactive voice response system (PSQ-IVRS) to evaluate the subjective sleep effects of ramelteon in adults with chronic insomnia. Sleep Med. 2011, 12, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Zammit, G.; Erman, M.; Wang-Weigand, S.; Sainati, S.; Zhang, J.; Roth, T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J. Clin. Sleep Med. 2007, 3, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Afonso, R.F.; Hachul, H.; Kozasa, E.H.; Oliveira, D.D.S.; Goto, V.; Rodrigues, D.; Tufik, S.; Leite, J.R. Yoga decreases insomnia in postmenopausal women. Menopause 2012, 19, 186–193. [Google Scholar] [CrossRef]
- Garcia, M.C.; Kozasa, E.H.; Tufik, S.; Mello, L.E.A.M.; Hachul, H. The effects of mindfulness and relaxation training for insomnia (MRTI) on postmenopausal women. Menopause 2018, 25, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.N.; Carlson, L.E.; Stephens, A.J.; Antle, M.C.; Samuels, C.; Campbell, T.S. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J. Clin. Oncol. 2014, 32, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.R.; Kreitzer, M.J.; Reilly-Spong, M.; Wall, M.; Winbush, N.Y.; Patterson, R.; Mahowald, M.; Cramer-Bornemann, M. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: A randomized controlled clinical trial. Explore 2011, 7, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.R.; Olmstead, R.; Breen, E.C.; Witarama, T.; Carrillo, C.; Sadeghi, N.; Arevalo, J.M.G.; Ma, J.; Nicassio, P.; Ganz, P.A.; et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. J. Natl. Cancer Inst. Monogr. 2014, 2014, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R.; Olmstead, R.; Carrillo, C.; Sadeghi, N.; Nicassio, P.; Ganz, P.A.; Bower, J.E. Tai chi chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast cancer: A randomized, partially blinded, noninferiority trial. J. Clin. Oncol. 2017, 35, 2656–2665. [Google Scholar] [CrossRef] [Green Version]
- Ong, J.C.; Manber, R.; Segal, Z.; Xia, Y.; Shapiro, S.; Wyatt, J.K. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep 2014, 37, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.C.; Xia, Y.; Smith-Mason, C.E.; Manber, R. A randomized controlled trial of mindfulness meditation for chronic insomnia: Effects on daytime symptoms and cognitive-emotional arousal. Mindfulness 2018, 9, 1702–1712. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Liu, X.-H.; Xie, X.-H.; Zhao, D.; Shan, M.-S.; Zhang, X.-L.; Kong, X.-M.; Cui, H. Mindfulness-based stress reduction for chronic insomnia in adults older than 75 years: A randomized, controlled, single-blind clinical trial. Explore 2015, 11, 180–185. [Google Scholar] [CrossRef] [PubMed]
- D’Aurea, C.V.; Poyares, D.; Passos, G.S.; Santana, M.G.; Youngstedt, S.D.; Souza, A.A.; Bicudo, J.; Tufik, S.; De Mello, M.T. Effects of resistance exercise training and stretching on chronic insomnia. Rev. Bras. Psiquiatr. 2019, 41, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartescu, I.; Morgan, K.; Stevinson, C.D. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. J. Sleep Res. 2015, 24, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Hartescu, I.; Morgan, K.; Stevinson, C.D. psychomotor performance decrements following a successful physical activity intervention for insomnia. Behav. Sleep Med. 2019, 18, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Passos, G.S.; Poyares, D.; Santana, M.G.; Garbuio, S.A.; Tufik, S.; Mello, M.T. Effect of acute physical exercise on patients with chronic primary insomnia. J. Clin. Sleep Med. 2010, 6, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Reid, K.J.; Baron, K.G.; Lu, B.; Naylor, E.; Wolfe, L.; Zee, P. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010, 11, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Alén, M.; Wiklund, P.; Partinen, M.; Cheng, S. Effects of aerobic exercise on home-based sleep among overweight and obese men with chronic insomnia symptoms: A randomized controlled trial. Sleep Med. 2016, 25, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Yeung, W.F.; Ho, F.Y.-Y.; Chung, K.-F.; Zhang, Z.-J.; Yu, B.Y.-M.; Suen, L.K.; Chan, L.Y.-T.; Chen, H.-Y.; Ho, L.-M.; Lao, L.-X. Self-administered acupressure for insomnia disorder: A pilot randomized controlled trial. J. Sleep Res. 2017, 27, 220–231. [Google Scholar] [CrossRef]
- Dorn, M. Wirksamkeit und verträglichkeit von baldrian versus oxazepam bei nichtorganischen und nichtpsychiatrischen insomnien: Eine randomisierte, doppelblinde, klinische Vergleichsstudie. Complementary Med. Res. 2000, 7, 79–84. [Google Scholar] [CrossRef]
- Morin, C.M.; Koetter, U.; Bastien, C.; Ware, J.C.; Wooten, V. Valerian-hops combination and diphenhydramine for treating insomnia: A randomized placebo-controlled clinical trial. Sleep 2005, 28, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, B.; Poddighe, D.; Vadalà, M.; Laurino, C.; Carnovale, C.; Clementi, E. Severe somatoform and dysautonomic syndromes after HPV vaccination: Case series and review of literature. Immunol. Res. 2017, 65, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyares, D.R.; Guilleminault, C.; Ohayon, M.M.; Tufik, S. Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 539–545. [Google Scholar] [CrossRef]
- Rodenbeck, A.; Simen, S.; Cohrs, S.; Jordan, W.; Kinkelbur, J.; Staedt, J.; Hajak, G. Veränderte schlafstadienstruktur als hinweis auf die GABAerge wirkung eines baldrian-hopfen-präparates bei patienten mit psychophysiologischer insomnie. Somnologie-Schlafforschung Schlafmed. 1998, 2, 26–31. [Google Scholar] [CrossRef]
- Friedman, L.; Zeitzer, J.M.; Kushida, C.; Zhdanova, I.; Noda, A.; Lee, T.; Schneider, B.; Guilleminault, C.; Sheikh, J.; Yesavage, J.A. Scheduled bright light for treatment of insomnia in older adults. J. Am. Geriatr. Soc. 2009, 57, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Lack, L.; Wright, H.; Paynter, D. The treatment of sleep onset insomnia with bright morning light. Sleep Biol. Rhythm. 2007, 5, 173–179. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Bianchi, M.T.; Zhan, S.; Jiang, F.; Li, N.; Ding, Y.; Hou, Y.; Wang, L.; Ouyang, Q.; et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018, 11, 1103–1109. [Google Scholar] [CrossRef]
- Jiang, C.-G.; Zhang, T.; Yue, F.; Yi, M.-L.; Gao, D. Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biophys. 2013, 67, 169–173. [Google Scholar] [CrossRef]
- Michael, J.; Singh, S.; Sadhukhan, S.; Nath, A.; Kundu, N.; Magotra, N.; Dutta, S.; Parewa, M.; Koley, M.; Saha, S.; et al. Efficacy of individualized homeopathic treatment of insomnia: Double-blind, randomized, placebo-controlled clinical trial. Complementary Ther. Med. 2019, 43, 53–59. [Google Scholar] [CrossRef]
