Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa
Abstract
1. Introduction
1.1. Introduction to Retinitis Pigmentosa
1.2. Genetic Heterogeneity of Retinitis Pigmentosa
1.3. Toward a Mutation-Independent Treatment
2. Main Text
2.1. Human Phenotypes of NRL Pathway Mutations
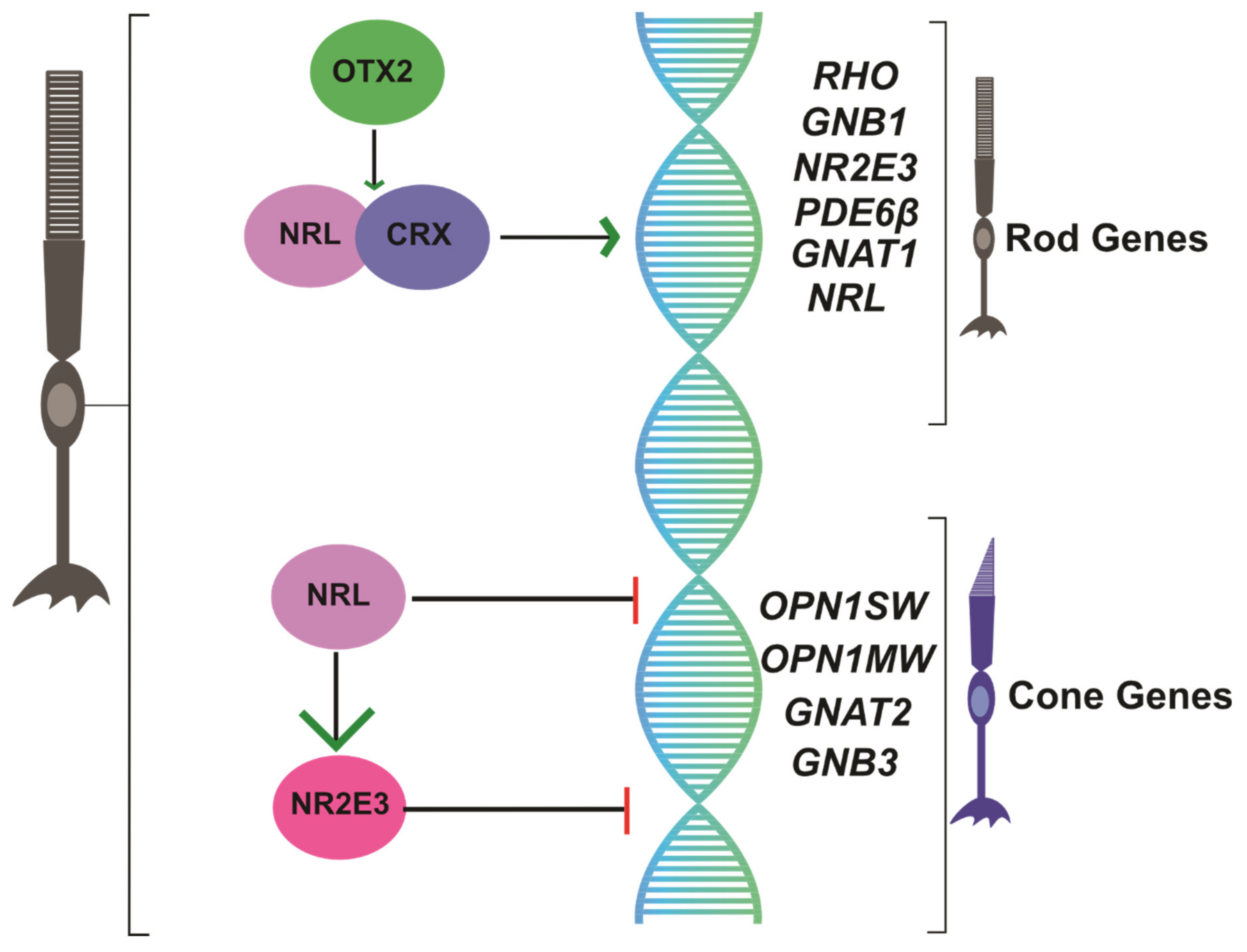
2.2. NRL as a Regulator of Rod Photoreceptor Transcription
2.3. NR2E3 Suppresses Cone Transcription
2.4. Manipulating the NRL Pathway as a Neuroprotective Strategy in RP
2.5. Manipulating the NR2E3 Pathway as a Neuroprotective Strategy in RP
3. Conclusions/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Retinitis Pigmentosa. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519518/ (accessed on 17 April 2020).
