New Antithrombotic Drugs in Acute Coronary Syndrome
Abstract
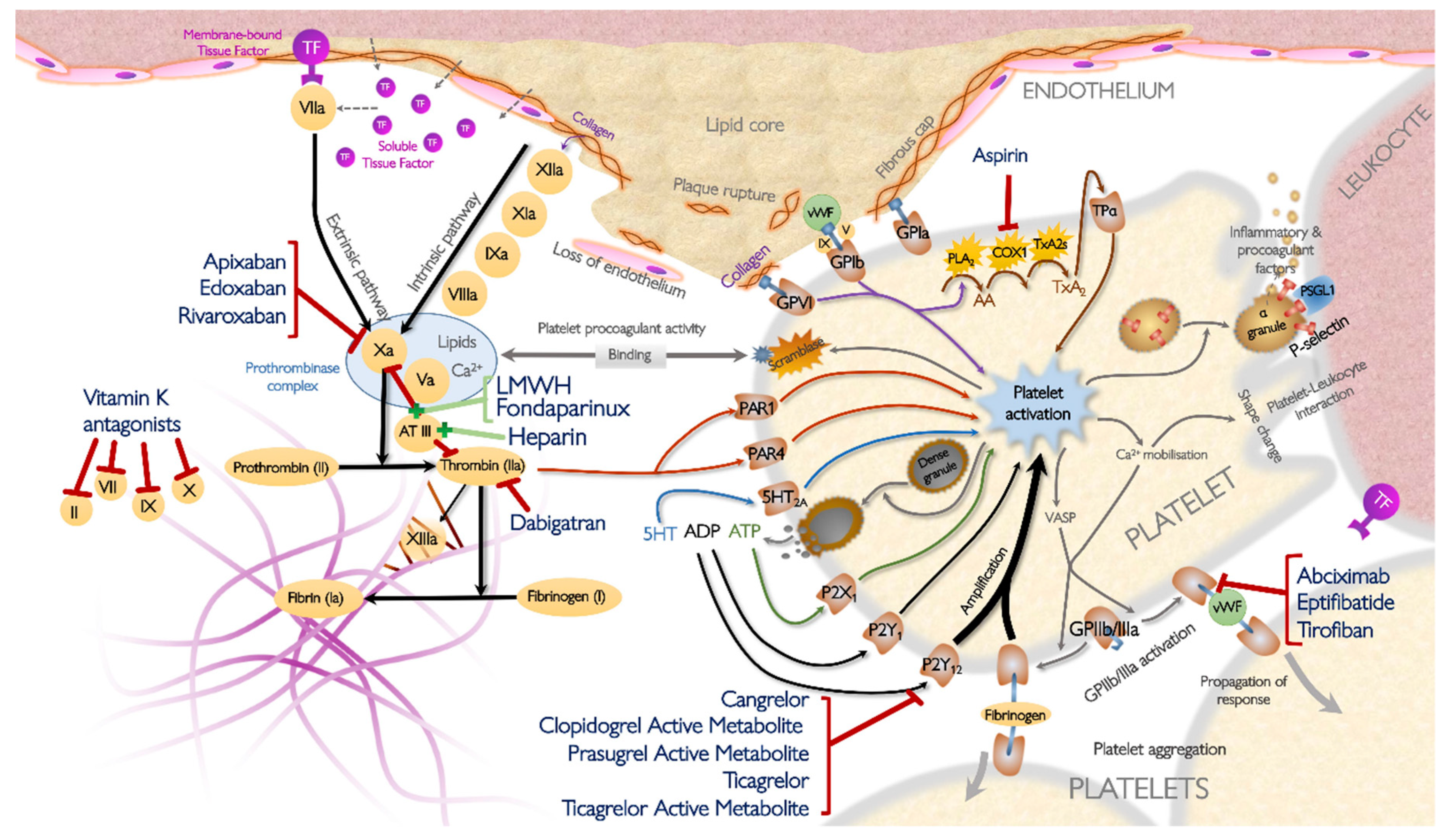
1. Introduction
2. Cangrelor
3. Novel Antiplatelet Drugs
3.1. Selatogrel
3.2. Phosphoinositide 3-Kinase β
3.3. GP IIb/IIIa Outside-In Signaling
3.4. Conformation-Specific Targeting of GP IIb/IIIa
3.5. Activated Platelet-Targeted CD39 Therapy
3.6. Inhibitors of Platelet GP VI
3.7. Inhibition of Protein Disulfide Isomerase
3.8. Inhibition of Protease-Activated Receptors
3.9. Caplacizumab
4. Anticoagulation in ACS
4.1. Enoxaparin in STEMI
4.2. Non-Vitamin-K-Antagonist Oral Anticoagulants (NOACs)
4.3. Development of Factor IX, XI, and XII Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Handin, R.I. Platelets and coronary artery disease. N. Engl. J. Med. 1996, 334, 1126–1127. [Google Scholar] [CrossRef]
- EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N. Engl. J. Med. 1994, 330, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Restore Investigators. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation 1997, 96, 1445–1453. [Google Scholar] [CrossRef]
- Schömig, A.; Neumann, F.J.; Kastrati, A.; Schühlen, H.; Blasini, R.; Hadamitzky, M.; Walter, H.; Zitzmann-Roth, E.M.; Richardt, G.; Alt, E.; et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N. Engl. J. Med. 1996, 334, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Baim, D.S.; Popma, J.J.; Gordon, P.C.; Cutlip, D.E.; Ho, K.K.; Giambartolomei, A.; Diver, D.J.; Lasorda, D.M.; Williams, D.O.; et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 1998, 339, 1665–1671. [Google Scholar] [CrossRef]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.-P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2017. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Tantry, U.S.; Gesheff, T.; Wei, C.; Teng, R.; Antonino, M.J.; Patil, S.B.; Karunakaran, A.; et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation 2009, 120, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Varenhorst, C.; James, S.; Erlinge, D.; Braun, O.O.; Jakubowski, J.A.; Sugidachi, A.; Winters, K.J.; Siegbahn, A. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 2008, 29, 21–30. [Google Scholar] [CrossRef]
- Thomas, M.R.; Morton, A.C.; Hossain, R.; Chen, B.; Luo, L.; Shahari, N.N.B.M.; Hua, P.; Beniston, R.G.; Judge, H.M.; Storey, R.F. Morphine delays the onset of action of prasugrel in patients with prior history of ST-elevation myocardial infarction. Thromb. Haemost. 2016, 116, 96–102. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Ostrowska, M.; Sikora, J.; Kubica, J.M.; Sroka, W.D.; Stankowska, K.; Buszko, K.; Navarese, E.P.; Jilma, B.; et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: The randomized, double-blind, placebo-controlled IMPRESSION trial. Eur. Heart J. 2016, 37, 245–252. [Google Scholar] [CrossRef]
- Parodi, G.; Bellandi, B.; Xanthopoulou, I.; Capranzano, P.; Capodanno, D.; Valenti, R.; Stavrou, K.; Migliorini, A.; Antoniucci, D.; Tamburino, C.; et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2015, 8. [Google Scholar] [CrossRef]
- Silvain, J.; Storey, R.F.; Cayla, G.; Esteve, J.-B.; Dillinger, J.-G.; Rousseau, H.; Tsatsaris, A.; Baradat, C.; Salhi, N.; Hamm, C.W.; et al. P2Y12 receptor inhibition and effect of morphine in patients undergoing primary PCI for ST-segment elevation myocardial infarction. The PRIVATE-ATLANTIC study. Thromb. Haemost. 2016, 116, 369–378. [Google Scholar] [CrossRef]
