Delayed Thrombin Generation Is Associated with Minor Bleedings in Venous Thromboembolism Patients on Rivaroxaban: Usefulness of Calibrated Automated Thrombography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Preparation
2.2. Laboratory Measurements
2.3. Thrombophilia Testing
2.4. Rivaroxaban Concentration
2.5. Thrombin Generation Assay
2.6. Follow-up
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Bleeding and Thromboembolic Events During Follow-up
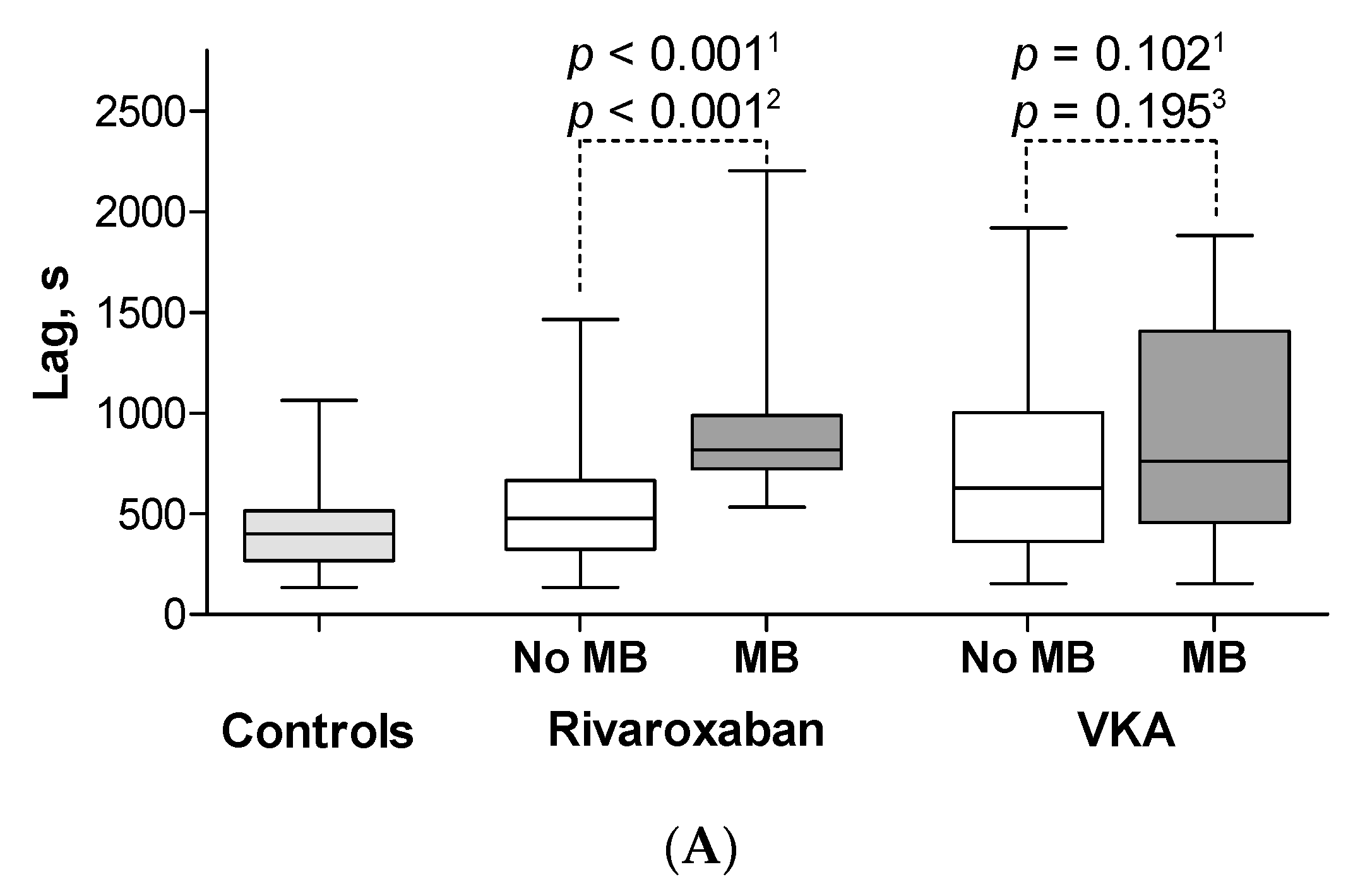
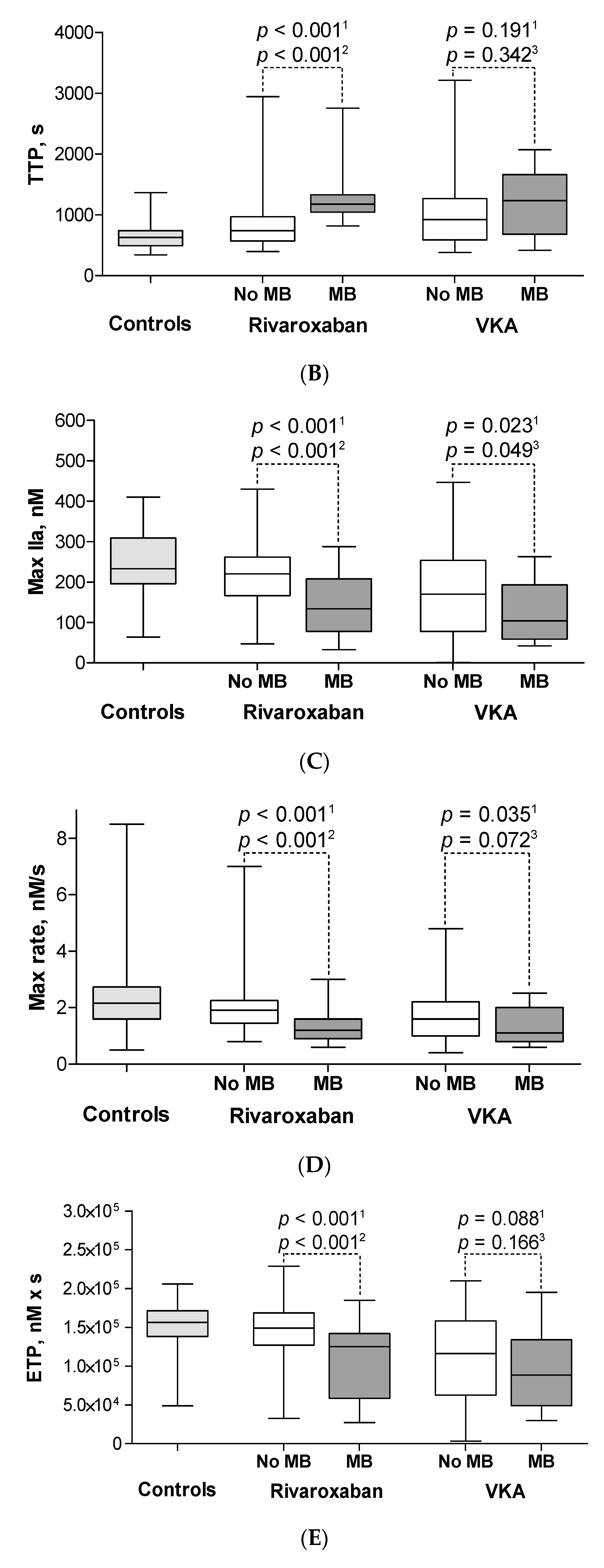
3.3. Minor Bleeding on Rivaroxaban
3.4. Minor Bleeding on VKA
3.5. CAT Parameters in Patients with Major Bleeding
3.6. ROC Curves
3.7. Predictors of Minor Bleedings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schulman, S.; Angerås, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Giménez, N.; Suárez, C.; González, R.; Nieto, J.A.; Todolí, J.A.; Samperiz, A.L.; Monreal, M. RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008, 100, 26–31. [Google Scholar] [CrossRef]
- Weitz, J.I.; Haas, S.; Ageno, W.; Angchaisuksiri, P.; Bounameaux, H.; Nielsen, J.D.; Goldhaber, S.Z.; Goto, S.; Kayani, G.; Mantovani, L.; et al. Global Anticoagulant Registry in the Field—Venous Thromboembolism (GARFIELD-VTE). Rationale and design. Thromb. Haemost. 2016, 116, 1172–1179. [Google Scholar] [CrossRef]
- Ageno, W.; Mantovani, L.G.; Haas, S.; Kreutz, R.; Monje, D.; Schneider, J.; van Eickels, M.; Gebel, M.; Zell, E.; Turpie, A.G. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): An international, prospective, non-interventional study. Lancet Haematol. 2016, 3, e12–e21. [Google Scholar] [CrossRef]
- Einstein Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef] [Green Version]
- Einstein–PE Investigators; Büller, H.R.; Prins, M.H.; Lensin, A.W.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Själander, A.; Friberg, B.; Svensson, P.; Stigendal, L.; Lethagen, S. Menorrhagia and minor bleeding symptoms in women on oral anticoagulation. J. Thromb. Thrombolysis. 