Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
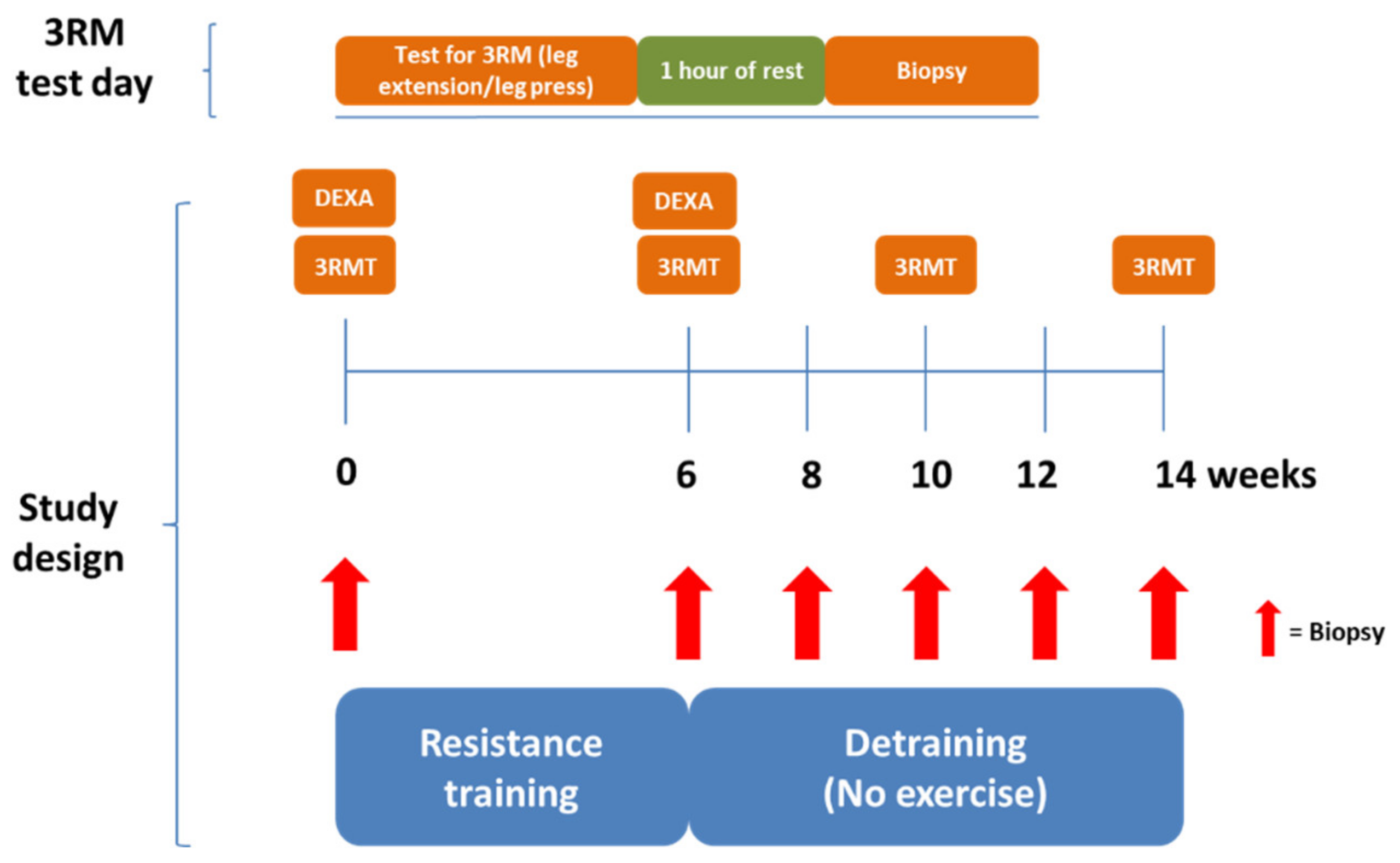
2.2. Study Design
2.3. DEXA Scanning
2.4. Exercise Equipment and Protocol
2.5. Strength Testing
2.6. Resistance Exercise Training and Deconditioning Interventions
2.7. Skeletal Muscle Biopsy
2.8. Western Blotting Analysis
2.9. Muscle Histology and Immunohistochemistry
2.10. Mitochondrial Citrate Synthase Enzyme Activity
2.11. Statistical Analysis
3. Results
3.1. Anthropometry
3.2. Muscle Strength
3.3. Muscle Fiber Type Composition
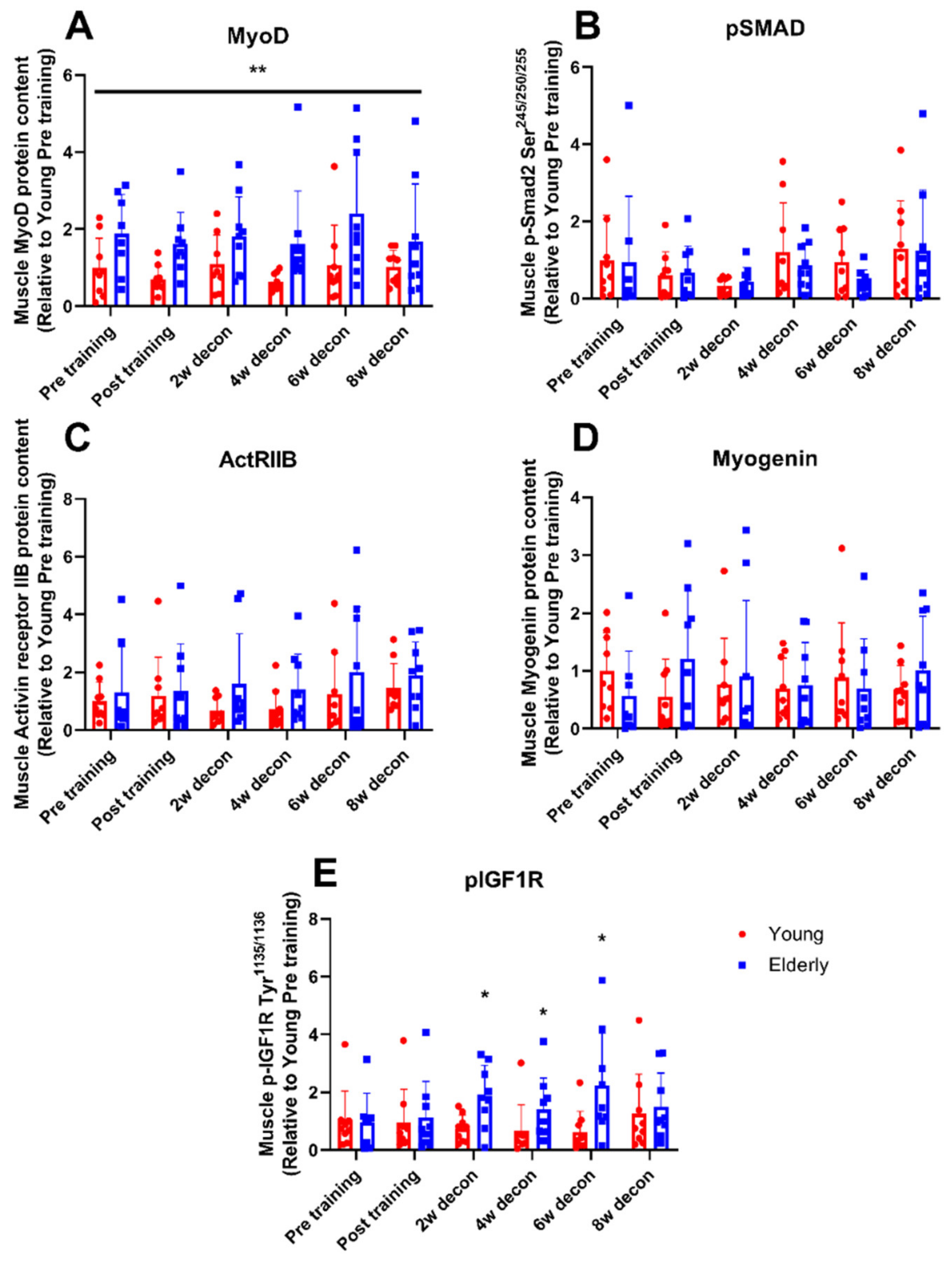
3.4. Myogenic Regulatory Factors
3.5. Mitochondrial Markers: Porin and Citrate Synthase
3.6. Correlation between Myogenic and Mitochondrial Factors and Muscle Strength
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuggle, N.; Shaw, S.; Dennison, E.; Cooper, C. Sarcopenia. Best Pract. Res. Clin. Rheumatol. 2017, 31, 218–242. [Google Scholar] [CrossRef] [PubMed]
- Drey, M. Sarcopenia—Pathophysiology and clinical relevance. Wien Med. Wochenschr. 2011, 161, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Newton, R.U.; Hakkinen, K.; Hakkinen, A.; McCormick, M.; Volek, J.; Kraemer, W.J. Mixed-methods resistance training increases power and strength of young and older men. Med. Sci. Sports Exerc. 2002, 34, 1367–1375. [Google Scholar] [CrossRef]
- HÃkkinen, K.; Newton, R.U.; Gordon, S.E.; McCormick, M.; Volek, J.S.; Nindl, B.C.; Gotshalk, L.A.; Campbell, W.W.; Evans, W.J.; Häkkinen, A.; et al. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B415–B423. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, J.T.; Hurlbut, D.E.; Martel, G.F.; Tracy, B.L.; Ivey, F.M.; Metter, E.J.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; De Vito, G.; Felici, F.; Nimmo, M.A. Electromyogram changes during sustained contraction after resistance training in women in their 3rd and 8th decades. Eur. J. Appl. Physiol. 2000, 82, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. Functional consequences of sarcopenia and dynapenia in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 271–276. [Google Scholar] [CrossRef]
- Karlsen, A.; Bechshoft, R.L.; Malmgaard-Clausen, N.M.; Andersen, J.L.; Schjerling, P.; Kjaer, M.; Mackey, A.L. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83-94-year-old men and women. Acta Physiol. 2019, 227, e13271. [Google Scholar] [CrossRef]