- Zwart, B.; Yazdani, M.; Ow, K.W.; Richardson, J.D.; Iqbal, J.; Gunn, J.P.; Storey, R.F. Use of glycoprotein IIb/IIIa antagonists to prevent stent thrombosis in morphine-treated patients with ST-elevation myocardial infarction. Platelets 2020, 31, 174–178. [Google Scholar] [CrossRef]
- Roule, V.; Agueznai, M.; Sabatier, R.; Blanchart, K.; Lemaître, A.; Ardouin, P.; Collet, J.-P.; Milliez, P.; Montalescot, G.; Beygui, F. Safety and efficacy of IIb/IIIa inhibitors in combination with highly active oral antiplatelet regimens in acute coronary syndromes: A meta-analysis of pivotal trials. Platelets 2017, 28, 174–181. [Google Scholar] [CrossRef]
- Storey, R.F.; Sinha, A. Cangrelor for the management and prevention of arterial thrombosis. Expert Rev. Cardiovasc. Ther. 2016, 14, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Bliden, K.P.; Ecob, R.; Karunakaran, A.; Butler, K.; Wei, C.; Tantry, U.; Gurbel, P.A. Earlier recovery of platelet function after discontinuation of treatment with ticagrelor compared with clopidogrel in patients with high antiplatelet responses. J. Thromb. Haemost. 2011, 9, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Oldroyd, K.G.; Wilcox, R.G. Open multicentre study of the P2T receptor antagonist AR-C69931MX assessing safety, tolerability and activity in patients with acute coronary syndromes. Thromb. Haemost. 2001, 85, 401–407. [Google Scholar]
- Akers, W.S.; Oh, J.J.; Oestreich, J.H.; Ferraris, S.; Wethington, M.; Steinhubl, S.R. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: A direct, parenteral P2Y12 receptor antagonist. J Clin Pharm. 2010, 50, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Hamm, C.W.; Stone, G.W.; Gibson, C.M.; Mahaffey, K.W.; Leonardi, S.; Liu, T.; Skerjanec, S.; Day, J.R.; et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 2013, 382, 1981–1992. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Pappas, C.; Sfantou, D.; Lekakis, J. Cangrelor in Percutaneous Coronary Intervention: Current Status and Perspectives. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 13–22. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Qamar, A.; Bhatt, D.L. Optimizing the Use of Cangrelor in the Real World. Am. J. Cardiovasc. Drugs 2017, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic therapy in patients with acute coronary syndrome complicated by cardiogenic shock or out-of-hospital cardiac arrest: A Joint Position Paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. Cardiovasc. Pharm. 2020. [Google Scholar] [CrossRef]
- Parker, W.A.E.; Storey, R.F. Novel approaches to P2Y12 inhibition and aspirin dosing. Platelets 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.A.E.; Storey, R.F. Pharmacology and potential role of selatogrel, a subcutaneous platelet P2Y12 receptor antagonist. Expert Opin. Emerg. Drugs 2020, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Caroff, E.; Meyer, E.; Treiber, A.; Hilpert, K.; Riederer, M.A. Optimization of 2-phenyl-pyrimidine-4-carboxamides towards potent, orally bioavailable and selective P2Y(12) antagonists for inhibition of platelet aggregation. Bioorg. Med. Chem. Lett. 2014, 24, 4323–4331. [Google Scholar] [CrossRef]