2007, 24, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Konieczyńska, M.; Bijak, P.; Desteghe, L.; Heidbuchel, H.; Undas, A. Knowledge gaps in patients with venous thromboembolism: Usefulness of a new questionnaire. Pol. Arch. Intern. Med. 2019, 129, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Konieczyńska, M.; Sobieraj, E.; Bryk, A.H.; Dębski, M.; Polak, M.; Podolec, P.; Małecka, B.; Pająk, A.; Desteghe, L.; Heidbuchel, H.; et al. Differences in knowledge among patients with atrial fibrillation receiving non-vitamin K antagonist oral anticoagulants and vitamin K antagonists. Kardiol. Pol. 2018, 76, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Beyer-Westendorf, J.; Förster, K.; Pannach, S.; Ebertz, F.; Gelbricht, V.; Thieme, C.; Michalski, F.; Köhler, C.; Werth, S.; Sahin, K.; et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: Results from the Dresden NOAC registry. Blood 2014, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Dawwas, G.K.; Brown, J.; Dietrich, E.; Park, H. Effectiveness and safety of apixaban versus rivaroxaban for prevention of recurrent venous thromboembolism and adverse bleeding events in patients with venous thromboembolism: A retrospective population-based cohort analysis. Lancet Haematol. 2019, 6, e20–e28. [Google Scholar] [CrossRef]
- Veeger, N.J.; Piersma-Wichers, M.; Meijer, K.; Hillege, H.L. Minor bleeds alert for subsequent major bleeding in patients using vitamin K antagonists. Br. J. Haematol. 2011, 153, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rein, N.; le Cessie, S.; van Vliet, I.P.; Reitsma, P.H.; van der Meer, F.J.; Lijfering, W.M.; Cannegieter, S.C. Increased risk of major bleeding after a minor bleed during treatment with vitamin K antagonists is determined by fixed common risk factors. J. Thromb. Haemost. 2016, 14, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Bahit, M.C.; Lopes, R.D.; Wojdyla, D.M.; Held, C.; Hanna, M.; Vinereanu, D.; Hylek, E.M.; Verheugt, F.; Goto, S.; Alexander, J.H.; et al. Non-major bleeding with apixaban versus warfarin in patients with atrial fibrillation. Heart 2017, 103, 623–628. [Google Scholar] [CrossRef]
- Kremers, R.M.; Peters, T.C.; Wagenvoord, R.J.; Hemker, H.C. The balance of pro- and anticoagulant processes underlying thrombin generation. J. Thromb. Haemost. 2015, 13, 437–447. [Google Scholar] [CrossRef]
- Al Dieri, R.; Peyvandi, F.; Santagostino, E.; Giansily, M.; Mannucci, P.M.; Schved, J.F.; Béguin, S.; Hemker, H.C. The thrombogram in rare inherited coagulation disorders: Its relation to clinical bleeding. Thromb. Haemost. 2002, 88, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Shima, M.; Takeyama, M.; Yoshida, K.; Tanaka, I.; Sakurai, Y.; Giles, A.R.; Yoshioka, A. The measurement of low levels of factor VIII or factor IX in hemophilia A and hemophilia B plasma by clot waveform analysis and thrombin generation assay. J. Thromb. Haemost. 2006, 4, 377–384. [Google Scholar] [CrossRef]