- Ivanišević, M. First look into the eye. Eur. J. Ophthalmol. 2018, 29, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; Van Huet, R.A.; Boon, C.J.F.; Hollander, A.I.D.; Collin, R.W.; Klaver, C.C.W.; Hoyng, C.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Koenekoop, R.K.; Loyer, M.; Hand, C.; Al Mahdi, H.; Dembinska, O.; Beneish, R.; Racine, J.; Rouleau, G.A. Novel RPGR mutations with distinct retinitis pigmentosa phenotypes in French-Canadian families. Am. J. Ophthalmol. 2003, 136, 678–687. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Q.; Huang, X.; Qu, C.; Ma, S.; Mao, Y.; Yang, J.; Li, Y.; Li, Y.; Tan, C.; et al. Mutation screening in genes known to be responsible for Retinitis Pigmentosa in 98 Small Han Chinese Families. Sci. Rep. 2017, 7, 1948. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Reichel, E.; Hahn, L.B.; Cowley, G.S.; Yandell, D.W.; Sandberg, M.A.; Berson, E.L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 1990, 343, 364–366. [Google Scholar] [CrossRef]
- Ziviello, C.; Simonelli, F.; Testa, F.; Anastasi, M.; Marzoli, S.B.; Falsini, B.; Ghiglione, D.; Macaluso, C.; Manitto, M.P.; Garrè, C.; et al. Molecular genetics of autosomal dominant retinitis pigmentosa (ADRP): A comprehensive study of 43 Italian families. J. Med. Gen. 2005, 42, e47. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Cowley, G.S.; McGee, T.L.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. A Null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat. Genet. 1992, 1, 209–213. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. Retinitis Pigmentosa (Non-syndromic). Pl. Prom. Transc. Fact. 2018, 1085, 125–130. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Buraczynska, M.; Milam, A.H.; Chen, C.; Järvaläinen, M.; Fujita, R.; Wu, W.; Huang, Y.; Cideciyan, A.V.; Swaroop, A. Disease expression in X-linked retinitis pigmentosa caused by a putative null mutation in the RPGR gene. Investig. Ophthalmol. Vis. Sci. 1997, 1983–1997. [Google Scholar]
- Parmeggiani, F.; Sorrentino, F.S.; Ponzin, D.; Barbaro, V.; Ferrari, S.; Di Iorio, E. Retinitis Pigmentosa: Genes and Disease Mechanisms. Curr. Genom. 2011, 12, 238–249. [Google Scholar] [CrossRef]
- Bessant, D.A.; Payne, A.; Mitton, K.P.; Wang, Q.-L.; Swain, P.K.; Plant, C.; Bird, A.C.; Zack, D.J.; Swaroop, A.; Bhattacharya, S.S. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat. Genet. 1999, 21, 355–356. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, M.M.; Grimsby, J.L.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. Novel mutations in the NRL gene and associated clinical findings in patients with dominant retinitis pigmentosa. Arch. Ophthalmol. 2002, 120, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, K.M.; Friedman, J.S.; Sandberg, M.A.; Swaroop, A.; Berson, E.L.; Dryja, T.P. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc. Natl. Acad. Sci. USA 2004, 101, 17819–17824. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Chen, L.J.; Chiang, S.W.Y.; Tam, P.O.S.; Lai, T.Y.Y.; Chan, C.K.M.; Wang, N.; Lam, D.S.; Pang, C.-P. Association ofNR2E3but NotNRLMutations with Retinitis Pigmentosa in the Chinese Population. Investig. Opthalmology Vis. Sci. 2010, 51, 2229–2235. [Google Scholar] [CrossRef]