- Rey, M.; Kramberg, M.; Hess, P.; Morrison, K.; Ernst, R.; Haag, F.; Weber, E.; Clozel, M.; Baumann, M.; Caroff, E.; et al. The reversible P2Y12 antagonist ACT-246475 causes significantly less blood loss than ticagrelor at equivalent antithrombotic efficacy in rat. Pharm. Res. Perspect. 2017, 5. [Google Scholar] [CrossRef]
- Baldoni, D.; Bruderer, S.; Krause, A.; Gutierrez, M.; Gueret, P.; Astruc, B.; Dingemanse, J. A new reversible and potent P2Y12 receptor antagonist (ACT-246475): Tolerability, pharmacokinetics, and pharmacodynamics in a first-in-man trial. Clin. Drug Investig. 2014, 34, 807–818. [Google Scholar] [CrossRef]
- Juif, P.-E.; Boehler, M.; Dobrow, M.; Ufer, M.; Dingemanse, J. Clinical Pharmacology of the Reversible and Potent P2Y12 Receptor Antagonist ACT-246475 After Single Subcutaneous Administration in Healthy Male Subjects. J. Clin. Pharm. 2019, 59, 123–130. [Google Scholar] [CrossRef]
- Ufer, M.; Huynh, C.; van Lier, J.J.; Caroff, E.; Fischer, H.; Dingemanse, J. Absorption, distribution, metabolism and excretion of the P2Y12 receptor antagonist selatogrel after subcutaneous administration in healthy subjects. Xenobiotica 2020, 50, 427–434. [Google Scholar] [CrossRef]
- Storey, R.F.; Gurbel, P.A.; Ten Berg, J.; Bernaud, C.; Dangas, G.D.; Frenoux, J.-M.; Gorog, D.A.; Hmissi, A.; Kunadian, V.; James, S.K.; et al. Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Sinnaeve, P.R.; Fahrni, G.; Schelfaut, D.; Spirito, A.S.; Mueller, C.H.; Frenoux, J.-M.; Hmissi, A.; Bernaud, C.; Moccetti, T.; Atar, S.A.; et al. Inhibition of platelet aggregation after subcutaneous administration of a single-dose of selatogrel, a novel P2Y12 antagonist, in acute myocardial infarction: A randomised open-label phase 2 study. Eur Heart J. 2019, 40 (Suppl. 1), ehz746.0078. [Google Scholar] [CrossRef]
- Durrant, T.N.; Hers, I. PI3K inhibitors in thrombosis and cardiovascular disease. Clin. Transl. Med. 2020, 9, 8. [Google Scholar] [CrossRef]
- Garcia, A.; Kim, S.; Bhavaraju, K.; Schoenwaelder, S.M.; Kunapuli, S.P. Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem. J. 2010, 429, 369–377. [Google Scholar] [CrossRef]
- Sturgeon, S.A.; Jones, C.; Angus, J.A.; Wright, C.E. Advantages of a selective beta-isoform phosphoinositide 3-kinase antagonist, an anti-thrombotic agent devoid of other cardiovascular actions in the rat. Eur. J. Pharmacol. 2008, 587, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Nylander, S.; Kull, B.; Björkman, J.A.; Ulvinge, J.C.; Oakes, N.; Emanuelsson, B.M.; Andersson, M.; Skärby, T.; Inghardt, T.; Fjellström, O.; et al. Human target validation of phosphoinositide 3-kinase (PI3K)β: Effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kβ inhibitor. J. Thromb. Haemost. 2012, 10, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Ganji, G.; Lemech, C.; Burris, H.A.; Han, S.-W.; Swales, K.; Decordova, S.; DeYoung, M.P.; Smith, D.A.; Kalyana-Sundaram, S.; et al. A First-Time-in-Human Study of GSK2636771, a Phosphoinositide 3 Kinase Beta-Selective Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 5981–5992. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.A.E.; Storey, R.F. ‘Thrombotic Response’ in ESC Textbook of Cardiovascular Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Storey, R.F.; Wilcox, R.G.; Heptinstall, S. Differential effects of glycoprotein IIb/IIIa antagonists on platelet microaggregate and macroaggregate formation and effect of anticoagulant on antagonist potency. Implications for assay methodology and comparison of different antagonists. Circulation 1998, 98, 1616–1621. [Google Scholar] [CrossRef]