- Barco, S.; Whitney Cheung, Y.; Coppens, M.; Hutten, B.A.; Meijers, J.C.; Middeldorp, S. In vivo reversal of the anticoagulant effect of rivaroxaban with four-factor prothrombin complex concentrate. Br. J. Haematol. 2016, 172, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Crowther, M.; Cuker, A. How can we reverse bleeding in patients on direct oral anticoagulants? Kardiol. Pol. 2019, 77, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Béguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.G.; Whelihan, M.F.; Butenas, S.; Orfeo, T. Citrate anticoagulation and the dynamics of thrombin generation. J. Thromb. Haemost. 2007, 5, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Orfeo, T.; Gissel, M.; Butenas, S.; Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Anticoagulants and the propagation phase of thrombin generation. PLoS ONE 2011, 6, e27852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigano, J.; Ng, C.; Nandurkar, H.; Ho, P. Thrombin generation estimates the anticoagulation effect of direct oral anticoagulants with significant interindividual variability observed. Blood Coagul. Fibrinolysis. 2018, 29, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.R.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Rivaroxaban and warfarin achieve effective anticoagulation, as assessed by inhibition of TG and in-vivo markers of coagulation activation, in patients with venous thromboembolism. Thromb. Res. 2015, 135, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Barcellona, D.; Vannini, M.L.; Fenu, L.; Balestrieri, C.; Marongiu, F. Warfarin or acenocoumarol: Which is better in the management of oral anticoagulants? Thromb. Haemost. 1998, 80, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, J.; Mrozinska, S.; Broniatowska, E.; Undas, A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: A cohort study. Blood 2018, 131, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wypasek, E.; Corral, J.; Alhenc-Gelas, M.; Sydor, W.; Iwaniec, T.; Celińska-Lowenhoff, M.; Potaczek, D.P.; Blecharczyk, A.; Zawilska, K.; Musiał, J.; et