PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategies and Study Selection
2.2. Selection Criteria
2.3. Statistical Analysis
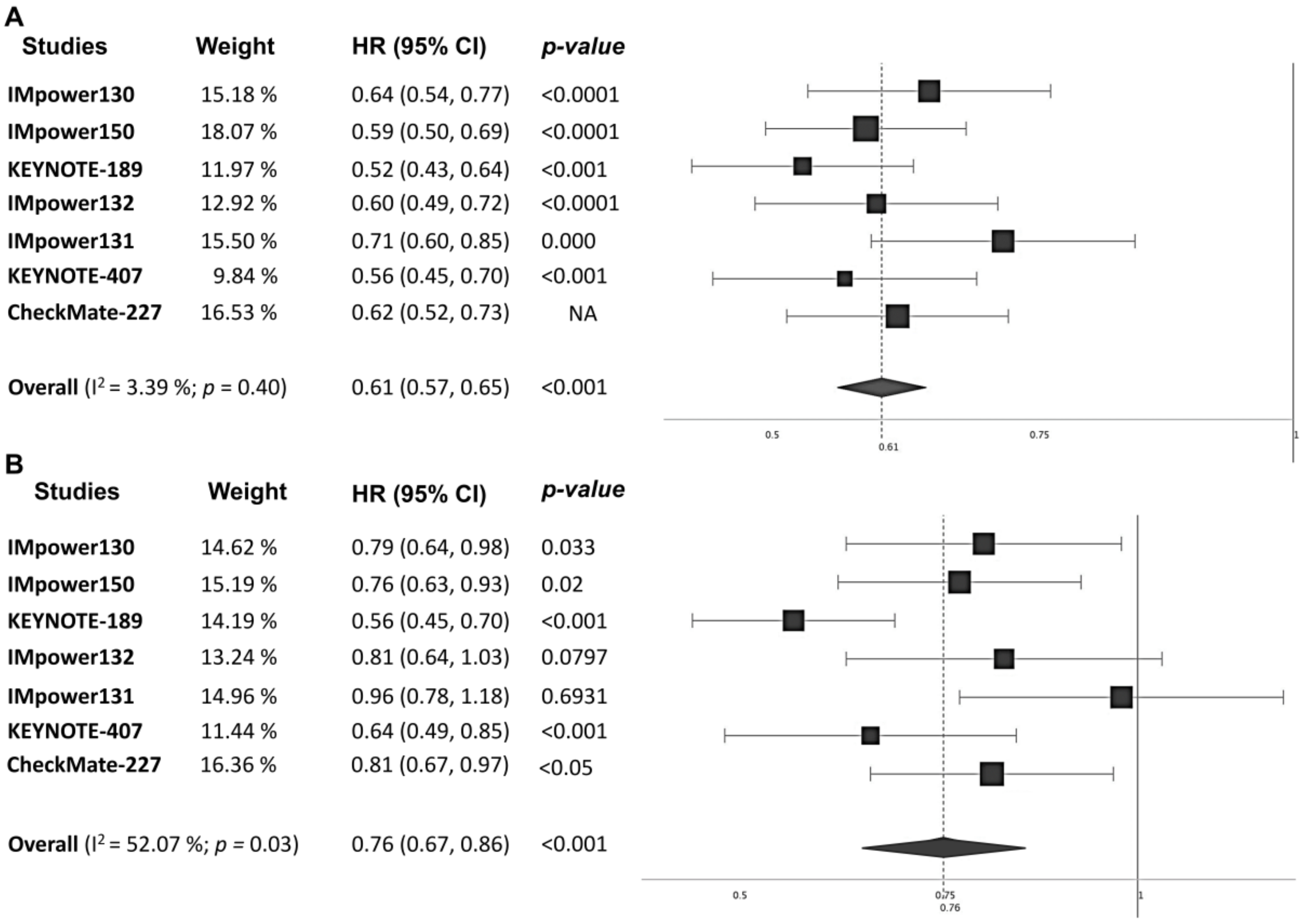
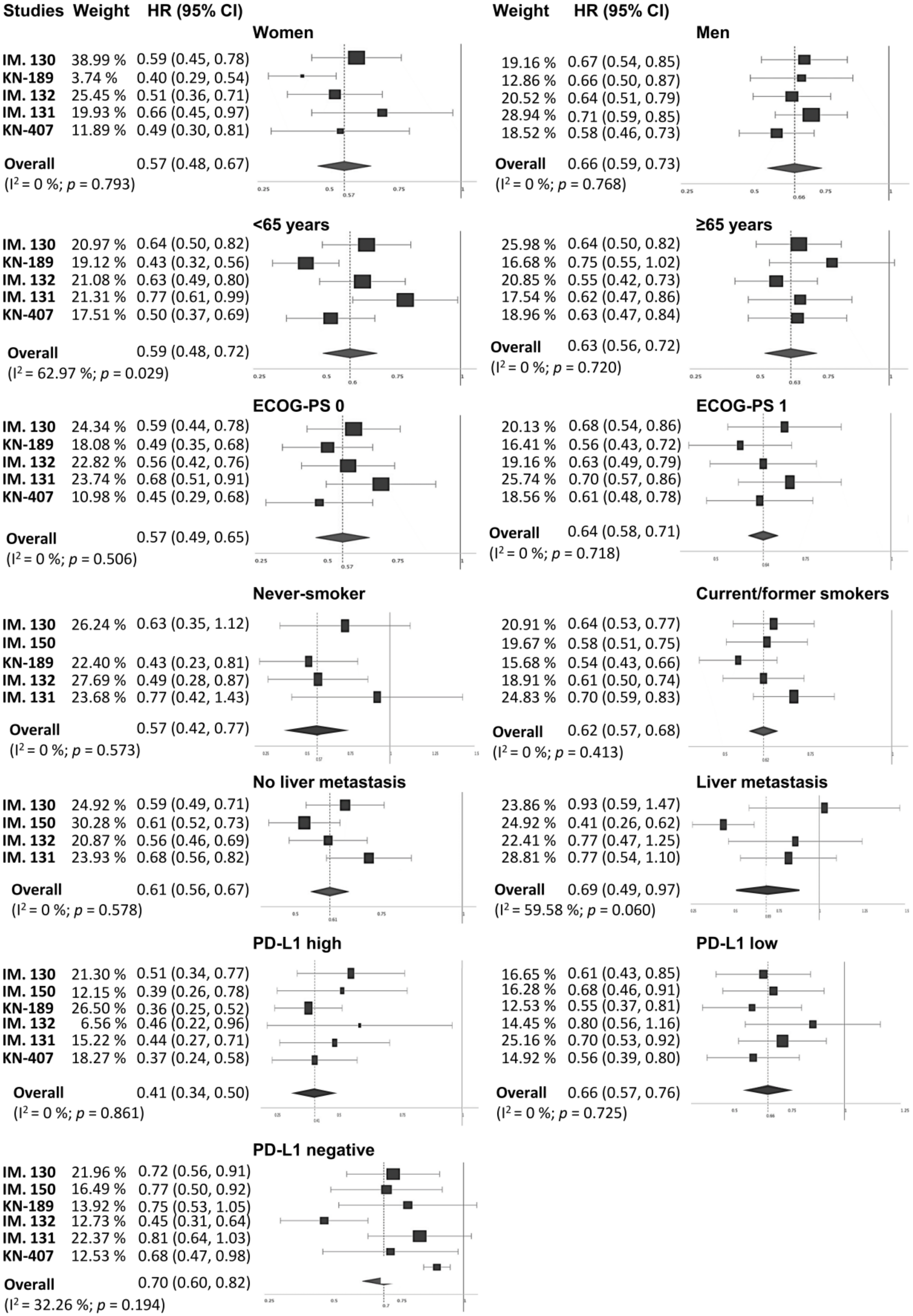
3. Results
3.1. Studies Included in the Meta-Analysis
3.2. Study Characteristics
3.3. Efficacy Endpoints in the Overall Population
3.4. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Lu, M.; Su, Y. Immunotherapy in non-small cell lung cancer: The past, the present, and the future. Thorac. Cancer 2019, 10, 585–586. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Ponce Aix, S.; Han, J.Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L.; et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef] [Green Version]
- Font, E.; Gettinger, S.N.; Burgio, M.; Antonia, S.J.; Holgado, E.; Spigel, D.R.; Arrieta, O.; Domine, M.; Aren, O.; Brahmer, J.; et al. 1301PD Three-year follow-up from CheckMate 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 2017. [Google Scholar] [CrossRef]
- Herbst, R.; Baas, P.; Kim, D.-S.; Felip, E.; Perez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-P.; Arvis, C.; Ahn, M.-J.; et al. Factors associated with better overall survival (OS) in patients with previously treated, PD-L1–expressing, advanced NSCLC: Multivariate analysis of KEYNOTE-010. J. Clin. Oncol. 2017, 35, 9090. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Lopes, G.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.; Cho, B.C.; Castro, G.; Srimuninnimit, V.; Bondarenko, I.; Kubota, K.; Lubiniecki, G.M.; et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ladoire, S.; Coukos, G.; Ghiringhelli, F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann. Oncol. 2015, 26, 1813–1823. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Garassino, M.C.; Esteban, E.; Speranza, G.; Felip, E.; Hochmair, M.J.; Powell, S.F.; Cheng, S.Y.; Bischoff, H.; Peled, N.; et al. KEYNOTE-189: Updated OS and progression after the next line of therapy (PFS2) with pembrolizumab (pembro) plus chemo with pemetrexed and platinum vs placebo plus chemo for metastatic nonsquamous NSCLC. J. Clin. Oncol. 2019, 37, 9013. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodriguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.; et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous Non-Small-Cell Lung Cancer (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.; Cobo, M.; Bordoni, R.; Dubray-Longeras, P.; Szalai, Z.; Ursol, G.; Novello, S.; Orlandi, F.; Ball, S.; Goldschmidt, J.; et al. OA05.07 IMpower132: PFS and Safety Results with 1L Atezolizumab + Carboplatin/Cisplatin + Pemetrexed in Stage IV Non-Squamous NSCLC. J. Thorac. Oncol. 