- Durrant, T.N.; van den Bosch, M.T.; Hers, I. Integrin αIIbβ3 outside-in signaling. Blood 2017, 130, 1607–1619. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, X.; O’Brien, K.A.; Stojanovic-Terpo, A.; Delaney, M.K.; Kim, K.; Cho, J.; Lam, S.C.-T.; Du, X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 2013, 503, 131–135. [Google Scholar] [CrossRef]
- Schwarz, M.; Meade, G.; Stoll, P.; Ylanne, J.; Bassler, N.; Chen, Y.C.; Hagemeyer, C.E.; Ahrens, I.; Moran, N.; Kenny, D.; et al. Conformation-specific blockade of the integrin GPIIb/IIIa: A novel antiplatelet strategy that selectively targets activated platelets. Circ. Res. 2006, 99, 25–33. [Google Scholar] [CrossRef]
- Hohmann, J.D.; Wang, X.; Krajewski, S.; Selan, C.; Haller, C.A.; Straub, A.; Chaikof, E.L.; Nandurkar, H.H.; Hagemeyer, C.E.; Peter, K. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: Breaking the link between antithrombotic potency and bleeding? Blood 2013, 121, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Sarratt, K.L.; Chen, H.; Zutter, M.M.; Santoro, S.A.; Hammer, D.A.; Kahn, M.L. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood 2005, 106, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.; Rosport, K.; Bültmann, A.; Piechatzek, R.; Uhland, K.; Schlieper, P.; Gawaz, M.; Münch, G. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation 2011, 123, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Schüpke, S.; Hein-Rothweiler, R.; Mayer, K.; Janisch, M.; Sibbing, D.; Ndrepepa, G.; Hilz, R.; Laugwitz, K.-L.; Bernlochner, I.; Gschwendtner, S.; et al. Revacept, a Novel Inhibitor of Platelet Adhesion, in Patients Undergoing Elective PCI-Design and Rationale of the Randomized ISAR-PLASTER Trial. Thromb. Haemost. 2019, 119, 1539–1545. [Google Scholar] [CrossRef]
- Lebozec, K.; Jandrot-Perrus, M.; Avenard, G.; Favre-Bulle, O.; Billiald, P. Design, development and characterization of ACT017, a humanized Fab that blocks platelet’s glycoprotein VI function without causing bleeding risks. MAbs 2017, 9, 945–958. [Google Scholar] [CrossRef]
- Voors-Pette, C.; Lebozec, K.; Dogterom, P.; Jullien, L.; Billiald, P.; Ferlan, P.; Renaud, L.; Favre-Bulle, O.; Avenard, G.; Machacek, M.; et al. Safety and Tolerability, Pharmacokinetics, and Pharmacodynamics of ACT017, an Antiplatelet GPVI (Glycoprotein VI) Fab. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 956–964. [Google Scholar] [CrossRef]
- O’Neill, S.; Robinson, A.; Deering, A.; Ryan, M.; Fitzgerald, D.J.; Moran, N. The platelet integrin alpha IIbbeta 3 has an endogenous thiol isomerase activity. J. Biol. Chem. 2000, 275, 36984–36990. [Google Scholar] [CrossRef]