al. Genetic characterization of antithrombin, protein C, and protein S deficiencies in Polish patients. Pol. Arch. Intern. Med. 2017, 127, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Zalewski, J.; Rychlak, R.; Góralczyk, T.; Undas, A. Rivaroxaban concentration in patients with deep vein thrombosis who reported thrombus progression or minor hemorrhagic complications: First Polish experience. Pol. Arch. Med. Wewn. 2014, 124, 553–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripodi, A.; Braham, S.; Scimeca, B.; Moia, M.; Peyvandi, F. How and when to measure anticoagulant effects of direct oral anticoagulants? Practical issues. Pol. Arch. Intern. Med. 2018, 128, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frączek, P.; Krzysztofik, M.; Stanisz, A.; Undas, A. Clinical outcomes and plasma clot permeability and lysability in patients with venous thromboembolism on rivaroxaban: A cohort study. Pol. Arch. Intern. Med. 2019, 129, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwaveling, S.; Bloemen, S.; de Laat, B.; Ten Cate, H.; Ten Cate-Hoek, A. Calibrated automated thrombinography (CAT), a tool to identify patients at risk of bleeding during anticoagulant therapy: A systematic review. TH Open 2018, 2, e291–e302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloemen, S.; Zwaveling, S.; Ten Cate, H.; Ten Cate-Hoek, A.; de Laat, B. Prediction of bleeding risk in patients taking vitamin K antagonists using thrombin generation testing. PLoS ONE 2017, 12, e0176967. [Google Scholar] [CrossRef]
- Dargaud, Y.; Hoffman, M.; Lefrapper, L.; Lin, F.C.; Genty, A.; Chatard, B.; Marin, S.; Négrier, C.; Monroe, D.M. Bleeding risk in warfarinized patients with a therapeutic international normalized ratio: The effect of low factor IX levels. J. Thromb. Haemost. 2013, 11, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Noventa, F.; Ghirarduzzi, A.; Pengo, V.; Bernardi, E.; Pesavento, R.; Iotti, M.; Tormene, D.; Simioni, P.; Pagnan, A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007, 92, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Tritschler, T.; Wells, P.S. Extended therapy for unprovoked venous thromboembolism: When is it indicated? Blood Adv. 2019, 3, 499. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Klok, F.A.; Barco, S.; Turpie, A.G.G.; Haas, S.; Kreutz, R.; Mantovani, L.G.; Gebel, M.; Herpers, M.; Bugge, J.P.; Kostantinides, S.V.; et al. Predictive value of venous thromboembolism (VTE)-BLEED to predict major bleeding and other adverse events in a practice-based cohort of patients with VTE: Results of the XALIA study. Br. J. Haematol. 2018, 183, 457–465. [Google Scholar] [CrossRef]