2018, 13, S332–S333. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ares, L.; Ciuleanu, T.E.; Yu, X.; Salman, P.; Pluzanski, A.; Nagrial, A.; Havel, L.; Kowalyszyn, R.; Audigier-Valette, C.; Wu, Y.L.; et al. LBA3 Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (aNSCLC): CheckMate 227 - part 2 final analysis. Ann. Oncol. 2019, 30, xi67–xi68. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK; Hoboken, NJ, USA, 2008. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodgríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; Angelis, F.D.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.; et al. Abstract CT075: KEYNOTE-189: Randomized, double-blind, phase 3 study of pembrolizumab (pembro) or placebo plus pemetrexed (pem) and platinum as first-line therapy for metastatic NSCLC. Cancer Res. 2018, 78, CT075. [Google Scholar]
- Jotte, R.M.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodriguez Abreu, D.; Hussein, M.A.; Soo, R.A.; Conter, H.J.; Kozuki, T.; Silva, C.; et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J. Clin. Oncol. 2018, 36, LBA9000. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Tan, P.S.; Aguiar, P.; Haaland, B.; Lopes, G. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer—A systematic review and network meta-analysis of 3024 participants. Lung Cancer 2018, 115, 84–88. [Google Scholar] [CrossRef]
- You, W.; Liu, M.; Miao, J.D.; Liao, Y.Q.; Song, Y.B.; Cai, D.K.; Gao, Y.; Peng, H. A Network Meta-analysis Comparing the Efficacy and Safety of Anti-PD-1 with Anti-PD-L1 in Non-small Cell Lung Cancer. J. Cancer 2018, 9, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.-W.; Xiong, Y.; Chen, S.; Xia, F.; Li, Q.; Hu, J. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials. Medicine 2016, 95, e4611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xie, R.; Lin, S.; You, X.; Weng, X. Anti-PD-1/PD-L1 Antibody Therapy for Pretreated Advanced or Metastatic Nonsmall Cell Lung Carcinomas and the Correlation between PD-L1 Expression and Treatment Effectiveness: An Update Meta-Analysis of Randomized Clinical Trials. BioMed. Res. Int. 2018, 2018, 3820956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, A.M.; Thein, K.Z.; Thein, W.L.; Guevara, E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: A systematic review and meta-analysis of randomized controlled trials. Future Sci. OA 2019, 5, Fso421. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Tang, L.; Peng, X.; Jiang, H.; Wang, G.; Zhuang, W. Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Cancer 2019, 10, 6261–6268. [Google Scholar] [CrossRef]