- Stopa, J.D.; Neuberg, D.; Puligandla, M.; Furie, B.; Flaumenhaft, R.; Zwicker, J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight. 2017, 2, e89373. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Kung, P.-H.; Hsieh, P.-W.; Lin, Y.-T.; Lee, J.-H.; Chen, I.-H.; Wu, C.-C. HPW-RX40 prevents human platelet activation by attenuating cell surface protein disulfide isomerases. Redox Biol. 2017, 13, 266–277. [Google Scholar] [CrossRef]
- Judge, H.M.; Jennings, L.K.; Moliterno, D.J.; Hord, E.; Ecob, R.; Tricoci, P.; Rorick, T.; Kotha, J.; Storey, R.F. PAR1 antagonists inhibit thrombin-induced platelet activation whilst leaving the PAR4-mediated response intact. Platelets 2015, 26, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tricoci, P.; Huang, Z.; Held, C.; Moliterno, D.J.; Armstrong, P.W.; Van de Werf, F.; White, H.D.; Aylward, P.E.; Wallentin, L.; Chen, E.; et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 2012, 366, 20–33. [Google Scholar] [CrossRef]
- Morrow, D.A.; Braunwald, E.; Bonaca, M.P.; Ameriso, S.F.; Dalby, A.J.; Fish, M.P.; Fox, K.A.A.; Lipka, L.J.; Liu, X.; Nicolau, J.C.; et al. Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med. 2012, 366, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Aisiku, O.; Peters, C.G.; De Ceunynck, K.; Ghosh, C.C.; Dilks, J.R.; Fustolo-Gunnink, S.F.; Huang, M.; Dockendorff, C.; Parikh, S.M.; Flaumenhaft, R. Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar. Blood 2015, 125, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Seiffert, D.; Bird, J.E.; Watson, C.A.; Bostwick, J.S.; Giancarli, M.; Allegretto, N.; Hua, J.; Harden, D.; Guay, J.; et al. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Wilson, S.J.; Ismat, F.A.; Wang, Z.; Cerra, M.; Narayan, H.; Raftis, J.; Gray, T.J.; Connell, S.; Garonzik, S.; Ma, X.; et al. PAR4 (Protease-Activated Receptor 4) Antagonism With BMS-986120 Inhibits Human Ex Vivo Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Sargentini-Maier, M.L.; De Decker, P.; Tersteeg, C.; Canvin, J.; Callewaert, F.; De Winter, H. Clinical pharmacology of caplacizumab for the treatment of patients with acquired thrombotic thrombocytopenic purpura. Expert Rev. Clin. Pharm. 2019, 12, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Yusuf, S.; Mehta, S.R.; Chrolavicius, S.; Afzal, R.; Pogue, J.; Granger, C.B.; Budaj, A.; Peters, R.J.G.; Bassand, J.-P. Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N. Engl. J. Med. 2006, 354, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; Ohman, E.M. Anticoagulant therapy for percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2010, 3, 80–88. [Google Scholar] [CrossRef]
- Montalescot, G.; Cohen, M.; Salette, G.; Desmet, W.J.; Macaya, C.; Aylward, P.E.G.; Steg, P.G.; White, H.D.; Gallo, R.; Steinhubl, S.R.; et al. Impact of anticoagulation levels on outcomes in patients undergoing elective percutaneous coronary intervention: Insights from the STEEPLE trial. Eur. Heart J. 2008, 29, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Sumaya, W.; Parker, W.A.E.; Fretwell, R.; Hall, I.R.; Barmby, D.S.; Richardson, J.D.; Iqbal, J.; Adam, Z.; Morgan, K.P.; Gunn, J.P.; et al. Pharmacodynamic Effects of a 6-Hour Regimen of Enoxaparin in Patients Undergoing Primary Percutaneous Coronary Intervention (PENNY PCI Study). Thromb. Haemost. 2018, 118, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Zeymer, U.; Silvain, J.; Boulanger, B.; Cohen, M.; Goldstein, P.; Ecollan, P.; Combes, X.; Huber, K.; Pollack, C.; et al. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: The international randomised open-label ATOLL trial. Lancet 2011, 378, 693–703. [Google Scholar] [CrossRef]