- Hemker, H.C.; Al Dieri, R.; De Smedt, E.; Beguin, S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb. Haemost. 2006, 96, 553–561. [Google Scholar] [PubMed]




| Controls n = 31 | Rivaroxaban without MB n = 105 | Rivaroxaban with MB n = 27 | VKA without MB n = 120 | VKA with MB n = 25 | |
|---|---|---|---|---|---|
| Age, year | 42 (36–48) | 46 (36–57) | 47 (42–52) | 47 (37–57) | 50 (38–61) |
| Male, n (%) | 12 (38.7) | 38 (36.2) | 15 (55.6) | 55 (45.8) | 10 (40.0) |
| Body mass index, kg/m2 | 25.1 (22.0–28.3) | 27.3 (22.3–30.7) | 26.6 (25.6–29.8) | 28 (24.1–30.5) * | 26.8 (23.6–30.4) |
| Currently smoking, n (%) | 7 (22.6) | 15 (14.3) | 7 (25.9) | 31 (25.8) | 7 (28.0) |
| VTE characteristics, n (%) | |||||
| Baseline VTE diagnosis | |||||
| DVT alone | 15 (48.4) | 40 (38.1) | 9 (33.3) | 43 (35.8) | 12 (48.0) |
| Pulmonary embolism alone | 6 (19.3) | 25 (23.8) | 9 (33.3) | 28 (23.4) | 6 (24.0) |
| Pulmonary embolism and DVT | 7 (22.6) | 29 (27.6) | 9 (33.3) | 43 (35.8) | 7 (28.0) |
| Other | 3 (9.7) | 11 (10.5) | 0 | 6 (5.0) | 0 |
| Unprovoked VTE | 15 (48.4) | 55 (52.3) | 24 (88.9) # | 67 (55.8) | 11 (44.0) |
| Proximal DVT | 16 (51.6) | 62 (59.0) | 16 (59.3) | 71 (59.2) | 19 (76) |
| Bilateral DVT | 3 (9.7) | 5 (4.8) | 0 | 3 (2.5) | 1 (4) |
| Time since last VTE, month | 10 (6–22) | 10 (6–17) | 8 (6–17) | 12 (8–23) | 8 (7–16) |
| Duration of anticoagulation, month | 6 (4–12) | 7 (5–14) | 8 (6–17) | 10 (6–18) | 8 (6–13) |
| Family history of VTE | 14 (45.2) | 34 (32.4) | 8 (29.6) | 35 (29.2) | 4 (16.0) |
| Varices | 11 (35.5) | 37 (35.2) | 11 (40.7) | 37 (30.8) | 9 (36.0) |
| Comorbidities, n (%) | |||||
| Coronary artery disease | 0 | 3 (2.9) | 0 | 3 (2.5) | 1 (4.0) |
| Prior ischemic stroke | 0 | 6 (5.7) | 1 (3.7) | 3 (2.5) | 1 (4.0) |
| Hypertension | 4 (12.9) | 31 (29.5) | 7 (25.9) | 39 (32.5) * | 10 (40.0) |
| Heart failure | 1 (3.2) | 1 (1.0) | 0 | 2 (1.7) | 1 (4.0) |
| Diabetes mellitus | 2 (6.5) | 4 (3.8) | 0 | 5 (4.2) | 4 (16.0) ** |
| Chronic kidney disease | 1 (3.2) | 3 (2.9) | 0 | 0 | 0 |