- Shen, K.; Cui, J.; Wei, Y.; Chen, X.; Liu, G.; Gao, X.; Li, W.; Lu, H.; Zhan, P.; Lv, T.; et al. Effectiveness and safety of PD-1/PD-L1 or CTLA4 inhibitors combined with chemotherapy as a first-line treatment for lung cancer: A meta-analysis. J. Thorac. Dis. 2018, 10, 6636–6652. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Banna, G.L.; Metro, G.; Di Maio, M. Chemotherapy in Combination with Immune Checkpoint Inhibitors for the First-Line Treatment of Patients With Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front. Oncol. 2019, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuzzo, F.M.M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.; Daniel, D.; McCune, S.; Mekhail, T.; Zer, A.; et al. IMpower130: Progression-free survival (PFS) and safety analysis from a randomised phase 3 study of carboplatin + nab-paclitaxel (CnP) with or without atezolizumab (atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. Ann. Oncol. 2018, 29, mdy424-065. [Google Scholar] [CrossRef]
- Barlesi, F.N.M.; Cobo, M.; Steele, N.; Paramonov, V.; Parente, B.; Dear, R.; Berard, H.; Peled, N.; Seneviratne, L.C.; Baldini, E.; et al. IMpower132: Efficacy of atezolizumab (atezo)+carboplatin (carbo)/cisplatin (cis)+pemetrexed (pem) as 1L treatment in key subgroups with stage IV non-squamous non-small cell lung cancer (NSCLC). Ann. Oncol. 2018, 29, mdy424-066. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; Angelis, F.D.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Abstract CT043: Outcomes among patients (pts) with metastatic nonsquamous NSCLC with liver metastases or brain metastases treated with pembrolizumab (pembro) plus pemetrexed-platinum: Results from the KEYNOTE-189 study. Cancer Res. 2019, 79, CT043. [Google Scholar]



| Study | Histology Expression | PD-L1 Expression | Primary Endpoint | Experimental Arm | Control Arm | Analysis Timing |
|---|---|---|---|---|---|---|
| IMpower130 [25] | Nonsquamous | All | PFS (ITT-WT *) OS (ITT-WT *) | Atezolizumab + (carboplatin + nab-paclitaxel) (n = 451) | Carboplatin + nab-paclitaxel (n = 228) | PFS: Final OS: Interim |