- Snijders, T.; Verdijk, L.B.; Smeets, J.S.; McKay, B.R.; Senden, J.M.; Hartgens, F.; Parise, G.; Greenhaff, P.; van Loon, L.J. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age 2014, 36, 9699. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.; Meijer, K.; Savelberg, H.H.; Dendale, P.; van Loon, L.J. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Elderly men and women benefit equally from prolonged resistance-type exercise training. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Nederveen, J.P.; Joanisse, S.; Leenders, M.; Verdijk, L.B.; van Loon, L.J.; Parise, G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J. Cachexia Sarcopenia Muscle 2017, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Soendenbroe, C.; Malmgaard-Clausen, N.M.; Wagener, F.; Moeller, C.E.; Senhaji, Z.; Damberg, K.; Andersen, J.L.; Schjerling, P.; Kjaer, M.; et al. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J. 2020, 34, 6418–6436. [Google Scholar] [CrossRef] [PubMed]
- Blocquiaux, S.; Gorski, T.; Van Roie, E.; Ramaekers, M.; Van Thienen, R.; Nielens, H.; Delecluse, C.; De Bock, K.; Thomis, M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp. Gerontol. 2020, 133, 110860. [Google Scholar] [CrossRef] [PubMed]
- Kosek, D.J.; Kim, J.S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006, 101, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.A.; Hulmi, J.J.; Salmijarvi, H.; Katajavuori, M.; Haverinen, M.; Holviala, J.; Ridanpaa, T.; Hakkinen, K.; Kovanen, V.; Ahtiainen, J.P.; et al. Resistance training induced increase in muscle fiber size in young and older men. Eur. J. Appl. Physiol. 2013, 113, 641–650. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016, 594, 7399–7417. [Google Scholar] [CrossRef]
- Hwa, V.; Fang, P.; Derr, M.A.; Fiegerlova, E.; Rosenfeld, R.G. IGF-I in human growth: Lessons from defects in the GH-IGF-I axis. Nestle Nutr. Inst. Workshop Ser. 2013, 71, 43–55. [Google Scholar] [CrossRef]
- Stilling, F.; Wallenius, S.; Michaelsson, K.; Dalgard, C.; Brismar, K.; Wolk, A. High insulin-like growth factor-binding protein-1 (IGFBP-1) is associated with low relative muscle mass in older women. Metabolism 2017, 73, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Gailly, P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Seward, D.J.; Haney, J.C.; Rudnicki, M.A.; Swoap, S.J. bHLH transcription factor MyoD affects myosin heavy chain expression pattern in a muscle-specific fashion. Am. J. Physiol. Cell Physiol. 2001, 280, C408–C413. [Google Scholar] [CrossRef] [PubMed]