- Dewilde, W.J.M.; Oirbans, T.; Verheugt, F.W.A.; Kelder, J.C.; De Smet, B.J.G.L.; Herrman, J.-P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.C.M.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Lamberts, M.; Gislason, G.H.; Olesen, J.B.; Kristensen, S.L.; Schjerning Olsen, A.-M.; Mikkelsen, A.; Christensen, C.B.; Lip, G.Y.H.; Køber, L.; Torp-Pedersen, C.; et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J. Am. Coll. Cardiol. 2013, 62, 981–989. [Google Scholar] [CrossRef]
- Oldgren, J.; Steg, P.G.; Hohnloser, S.H.; Lip, G.Y.H.; Kimura, T.; Nordaby, M.; Brueckmann, M.; Kleine, E.; Ten Berg, J.M.; Bhatt, D.L.; et al. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: A subgroup analysis from the RE-DUAL PCI trial. Eur. Heart J. 2019, 40, 1553–1562. [Google Scholar] [CrossRef]
- Alexander, J.H.; Lopes, R.D.; James, S.; Kilaru, R.; He, Y.; Mohan, P.; Bhatt, D.L.; Goodman, S.; Verheugt, F.W.; Flather, M.; et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N. Engl. J. Med. 2011, 365, 699–708. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Mega, J.L.; Braunwald, E.; Mohanavelu, S.; Burton, P.; Poulter, R.; Misselwitz, F.; Hricak, V.; Barnathan, E.S.; Bordes, P.; Witkowski, A.; et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): A randomised, double-blind, phase II trial. Lancet 2009, 374, 29–38. [Google Scholar] [CrossRef]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.-P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.A.; et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Mehta, S.R.; Jukema, J.W.; Lip, G.Y.H.; Gibson, C.M.; Kovar, F.; Kala, P.; Garcia-Hernandez, A.; Renfurm, R.W.; Granger, C.B.; et al. RUBY-1: A randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome. Eur. Heart J. 2011, 32, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Bates, E.R.; Bhatt, D.L.; Cao, C.; Holmes, D.; Kupfer, S.; Martinez, F.; Spaeder, J.; Weitz, J.I.; Ye, Z.; et al. Phase 2 study of TAK-442, an oral factor Xa inhibitor, in patients following acute coronary syndrome. Thromb. Haemost. 2014, 111, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H.; Emsley, J.; Feener, E.P.; Gailani, D.; Govers-Riemslag, J.W.P.; Kaplan, A.P.; Maas, C.; Morrissey, J.H.; Renné, T.; Sidelmann, J.J.; et al. Nomenclature of factor XI and the contact system. J. Thromb. Haemost. 2019, 17, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Puy, C.; Rigg, R.A.; McCarty, O.J.T. The hemostatic role of factor XI. Thromb. Res. 2016, 141 (Suppl. 2), S8–S11. [Google Scholar] [CrossRef]
- Renné, T.; Pozgajová, M.; Grüner, S.; Schuh, K.; Pauer, H.-U.; Burfeind, P.; Gailani, D.; Nieswandt, B. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005, 202, 271–281. [Google Scholar] [CrossRef]
- Renné, T.; Oschatz, C.; Seifert, S.; Müller, F.; Antovic, J.; Karlman, M.; Benz, P.M. Factor XI deficiency in animal models. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 79–83. [Google Scholar] [CrossRef]