- Lam, T.-H.; Chung, K.-F.; Lee, C.-T.; Yeung, W.-F.; Yu, B.Y.-M. Hypnotherapy for insomnia: A randomized controlled trial comparing generic and disease-specific suggestions. Complementary Ther. Med. 2018, 41, 231–239. [Google Scholar] [CrossRef]
- Cornu, C.; Remontet, L.; Noel-Baron, F.; Nicolas, A.; Feugier-Favier, N.; Roy, P.; Claustrat, B.; Saadatian-Elahi, M.; Kassai, B. A dietary supplement to improve the quality of sleep: A randomized placebo controlled trial. BMC Complementary Altern. Med. 2010, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frase, L.; Nissen, C.; Riemann, D.; Spiegelhalder, K. Making sleep easier: Pharmacological interventions for insomnia. Expert Opin. Pharmacother. 2018, 19, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Spiegelhalder, K.; Lombardo, C.; Riemann, D. Sleep and emotions: A focus on insomnia. Sleep Med. Rev. 2010, 14, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Kyle, S.D.; Williams, C.; Ong, J.C.; Douglas, N.J.; Hames, P.; Brown, J.S. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep 2012, 35, 769–781. [Google Scholar] [CrossRef]
- Gruber, R.; Paquin, S.; Cassoff, J.; Wise, M.S. The impact of sleep on emotion in typically developing children. In Sleep and Affect; Elsevier: Amsterdam, The Netherlands, 2015; pp. 399–419. [Google Scholar]










| Study | Intervention of Interest | Intervention of Reference | Age Range | Sex (% of Females) | Comorbidities | Insomnia Definition | Sleep/Insomnia Outcomes |
|---|---|---|---|---|---|---|---|
| Afonso et al. 2012 | Yoga + 500 mg Calcium supplement | 1. Passive stretching + 500 mg Calcium supplement; 2. Waiting List + 500 mg Calcium supplement | 50–65 years | 100 | other mental disorder not excluded | DSM-IV | Self-report questionnaires |
| Cornu et al. 2010 | Dietary supplement (soft gelatine capsules) | Placebo | 25–65 years | 68 | excluded | DSM-IV, ICSD 2 | Self-report questionnaires + Sleep diaries + Actigraphy |
| Cortesi et al. 2012 | Melatonin | 1.CBT-I; 2. CBT-I+melatonin; 3. Placebo | 4–10 years | 17.75 | partially excluded | SOL and WASO > 30min on 3 or more nights a week | Self-report questionnaires + Actigraphy |
| D’Aurea et al. 2019 | Resistance exercise training | 1. Stretching; 2. Non-intervention | n.r. | n.r. | partially excluded | DSM-IV | Self-report questionnaires + Actigraphy + Polysomnography |
| Dorn, 2000 | Valerian | Oxazepam | 18–70 years | 69.55 | partially excluded | ICD-10 | Self-report questionnaires |
| Friedman et al. 2009 | 1. Sleep hygiene + bright morning light 10.000lux; 2. Sleep hygiene + bright evening light 4000lux | 1. Sleep hygiene + dim morning light 50lux; 2. Sleep hygiene + dim evening light 50lux | 54–78 years | 60 | partially excluded | ICSD | Self-report questionnaires + Sleep diaries + Actigraphy + Polysomnography |
| Garcia et al. 2018 | Mindfulness and relaxation training for insomnia | 1. Placebo; 2. Waiting List | 50–65 years | 100 | partially excluded | DSM-V | Self-report questionnaires + Polysomnography |
| Garland et al. 2014 | Mindfulness-Based Stress Reduction | CBT-I | 35–88 years | 70.5 | other somatic disorder not excluded | Research diagnostic criteria for insomnia, DSM-IV and ICSD | Self-report questionnaires + Sleep diaries + Actigraphy |
| Gringras et al. 2012 | Melatonin | Placebo | 3–15.5 years | 33.5 | not excluded | SOL > 60min in three nights out of five or TST < 6h in three nights out of five as reported by parents | Self-report questionnaires + Sleep diaries + Actigraphy |