- Mozdziak, P.E.; Greaser, M.L.; Schultz, E. Myogenin, MyoD, and myosin heavy chain isoform expression following hindlimb suspension. Aviat. Space Environ. Med. 1999, 70, 511–516. [Google Scholar] [PubMed]
- Mozdziak, P.E.; Greaser, M.L.; Schultz, E. Myogenin, MyoD, and myosin expression after pharmacologically and surgically induced hypertrophy. J. Appl. Physiol. 1998, 84, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Schoene, D.; Kiesswetter, E.; Sieber, C.C.; Freiberger, E. Musculoskeletal factors, sarcopenia and falls in old age. Z. Gerontol. Geriatr. 2019, 52, 37–44. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Adams, G.R. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J. Appl. Physiol. 2006, 100, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Orrell, R.W.; Cobbold, M.; Goldspink, G.; Harridge, S.D. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J. Physiol. 2003, 547, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kosek, D.J.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J. Appl. Physiol. 2005, 99, 2149–2158. [Google Scholar] [CrossRef]
- Raue, U.; Slivka, D.; Jemiolo, B.; Hollon, C.; Trappe, S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J. Appl. Physiol. 2006, 101, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Ahtiainen, J.P.; Kaasalainen, T.; Pollanen, E.; Hakkinen, K.; Alen, M.; Selanne, H.; Kovanen, V.; Mero, A.A. Postexercise myostatin and activin IIb mRNA levels: Effects of strength training. Med. Sci. Sports Exerc. 2007, 39, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Kovanen, V.; Selanne, H.; Kraemer, W.J.; Hakkinen, K.; Mero, A.A. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 2009, 37, 297–308. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K. Impact of Resistance Training on Skeletal Muscle Mitochondrial Biogenesis, Content, and Function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef]
- Dordevic, A.L.; Bonham, M.; Ghasem-Zadeh, A.; Evans, A.; Barber, E.; Day, K.; Kwok, A.; Truby, H. Reliability of Compartmental Body Composition Measures in Weight-Stable Adults Using GE iDXA: Implications for Research and Practice. Nutrients 2018, 10, 1484. [Google Scholar] [CrossRef]