- Humphries, M.M.; Rancourt, D.; Farrar, G.J.; Kenna, P.F.; Hazel, M.; Bush, R.A.; Sieving, P.A.; Sheils, D.M.; Creighton, P.; Erven, A.; et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 1997, 15, 216–219. [Google Scholar] [CrossRef]
- Cai, X.; Conley, S.M.; Naash, M.I. RPE65: Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009, 30, 57–62. [Google Scholar] [CrossRef]
- Hong, D.-H.; Pawlyk, B.S.; Shang, J.; Sandberg, M.A.; Berson, E.L.; Li, T. A retinitis pigmentosa GTPase regulator (RPGR)- deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc. Natl. Acad. Sci. USA 2000, 97, 3649–3654. [Google Scholar] [CrossRef]
- Mears, A.J.; Kondo, M.; Swain, P.K.; Takada, Y.; Bush, R.A.; Saunders, T.L.; Sieving, P.A.; Swaroop, A. Nrl is required for rod photoreceptor development. Nat. Genet. 2001, 29, 447–452. [Google Scholar] [CrossRef]
- Chen, J.; Rattner, A.; Nathans, J. The Rod Photoreceptor-Specific Nuclear Receptor Nr2e3 Represses Transcription of Multiple Cone-Specific Genes. J. Neurosci. 2005, 25, 118–129. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Wada, Y.; Sukegawa, M.; Sawa, M.; Gomi, F.; Nishida, K.; Tano, Y. Age at Onset Curves of Retinitis Pigmentosa. Arch. Ophthalmol. 2008, 126, 337. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; A Wellman, J.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Ziccardi, L.; Cordeddu, V.; Gaddini, L.; Matteucci, A.; Parravano, M.; Malchiodi-Albedi, F.; Varano, M.; Albedi, M. Gene Therapy in Retinal Dystrophies. Int. J. Mol. Sci. 2019, 20, 5722. [Google Scholar] [CrossRef] [PubMed]
- Dyka, F.M.; Molday, L.L.; Chiodo, V.A.; Molday, R.S.; Hauswirth, W.W. Dual ABCA4-AAV Vector Treatment Reduces Pathogenic Retinal A2E Accumulation in a Mouse Model of Autosomal Recessive Stargardt Disease. Hum. Gene Ther. 2019, 30, 1361–1370. [Google Scholar] [CrossRef]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.S.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Bennett, L.D.; Duncan, J.L.; Weleber, R.G.; Pennesi, M.E. Long-term Follow-up of Patients With Retinitis Pigmentosa Receiving Intraocular Ciliary Neurotrophic Factor Implants. Am. J. Ophthalmol. 2016, 170, 10–14. [Google Scholar] [CrossRef]
- Talcott, K.E.; Ratnam, K.; Sundquist, S.M.; Lucero, A.S.; Lujan, B.J.; Tao, W.; Porco, T.C.; Roorda, A.; Duncan, J.L. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Investig. Opthalmol. Vis. Sci. 2011, 52, 2219–2226. [Google Scholar] [CrossRef]
- Falsini, B.; Iarossi, G.; Chiaretti, A.; Ruggiero, A.; Manni, L.; Galli-Resta, L.; Corbo, G.; Abed, E.; Manni, L. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J. Transl. Med. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Birch, D.G.; Bernstein, P.S.; Iannacone, A.; Pennesi, M.E.; Lam, B.L.; Heckenlively, J.; Csaky, K.; Hartnett, M.E.; Winthrop, K.L.; Jayasundera, K.T.; et al. Effect of Oral Valproic Acid vs Placebo for Vision Loss in Patients With Autosomal Dominant Retinitis Pigmentosa. JAMA Ophthalmol. 2018, 136, 849–856. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Iftikhar, M.; Hafiz, G.; Akhlaq, A.; Tsai, G.; Wehling, D.; Lu, L.; Wall, G.M.; Singh, M.S.; Kong, X. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J. Clin. Investig. 2020, 130, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-Derived Cone Viability Factor Promotes Cone Survival by Stimulating Aerobic Glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Mantelli, F.; Merlo, D.; Lambiase, A. Systematic Review of Randomized Clinical Trials on Safety and Efficacy of Pharmacological and Nonpharmacological Treatments for Retinitis Pigmentosa. J. Ophthalmol. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Barnea-Cramer, A.O.; Wang, W.; Lu, S.-J.; Singh, M.S.; Luo, C.; Huo, H.; McClements, M.E.; Barnard, A.R.; MacLaren, R.E.; Lanza, R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci. Rep. 2016, 6, 29784. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Mandai, M.; Takahashi, M. Gene and Induced Pluripotent Stem Cell Therapy for Retinal Diseases. Annu. Rev. Genom. Hum. Genet. 2019, 20, 201–216. [Google Scholar] [CrossRef]
- Radtke, N.D.; Aramant, R.B.; Seiler, M.; Petry, H.M.; Pidwell, D. Vision Change after Sheet Transplant of Fetal Retina With Retinal PigmentEpithelium to a Patient With Retinitis Pigmentosa. Arch. Ophthalmol. 2004, 122, 1159. [Google Scholar] [CrossRef]
- Simunovic, M.; Shen, W.; Lin, J.Y.; Protti, D.; Lisowski, L.; Gillies, M. Optogenetic approaches to vision restoration. Exp. Eye Res. 2019, 178, 15–26. [Google Scholar] [CrossRef]
- Tochitsky, I.; Kienzler, M.A.; Isacoff, E.Y.; Kramer, R.H. Restoring Vision to the Blind with Chemical Photoswitches. Chem. Rev. 2018, 118, 10748–10773. [Google Scholar] [CrossRef]
- Deisseroth, K.; Hegemann, P. The form and function of channelrhodopsin. Science. 2017, 357, eaan5544. [Google Scholar] [CrossRef]
- Da Cruz, L.; Dorn, J.D.; Humayun, M.S.; Dagnelie, G.; Handa, J.; Barale, P.-O.; Sahel, J.-A.; Stanga, P.E.; Hafezi, F.; Safran, A.B.; et al. Five-Year Safety and Performance Results from the Argus II Retinal Prosthesis System Clinical Trial. Ophthalmology 2016, 123, 2248–2254. [Google Scholar] [CrossRef]
- Chuang, A.T.; Margo, C.E.; Greenberg, P.B. Retinal implants: A systematic review. Br. J. Ophthalmol. 2014, 98, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Milam, A.H.; Rose, L.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.-X.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Sumaroka, A.; Aleman, T.S.; Cideciyan, A.V.; Schwartz, S.B.; Roman, A.J.; McInnes, R.R.; Sheffield, V.C.; Stone, E.M.; Swaroop, A.; et al. Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual degeneration. Hum. Mol. Genet. 2004, 13, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Bessant, D.A.; Payne, A.; Plant, C.; Bird, A.C.; Swaroop, A.; Bhattacharya, S.S. NRL S50T mutation and the importance of ‘founder effects’ in inherited retinal dystrophies. Eur. J. Hum. Genet. 2000, 8, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gimeno, M.; Maseras, M.; Baiget, M.; Beneito, M.; Antiñolo, G.; Ayuso, C.; Carballo, M. Mutations P51U and G122E in retinal transcription factor NRL associated with autosomal dominant and sporadic retinitis pigmentosa. Hum. Mutat. 2001, 17, 520. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, S.; Liu, C.; Qin, Y.; Archacki, S.; Jin, L.; Wang, Y.; Liu, F.; Chen, J.; Liu, Y.; et al. Whole exome sequencing identifies a novel NRL mutation in a Chinese family with autosomal dominant retinitis pigmentosa. Mol. Vis. 2016, 22, 234–242. [Google Scholar]