| Gringras et al. 2017 | Melatonin | Placebo | 2–17.5 years | 26.35 | not excluded | DSM-V | Self-report questionnaires + Sleep diaries + Actigraphy |
| Gross et al. 2011 | Mindfulness-Based Stress Reduction | Eszopiclone (LUNESTA™) | 21–65 years | 72.5 | partially excluded | DSM-IV-TR and ICSD-2 | Self-report questionnaires + Sleep diaries + Actigraphy |
| Hartescu et al. 2015 | Moderate intensity physical activity | Waiting List | n.r. (>40) | 73 | partially excluded | Research Diagnostic Criteria | Self-report questionnaires + Sleep diaries + Actigraphy |
| Hartescu et al. 2019 | Brisk walking | Waiting List | n.r. | 73.2 | partially excluded | Research Diagnostic Criteria | Self-report questionniares |
| Huang et al. 2018 | rTMS | sham rTMS | n.r. | 50 | partially excluded | DSM-IV | Self-report questionnaires |
| Irwin et al. 2017 | Tai Chi | CBT-I | 42–83 years | 100 | partially excluded | DSM-4-TR and ICSD II | Self-report questionnaires + Sleep diaries + Polysomnography |
| Irwin et al. 2014 | Tai Chi | 1. CBT-I; 2. Sleep hygiene seminar | 55–85 years | 71.53 | partially excluded | DSM-IV, ICSD | Self-report questionnaires + Sleep diaries + Polysomnography |
| James et al. 2019 | Individualized homeopathic therapy | Placebo | n.r. | 51.5 | n.r. | ICD-10 | Self-report questionnaires + Sleep diaries |
| Jiang et al. 2013 | rTMS | 1. CBT-I; 2. Pharmacotherapy | n.r. | 55.5 | partially excluded | DSM-IV | Self-report questionnaires + Polysomnography |
| Lack et al. 2007 | Bright light 2500lux | Dim red light 100lux | 18–56 years | 68.75 | n.r. | SOL > 45min, <30min WASO, difficulty waking spontaneously at the desired time, daytime symptoms | Self-report questionnaires + Actigraphy |
| Lam, 2018 | Hypnotherapy with disease-specific suggestions | Hypnotherapy with generic suggestions | n.r. | 78.5 | partially excluded | DSM-V | Self-report questionnaires + Sleep diaries |
| Luthringer et al. 2009 | Melatonin | Placebo | 55–68 years | 40 | partially excluded | DSM-IV | Self-report questionnaires + Polysomnography |
| Mayer et al. 2009 | Ramelteon | Placebo | 18–79 years | 63.2 | other mental disorder not excluded | SOL>45min or TST<6,5h or difficulty initiating/maintaining sleep or nonrestorative sleep or significant impairment due to insomnia | Sleep diaries + Polysomnography |
| Morin et al. 2005 | 1. Valerian-hops combination; 2. Diphenhydramine | Placebo | 25–65 years | 59.77 | excluded | SOL>30min or WASO>30min min 2 nights max 4 nights a week | Self-report questionnaires + Sleep diaries + Polysomnography |
| Ong et al. 2014 | Mindfulness-based therapy for insomnia | 1. Mindfulness-Based Stress Reduction; 2. Self monitoring | n.r. | 73.80 | partially excluded | Research Diagnostic Criteria for Insomnia Disorder | Self-report questionnaires + Sleep diaries + Actigraphy + Polysomnography |
| Ong et al. 2018 | 1. Mindfulness-Based Stress Reduction; 2. Mindfulness-based therapy for insomnia | Self-monitoring | n.r. | 72.67 | other mental or somatic disorder not excluded | Schedule for Sleep Disorders (Edinger et al. 2011) and ICSD-2 | Self-report questionnaires |
| Palmieri et al. 2017 | Herbal compound | Placebo | 43–65.5 years | 54.25 | partially excluded | DSM-IV | Self-report questionnaires |
| Passos et al. 2010 | 1. Moderate-intensity aerobic exercise; 2. High intensity aerobic exercise; 3. Moderate intensity resistance exercise | Waiting List | 30–55 years | 79.15 | partially excluded | DSM-IV and ICSD-2 | Sleep diaries + Polysomnography |