- Abdalla, P.P.; Carvalho, A.D.S.; Dos Santos, A.P.; Venturini, A.C.R.; Alves, T.C.; Mota, J.; Machado, D.R.L. One-repetition submaximal protocol to measure knee extensor muscle strength among older adults with and without sarcopenia: A validation study. BMC Sports Sci. Med. Rehabil. 2020, 12, 29. [Google Scholar] [CrossRef]
- Abdul-Hameed, U.; Rangra, P.; Shareef, M.Y.; Hussain, M.E. Reliability of 1-repetition maximum estimation for upper and lower body muscular strength measurement in untrained middle aged type 2 diabetic patients. Asian J. Sports Med. 2012, 3, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 1975, 35, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Thogersen, F.B.; Thybo, K.; Vissing, C.R.; Krag, T.O.; Ruiz-Ruiz, C.; Risom, L.; Wibrand, F.; Hoeg, L.D.; Kiens, B.; et al. Adaptations in Mitochondrial Enzymatic Activity Occurs Independent of Genomic Dosage in Response to Aerobic Exercise Training and Deconditioning in Human Skeletal Muscle. Cells 2019, 8, 237. [Google Scholar] [CrossRef]
- Ben-Hail, D.; Shoshan-Barmatz, V. VDAC1-interacting anion transport inhibitors inhibit VDAC1 oligomerization and apoptosis. Biochim. Biophys. Acta 2016, 1863, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Brioche, T.; Pagano, A.F.; Py, G.; Chopard, A. Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention. Mol. Aspects Med. 2016, 50, 56–87. [Google Scholar] [CrossRef]
- Kopantseva, E.E.; Belyavsky, A.V. Key regulators of skeletal myogenesis. Mol. Biol. (Mosk.) 2016, 50, 195–222. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Reitelseder, S.; Pedersen, T.G.; Doessing, S.; Schjerling, P.; Langberg, H.; Miller, B.F.; Aagaard, P.; Kjaer, M.; Holm, L. Myogenic, matrix, and growth factor mRNA expression in human skeletal muscle: Effect of contraction intensity and feeding. Muscle Nerve 2013, 47, 748–759. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Schjerling, P.; Haddad, F.; Langberg, H.; Baldwin, K.M.; Kjaer, M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: Differential effects of specific contraction types. J. Appl. Physiol. 2007, 102, 573–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, L.; Lu, A.M.; Wang, Y.; Hong, A.; Chen, Y.; Hu, J.; Li, X.; Qin, Z.H. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp. Gerontol. 2013, 48, 427–436. [Google Scholar] [CrossRef]