- Bessant, D.A.R.; Holder, G.; Fitzke, F.W.; Payne, A.; Bhattacharya, S.S.; Bird, A. Phenotype of Retinitis Pigmentosa Associated With the Ser50Thr Mutation in the NRL Gene. Arch. Ophthalmol. 2003, 121, 793. [Google Scholar] [CrossRef]
- Hernan, I.; Gamundi, M.; Borràs, E.; Maseras, M.; García-Sandoval, B.; Blanco-Kelly, F.; Ayuso, C.; Carballo, M. Novel p.M96T variant of NRL and shRNA-based suppression and replacement ofNRLmutants associated with autosomal dominant retinitis pigmentosa. Clin. Genet. 2011, 82, 446–452. [Google Scholar] [CrossRef]
- Newman, H.; Hanna, R.; Tiosano, B.; Perlman, I.; Ben-Yosef, T.; Blumen, S.C.; Braverman, I. Homozygosity for a Recessive Loss-of-Function Mutation of the NRL Gene Is Associated With a Variant of Enhanced S-Cone Syndrome. Investig. Opthalmol. Vis. Sci. 2016, 57, 5361–5371. [Google Scholar] [CrossRef]
- Beryozkin, A.; Shevah, E.; Kimchi, A.; Mizrahi-Meissonnier, L.; Khateb, S.; Ratnapriya, R.; Lazar, C.H.; Blumenfeld, A.; Ben-Yosef, T.; Hemo, Y.; et al. Whole Exome Sequencing Reveals Mutations in Known Retinal Disease Genes in 33 out of 68 Israeli Families with Inherited Retinopathies. Sci. Rep. 2015, 5, 13187. [Google Scholar] [CrossRef]
- To, K.W.; Adamian, M.; Jakobiec, F.A.; Berson, E.L. Clinical and Histopathologic Findings in Clumped Pigmentary Retinal Degeneration. Arch. Ophthalmol. 1996, 114, 950. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Michaelides, M.; Robson, A.; Hawlina, M.; Vaclavik, V.; Sandbach, J.M.; Neveu, M.M.; Hogg, C.R.; Hunt, D.M.; Moore, A.T.; et al. Phenotypic Variation in Enhanced S-cone Syndrome. Investig. Opthalmol. Vis. Sci. 2008, 49, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Rozet, J.-M.; Takezawa, S.-I.; Dos Santos, L.C.; Lopes, L.; Gribouval, O.; Penet, C.; Perrault, I.; Ducroq, D.; Souied, E.; et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Qual. Life Res. 2000, 107, 276–284. [Google Scholar] [CrossRef]
- Coppieters, F.; Leroy, B.P.; Beysen, D.; Hellemans, J.; De Bosscher, K.; Haegeman, G.; Robberecht, K.; Wuyts, W.; Coucke, P.J.; De Baere, E. Recurrent Mutation in the First Zinc Finger of the Orphan Nuclear Receptor NR2E3 Causes Autosomal Dominant Retinitis Pigmentosa. Am. J. Hum. Genet. 2007, 81, 147–157. [Google Scholar] [CrossRef]
- Hull, S.; Arno, G.; Sergouniotis, P.I.; Tiffin, P.; Borman, A.D.; Chandra, A.; Robson, A.; Holder, G.E.; Webster, A.R.; Moore, A.T. Clinical and Molecular Characterization of Enhanced S-Cone Syndrome in Children. JAMA Ophthalmol. 2014, 132, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.F.; Reddick, A.C.; Schwartz, S.B.; Ferguson, J.S.; Aleman, T.S.; Kellner, U.; Jurklies, B.; Schuster, A.; Zrenner, E.; Wissinger, B.; et al. Mutation analysis ofNR2E3 andNRL genes in Enhanced S Cone Syndrome. Hum. Mutat. 2004, 24, 439. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, A.; Kim, D.; Forrest, D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 2010, 11, 563–576. [Google Scholar] [CrossRef]