| Use of aspirin | 4 (12.9) | 8 (7.6) | 0 | 7 (5.8) | 2 (8.0) |
| Use of statin | 2 (6.5) | 15 (14.3) | 1 (3.7) | 17 (14.2) | 3 (12.0) |
| Proton pump inhibitor | 3 (9.7) | 24 (22.9) | 1 (3.7) ## | 25 (20.8) | 4 (16.0) |
| Thrombophilia, n (%) | |||||
| Factor V Leiden | 11 (35.5) | 26 (24.8) | 6 (22.2) | 23 (19.2) | 2 (8.0) |
| Prothrombin G20210A mutation | 1 (3.2) | 6 (5.7) | 0 | 6 (5.0) | 5 (20.0) ** |
| Deficiencies in natural anticoagulants | 1 (3.2) | 9 (9.6) | 3 (11.1) | 16 (13.3) | 7 (28.0) |
| Antiphospholipid syndrome | 2 (6.5) | 7 (6.7) | 5 (18.5) | 10 (8.3) | 2 (8.0) |
| Laboratory Investigations | |||||
| White blood cells, 103/μL | 5.90 (4.92–7.54) | 5.89 (4.85–7.1) | 6.07 (5.69–6.94) | 6.36 (5.27–7.66) | 6.08 (5.46–7.19) |
| Hemoglobin, g/dL | 13.6 (12.9–14.5) | 13.9 (13.1–14.9) | 13.9 (13.1–15.4) | 14.4 (13.4–15.5) * | 13.4 (13.1–14.5) |
| Platelets, 103/μL | 232 (195–265) | 245 (206–284) | 216 (186–265) | 244 (201–285) | 243 (212–293) |
| Glucose, mmol/L | 5.1 (4.8–5.3) | 5.1 (4.9–5.8) | 5.2 (5.0–5.4) | 5.2 (4.9–5.6) | 5.4 (5.0–5.7) |
| Creatinine, μmol/L | 70 (64–85) | 70 (61–79) | 74 (67–89) | 72 (65–83) | 70 (64–82) |
| eGFR, ml/min/1.73 m2 | 92 (83–114) | 101 (89–111) | 94 (86–106) | 98 (88–107) | 99 (90–1017) |
| hsCRP, ng/mL | 1.12 (0.71–1.87) | 1.1 (0.6–3.43) | 1.98 (0.9–5.6) | 1.76 (0.85–3.11) | 1.52 (0.74–3.58) |
| D-Dimer, ng/mL | 275 (183–447) | 265 (171–375) | 209 (171–348) | 203 (171–341) | 177 (171–226) |
| Rivaroxaban concentration, μg/L | - | 25 (13–44) | 35 (6–66) | - | - |
| APTT, s | 25.8 (24.7–29.1) | 25.8 (24.1–28.4) | 27.1 (24.9–34.1) | 28.7 (25.1–34.1) * | 31.0 (25.4–35.7) |
| INR | 0.99 (0.97–1.04) | 1.03 (0.99–1.14) | 1.04 (0.98–1.10) | 1.47 (1.02–2.20) | 2.19 (1.46–2.45) ** |
| Fibrinogen, g/L | 3.03 (2.61–4.0) | 3.06 (2.64–3.64) | 3.3 (2.8–4.05) | 3.13 (2.71–363) | 3.24 (2.91–3.48) |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Independent Variable | p-Value | OR | 95% CI for OR | p-Value | OR | 95% CI for OR |
| MB on Rivaroxaban | ||||||
| Age, per 1 year | 0.586 | 1.010 | 0.975–1.045 | 0.996 | 1.000 | 0.929–1.076 |
| Male gender, yes/no | 0.071 | 0.454 | 0.193–1.069 | 0.828 | 0.818 | 0.134–5.005 |
| Body mass index, per 1 kg/m2 | 0.935 | 0.997 | 0.927–1.073 | 0.787 | 0.977 | 0.827–1.155 |
| Creatinine, per 1 μmol/L | 0.069 | 1.029 | 0.998–1.061 | 0.503 | 0.980 | 0.926–1.039 |