| Poyares et al. 2002 | Valerian | 1. Placebo; 2. Healthy controls | n.r. | 79 | partially excluded | DSM-IV | Sleep diaries + Polysomnography |
| Reid et al. 2010 | Aerobic physical activity + sleep hygiene | Non-physical activity + sleep hygiene | >55 years | 92.85 | partially excluded | difficulty falling asleep and/or staying asleep, impairment in daytime functioning, SEI<80% or awakening earlier than 6AM or sleep less then 6,5h | Self-report questionnaires |
| Rodenbeck et al. 1998 | Valerian | Placebo | n.r. | n.r. | partially excluded | ICSD I | Polysomnography |
| Rondanelli et al. 2011 | Melatonin | Placebo | n.r. | 62.5 | partially excluded | DSM-IV | Self-report questionnaires |
| Roth et al. 2006 | 1. Ramelteon 4mg; 2. Ramelteon 8mg | Placebo | 64–93 years | 58.9 | partially excluded | DSM-IV-TR | Sleep diaries |
| Smits et al. 2001 | Melatonin | Placebo | 7–13 years | 29 | partially excluded | consistent with DSM-IV | Actigraphy |
| Tan et al. 2016 | Exercise | Waiting List | 30–65 years | 0 | partially excluded | DSM-IV-TR | Self-report questionnaires + Actigraphy |
| van Geijlswijk et al. 2010 | Melatonin: 1. 0,5 mg/kg; 2. 0,1 mg/kg; 3. 0,15 mg/kg | Placebo | 6–12 years | 56.75 | partially excluded | DSM-IV | Actigraphy |
| Wade et al. 2007 | Melatonin | Placebo | 55–80 years | 39.5 | excluded | DSM-IV and ICD-10 | Self-report questionnaires |
| Wang-Weigand et al. 2011 | Ramelteon | Placebo | 18–64 years | 64.7 | partially excluded | DSM-IV-TR | Polysomnography |
| Yeung et al. 2018 | Zero time exercise | Sleep hygiene | n.r. | 91.8 | partially excluded | DSM-IV | Self-report questionnaires + Actigraphy |
| Zammit et al. 2007 | Ramelteon: 16 mg and 8 mg | Placebo | 18–64 years | 32 | excluded | DSM-IV-TR | Sleep diaries + Polysomnography |
| Zhang et al. 2015 | Mindfulness-Based Stress Reduction | Waiting List | >75 years | 29.06 | partially excluded | DSM-IV | Self-report questionnaires |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglioni, C.; Bostanova, Z.; Bacaro, V.; Benz, F.; Hertenstein, E.; Spiegelhalder, K.; Rücker, G.; Frase, L.; Riemann, D.; Feige, B. A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder. J. Clin. Med. 2020, 9, 1949. https://doi.org/10.3390/jcm9061949
Baglioni C, Bostanova Z, Bacaro V, Benz F, Hertenstein E, Spiegelhalder K, Rücker G, Frase L, Riemann D, Feige B. A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder. Journal of Clinical Medicine. 2020; 9(6):1949. https://doi.org/10.3390/jcm9061949
Chicago/Turabian StyleBaglioni, Chiara, Zarina Bostanova, Valeria Bacaro, Fee Benz, Elisabeth Hertenstein, Kai Spiegelhalder, Gerta Rücker, Lukas Frase, Dieter Riemann, and Bernd Feige. 2020. "A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder" Journal of Clinical Medicine 9, no. 6: 1949. https://doi.org/10.3390/jcm9061949
APA StyleBaglioni, C., Bostanova, Z., Bacaro, V., Benz, F., Hertenstein, E., Spiegelhalder, K., Rücker, G., Frase, L., Riemann, D., & Feige, B. (2020). A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials Evaluating the Evidence Base of Melatonin, Light Exposure, Exercise, and Complementary and Alternative Medicine for Patients with Insomnia Disorder. Journal of Clinical Medicine, 9(6), 1949. https://doi.org/10.3390/jcm9061949