- Mathers, J.L.; Farnfield, M.M.; Garnham, A.P.; Caldow, M.K.; Cameron-Smith, D.; Peake, J.M. Early inflammatory and myogenic responses to resistance exercise in the elderly. Muscle Nerve 2012, 46, 407–412. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Bamman, M.M.; Ragan, R.C.; Kim, J.S.; Cross, J.M.; Hill, V.J.; Tuggle, S.C.; Allman, R.M. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J. Appl. Physiol. 2004, 97, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, G.R.; West, D.W.; Baar, K. The molecular basis for load-induced skeletal muscle hypertrophy. Calcif. Tissue Int. 2015, 96, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Bogdanovich, S.; Krag, T.O.; Barton, E.R.; Morris, L.D.; Whittemore, L.A.; Ahima, R.S.; Khurana, T.S. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002, 420, 418–421. [Google Scholar] [CrossRef]
- Cai, Q.; Zhao, M.; Liu, X.; Wang, X.; Nie, Y.; Li, P.; Liu, T.; Ge, R.; Han, F. Reduced expression of citrate synthase leads to excessive superoxide formation and cell apoptosis. Biochem. Biophys. Res. Commun. 2017, 485, 388–394. [Google Scholar] [CrossRef]
- Kadenbach, B. Introduction to mitochondrial oxidative phosphorylation. Adv. Exp. Med. Biol. 2012, 748, 1–11. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Proctor, D.N.; Joyner, M.J. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J. Appl. Physiol. 1997, 82, 1411–1415. [Google Scholar] [CrossRef]
- Meierhofer, D.; Mayr, J.A.; Foetschl, U.; Berger, A.; Fink, K.; Schmeller, N.; Hacker, G.W.; Hauser-Kronberger, C.; Kofler, B.; Sperl, W. Decrease of mitochondrial DNA content and energy metabolism in renal cell carcinoma. Carcinogenesis 2004, 25, 1005–1010. [Google Scholar] [CrossRef]
- Van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.A.; Jorgensen, J.A.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Duez, H.; Lefebvre, P.; Schaper, N.C.; et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5, 635–645. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Goreham, C.; Ouyang, J.; Ball-Burnett, M.; Ranney, D. Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am. J. Physiol. 1999, 276, R591–R596. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Carry, M.R.; Brass, E.P. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J. Appl. Physiol. 1996, 81, 780–788. [Google Scholar] [CrossRef]