| IMpower150 [23,24] | Nonsquamous | All | PFS (ITT-WT *) OS (ITT-WT *) | Atezolizumab + (carboplatin + paclitaxel + bevacizumab) (n = 356) | Carboplatin + paclitaxel + bevacizumab (n = 336) | PFS: Final OS: Interim |
| KEYNOTE-189 [17,28] | Nonsquamous | All | PFS (ITT) OS (ITT) | Pembrolizumab + (carboplatin or cisplatin + pemetrexed) (n = 410) | Carboplatin or cisplatin + pemetrexed (n = 206) | PFS: Final OS: Interim |
| IMpower132 [20] | Nonsquamous | All | PFS (ITT) OS (ITT) | Atezolizumab + (carboplatin or cisplatin + pemetrexed) (n = 292) | Carboplatin or cisplatin + pemetrexed (n = 286) | PFS: Final OS: Interim |
| IMpower131 [29] | Squamous | All | PFS (ITT) OS (ITT) | Atezolizumab + (carboplatin + nab-paclitaxel) (n = 343) | Carboplatin + nab-paclitaxel (n = 340) | PFS: Final OS: Interim |
| KEYNOTE-407 [22] | Squamous | All | PFS (ITT) OS (ITT) | Pembrolizumab + (carboplatin + paclitaxel or nab-paclitaxel) (n = 278) | Carboplatin + paclitaxel or nab-paclitaxel (n = 281) | PFS: Final OS: Interim |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, J.; Ruiz-Bañobre, J.; Afonso-Afonso, F.J.; Amenedo-Gancedo, M.; Areses-Manrique, M.d.C.; Campos-Balea, B.; Casal-Rubio, J.; Fernández-Núñez, N.; Fírvida Pérez, J.L.; Lázaro-Quintela, M.; et al. PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis. J. Clin. Med. 2020, 9, 2093. https://doi.org/10.3390/jcm9072093
García-González J, Ruiz-Bañobre J, Afonso-Afonso FJ, Amenedo-Gancedo M, Areses-Manrique MdC, Campos-Balea B, Casal-Rubio J, Fernández-Núñez N, Fírvida Pérez JL, Lázaro-Quintela M, et al. PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis. Journal of Clinical Medicine. 2020; 9(7):2093. https://doi.org/10.3390/jcm9072093
Chicago/Turabian StyleGarcía-González, Jorge, Juan Ruiz-Bañobre, Francisco J. Afonso-Afonso, Margarita Amenedo-Gancedo, María del Carmen Areses-Manrique, Begoña Campos-Balea, Joaquín Casal-Rubio, Natalia Fernández-Núñez, José Luis Fírvida Pérez, Martín Lázaro-Quintela, and et al. 2020. "PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis" Journal of Clinical Medicine 9, no. 7: 2093. https://doi.org/10.3390/jcm9072093
APA StyleGarcía-González, J., Ruiz-Bañobre, J., Afonso-Afonso, F. J., Amenedo-Gancedo, M., Areses-Manrique, M. d. C., Campos-Balea, B., Casal-Rubio, J., Fernández-Núñez, N., Fírvida Pérez, J. L., Lázaro-Quintela, M., Pérez Parente, D., Crama, L., Ruiz-Gracia, P., Santomé-Couto, L., & León-Mateos, L. (2020). PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis. Journal of Clinical Medicine, 9(7), 2093. https://doi.org/10.3390/jcm9072093