- Chan, N.C.; Weitz, J.I. Antithrombotic Agents. Circ. Res. 2019, 124, 426–436. [Google Scholar] [CrossRef]
- Buyue, Y.; Whinna, H.C.; Sheehan, J.P. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood 2008, 112, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, B.; Sullenger, B.; Becker, R.C. Antithrombotic therapy in acute coronary syndrome: How far up the coagulation cascade will we go? Curr. Cardiol. Rep. 2010, 12, 315–320. [Google Scholar] [CrossRef]
- Colman, R.W. Are hemostasis and thrombosis two sides of the same coin? J. Exp. Med. 2006, 203, 493–495. [Google Scholar] [CrossRef] [PubMed]
- DeLoughery, E.P.; Olson, S.R.; Puy, C.; McCarty, O.J.T.; Shatzel, J.J. The Safety and Efficacy of Novel Agents Targeting Factors XI and XII in Early Phase Human Trials. Semin. Thromb. Hemost. 2019, 45, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Balters, M.W.; Agrawal, D.K. Factors IX, XI, and XII: Potential therapeutic targets for anticoagulant therapy in atherothrombosis. Rev. Cardiovasc. Med. 2019, 20, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A. Factor XI(a) inhibitors for thrombosis: An updated patent review (2016-present). Expert Opin. Ther. Pat. 2020, 30, 39–55. [Google Scholar] [CrossRef] [PubMed]

| Type of Drugs | Class of Drugs | Drugs Name(s) | Route of Administration | Mechanism of Action | Main Study Findings | Stage of Development |
|---|---|---|---|---|---|---|
| Anticoagulant drugs | Low-molecular-weight heparin | Enoxaparin | Subcutaneous and intravenous | inhibiting factor FXa and FIIa | Sustained anti-Xa levels during infusion in STEMI patients undergoing PCI | Launched |
| Non-vitamin-K-antagonist oral anticoagulants | Rivaroxaban | Oral | direct FXa inhibitor | Addition of low-dose rivaroxaban (2.5 mg b.d.) reduced ischemic events and all-cause mortality with an increase in bleeding. No positive study results of other NOACs tested. | Launched | |
| Inhibitors of intrinsic pathway of coagulation | n/a | Intravenous, subcutaneous, and oral | Inhibitors of “upstream” anticoagulation factors FIX-, FXI-, and FXII. Various targets of action (e.g., hepatic synthesis, monoclonal antibodies) | Various phase I and phase II trials currently ongoing. First study results of FXI- and FXII-inhibitors more promising than FIX-inhibitors. | Phase I/II | |
| Antiplatelet drugs | P2Y12-receptor antagonist | Cangrelor | Intravenous | Adenosine triphosphate analogue blocking P2Y12-receptor | Phase III trials show reduced MACE and stent thrombosis versus oral clopidogrel. | Launched |
| P2Y12-receptor antagonist | Selatogrel | Subcutaneous | P2Y12 receptor antagonist | Potent platelet P2Y12 inhibition within 30 min, reversible by 24 h. No major bleeding events in the largest clinical study. | Phase II | |
| PI3Kβ-inhibitors | AZD6482; GSK2636771 | Intravenous and oral | Inhibiting the effect of PI3Kβ which acts through platelet cellular signaling systems | Mild effect on platelet activity, minimal effect on bleeding times in healthy volunteers. GSK2636771 has been evaluated in a phase I trial for its effect on tumor progression. | Phase I | |
| Platelet GP VI-inhibitors | Revacept; ACT017 | Intravenous | Inhibition of collagen-induced platelet aggregation | In phase I studies, drugs appeared to be effective and safe. Two phase II studies completed but results have not been fully disclosed yet. | Phase II | |