- Kirkendall, D.T.; Garrett, W.E., Jr. The effects of aging and training on skeletal muscle. Am. J. Sports Med. 1998, 26, 598–602. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]

| Young Group | Elderly Group | |||
|---|---|---|---|---|
| Age, years | 22.4 ± 2.2 | 82.3 ± 6.8 *** | ||
| Height, cm | 174.8 ± 10.4 | 167.5 ± 8.6 | ||
| Weight, kg | 70.9 ± 15.1 | 64.7 ± 9.7 | ||
| BMI, kg/m2 | 22.9 ± 2.4 | 23.5 ± 2.9 | ||
| Pre training | Post training | Pre training | Post training | |
| Body fat (%) | 26.2 ± 8.6 | 26.3 ± 9.5 | 27.9 ± 4.8 | 27.1 ± 5.8 |
| LBM (%) | 74.5 ± 8.9 | 74.5 ± 8.8 | 73.9 ± 6.6 | 73.7 ± 6.9 |
| LLM (kg) | 16.7 ± 4.2 | 17.0 ± 4.2 | 14.0 ± 2.9 | 14.2 ± 2.4 |
| Young Group | Elderly Group | |||||
|---|---|---|---|---|---|---|
| Pre Training | Post Training | Post Deconditioning | Pre Training | Post Training | Post Deconditioning | |
| Porin protein content relative to young pre training (AU) | 1.00 ± 0.4 | 0.98 ± 1.1 | 1.60 ± 1.3 | 1.19 ± 1.2 | 1.53 ± 1.3 | 2.40 ± 1.9 |
| CS activity, µmol/min/mg w.w. | 1.7 ± 1.0 | 2.2 ± 1.3 | 2.0 ± 0.6 | 1.0 ± 0.6 | 1.7 ± 1.9 | 1.4 ± 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritzen, A.M.; Thøgersen, F.D.; Qadri, K.A.N.; Krag, T.; Sveen, M.-L.; Vissing, J.; Jeppesen, T.D. Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning. J. Clin. Med. 2020, 9, 2188. https://doi.org/10.3390/jcm9072188
Fritzen AM, Thøgersen FD, Qadri KAN, Krag T, Sveen M-L, Vissing J, Jeppesen TD. Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning. Journal of Clinical Medicine. 2020; 9(7):2188. https://doi.org/10.3390/jcm9072188
Chicago/Turabian StyleFritzen, Andreas Mæchel, Frank D. Thøgersen, Khaled Abdul Nasser Qadri, Thomas Krag, Marie-Louise Sveen, John Vissing, and Tina D. Jeppesen. 2020. "Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning" Journal of Clinical Medicine 9, no. 7: 2188. https://doi.org/10.3390/jcm9072188
APA StyleFritzen, A. M., Thøgersen, F. D., Qadri, K. A. N., Krag, T., Sveen, M.-L., Vissing, J., & Jeppesen, T. D. (2020). Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning. Journal of Clinical Medicine, 9(7), 2188. https://doi.org/10.3390/jcm9072188