| INR, per 0.01 | 0.427 | 0.982 | 0.940–1.026 | 0.542 | 0.969 | 0.877–1.071 |
| Rivaroxaban concentration, per 1 μg/L | 0.349 | 1.011 | 0.989–1.033 | 0.947 | 1.001 | 0.970–1.034 |
| Unprovoked VTE, yes/no | 0.002 | 7.246 | 2.062–25.641 | 0.041 | 19.607 | 1.131–59.311 |
| Lag time, per 1 s | <0.001 | 1.004 | 1.002–1.006 | 0.007 | 1.006 | 1.002–1.010 |
| MB on VKA | ||||||
| Age, per 1 year | 0.811 | 1.004 | 0.969–1.042 | 0.851 | 0.996 | 0.956–1.038 |
| Male gender, yes/no | 0.594 | 1.269 | 0.528–3.051 | 0.928 | 0.948 | 0.297–3.024 |
| Body mass index, per 1 kg/m2 | 0.971 | 0.998 | 0.917–1.088 | 0.972 | 0.998 | 0.905–1.101 |
| Creatinine, per 1 μmol/L | 0.563 | 0.991 | 0.960–1.022 | 0.365 | 0.980 | 0.938–1.024 |
| INR, per 0.01 | 0.027 | 1.007 | 1.001–1.013 | 0.054 | 1.006 | 1.000–1.013 |
| Diabetes mellitus, yes/no | 0.038 | 4.386 | 1.086–17.544 | 0.059 | 4.098 | 0.948–17.857 |
| Max IIa, per 1 nM | 0.023 | 0.994 | 0.989–0.999 | 0.045 | 0.995 | 0.989–0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, J.; Stepien, K.; Nowak, K.; Caus, S.; Butenas, S.; Undas, A. Delayed Thrombin Generation Is Associated with Minor Bleedings in Venous Thromboembolism Patients on Rivaroxaban: Usefulness of Calibrated Automated Thrombography. J. Clin. Med. 2020, 9, 2018. https://doi.org/10.3390/jcm9072018
Zalewski J, Stepien K, Nowak K, Caus S, Butenas S, Undas A. Delayed Thrombin Generation Is Associated with Minor Bleedings in Venous Thromboembolism Patients on Rivaroxaban: Usefulness of Calibrated Automated Thrombography. Journal of Clinical Medicine. 2020; 9(7):2018. https://doi.org/10.3390/jcm9072018
Chicago/Turabian StyleZalewski, Jaroslaw, Konrad Stepien, Karol Nowak, Sandi Caus, Saulius Butenas, and Anetta Undas. 2020. "Delayed Thrombin Generation Is Associated with Minor Bleedings in Venous Thromboembolism Patients on Rivaroxaban: Usefulness of Calibrated Automated Thrombography" Journal of Clinical Medicine 9, no. 7: 2018. https://doi.org/10.3390/jcm9072018
APA StyleZalewski, J., Stepien, K., Nowak, K., Caus, S., Butenas, S., & Undas, A. (2020). Delayed Thrombin Generation Is Associated with Minor Bleedings in Venous Thromboembolism Patients on Rivaroxaban: Usefulness of Calibrated Automated Thrombography. Journal of Clinical Medicine, 9(7), 2018. https://doi.org/10.3390/jcm9072018