| Protein disulfide isomerase (PDI) inhibitors | Isoquercetin HPW-RX40 | Oral | Inhibition of PDI attenuates conformational changes in the activation of GP IIb/IIIa and inhibits the generation of thrombin generation | In the setting of prevention of cancer-associated thrombosis, isoquercetin caused a reduction in circulating levels of D-dimer and platelet-dependent thrombin generation was demonstrated. HPW-RX40 has only been tested in preclinical studies. | Phase II | |
| PAR1 signaling modulators | Parmodulins | n/a | Inhibition of PAR1 signaling pathways involved in platelet activation, but not those relevant to endothelial cytoprotective effects | Preclinical studies have demonstrated inhibition of thrombin-induced platelet activation. | Preclinical | |
| PAR4-inhibitors | BMS-986120 | Oral | Inhibition of PAR4 activation by thrombin | In a phase I study of healthy volunteers, BMS-986120 inhibited ex vivo platelet-rich thrombus formation upon stimulation with PAR4 agonist peptide in high-shear-stress conditions. | Phase I | |
| Platelet glycoprotein Ib-IX-V receptor inhibitor | Caplacizumab | Intravenous | Caplacizumab is a immunoglobulin fragment, which targets the A1 domain of von Willebrand factor, inhibiting interaction with the platelet glycoprotein Ib-IX-V receptor, which has an important role in platelet adhesion to damaged sub-endothelium. | Efficacy has been proven in a modestly sized phase III study for treatment of aTTP with an increase in gingival bleeding and epistaxis. No studies have yet been performed in other thrombotic conditions. | Launched (for aTTP) | |
| Confirmation-specific GPIIb/IIIa inhibitors | Anti-activated GPIIb/IIIa | n/a | Activated, but not unactivated GPIIb/IIIa is inhibited | Inhibited propagation of thrombosis in an animal model without prolonging bleeding time. | Preclinical | |
| Inhibitors of GPIIb/IIIa outside-in signaling | mP6 | n/a | Disrupts interaction between Gα13 and IIIa, inhibiting downstream signaling | Inhibited propagation of thrombosis in an animal model without prolonging bleeding time. | Preclinical | |
| Platelet-targeted CD39 | CD39-anti GPIIb/IIIa | n/a | CD39 breaks down ADP. Linking CD39 to anti-GPIIb/IIIa targets the enzyme to platelets. | Preclinical studies have shown greater antiplatelet efficacy of platelet-targeted CD39 compared to untargeted CD39. | Preclinical |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwart, B.; Parker, W.A.E.; Storey, R.F. New Antithrombotic Drugs in Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 2059. https://doi.org/10.3390/jcm9072059
Zwart B, Parker WAE, Storey RF. New Antithrombotic Drugs in Acute Coronary Syndrome. Journal of Clinical Medicine. 2020; 9(7):2059. https://doi.org/10.3390/jcm9072059
Chicago/Turabian StyleZwart, Bastiaan, William A. E. Parker, and Robert F. Storey. 2020. "New Antithrombotic Drugs in Acute Coronary Syndrome" Journal of Clinical Medicine 9, no. 7: 2059. https://doi.org/10.3390/jcm9072059
APA StyleZwart, B., Parker, W. A. E., & Storey, R. F. (2020). New Antithrombotic Drugs in Acute Coronary Syndrome. Journal of Clinical Medicine, 9(7), 2059. https://doi.org/10.3390/jcm9072059