- Farjo, Q.; Jackson, A.; Pieke-Dahl, S.; Scott, K.; Kimberling, W.J.; Sieving, P.A.; Richards, J.E.; Swaroop, A. Human bZIP Transcription Factor GeneNRL:Structure, Genomic Sequence, and Fine Linkage Mapping at 14q11.2 and Negative Mutation Analysis in Patients with Retinal Degeneration. Genomics 1997, 45, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yang-Feng, T.L.; Swaroop, A. Neural retina-specific leucine zipper gene NRL (D14S46E) maps to human chromosome 14q11.1-q11.2. Genomics 1992, 14, 491–492. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Chen, S.; Scheurer, D.; Wang, Q.-L.; Duh, E.; Sung, C.-H.; Rehemtulla, A.; Swaroop, A.; Adler, R.; Zack, D.J. The bZIP Transcription Factor Nrl Stimulates Rhodopsin Promoter Activity in Primary Retinal Cell Cultures. J. Boil. Chem. 1996, 271, 29612–29618. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Mears, A.J.; Friedman, J.S.; Carter, T.; He, S.; Oh, E.; Jing, Y.; Farjo, R.; Fleury, G.; Barlow, C.; et al. Expression profiling of the developing and mature Nrl/ mouse retina: Identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum. Mol. Genet. 2004, 13, 1487–1503. [Google Scholar] [CrossRef]
- Ng, L.; Lu, A.; Swaroop, A.; Sharlin, D.S.; Swaroop, A.; Forrest, U. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J. Neurosci. 2011, 31, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Zelinger, L.; Karakülah, G.; Chaitankar, V.; Kim, J.-W.; Yang, H.-J.; Brooks, M.J.; Swaroop, A. Regulation of Noncoding Transcriptome in Developing Photoreceptors by Rod Differentiation Factor NRL. Investig. Opthalmology Vis. Sci. 2017, 58, 4422–4435. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Yang, H.-J.; Brooks, M.J.; Zelinger, L.; Karakülah, G.; Gotoh, N.; Boleda, A.; Gieser, L.; Giuste, F.; Whitaker, D.T.; et al. NRL-Regulated Transcriptome Dynamics of Developing Rod Photoreceptors. Cell Rep. 2016, 17, 2460–2473. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, N.B.; Piriev, N.I.; Chang, B.; Rapoport, A.L.; Hawes, N.L.; Nishina, P.M.; Nusinowitz, S.; Heckenlively, J.R.; Roderick, T.H.; Kozak, C.A.; et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 5551–5556. [Google Scholar] [CrossRef]
- Cheng, H.; Khan, N.W.; E Roger, J.; Swaroop, A. Excess cones in the retinal degeneration rd7 mouse, caused by the loss of function of orphan nuclear receptor Nr2e3, originate from early-born photoreceptor precursors. Hum. Mol. Genet. 2011, 20, 4102–4115. [Google Scholar] [CrossRef]
- Oh, E.C.T.; Cheng, H.; Hao, H.; Jia, L.; Khan, N.W.; Swaroop, A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008, 1236, 16–29. [Google Scholar] [CrossRef]
- Cheng, H.; Aleman, T.S.; Cideciyan, A.V.; Khanna, R.; Jacobson, S.G.; Swaroop, A. In vivo function of the orphan nuclear receptor NR2E3 in establishing photoreceptor identity during mammalian retinal development. Hum. Mol. Genet. 2006, 15, 2588–2602. [Google Scholar] [CrossRef]
- A McIlvain, V.; Knox, B.E. Nr2e3 andNrl can reprogram retinal precursors to the rod fate inXenopus retina. Dev. Dyn. 2007, 236, 1970–1979. [Google Scholar] [CrossRef]
- Haider, N.B.; Naggert, J.; Nishina, P.M. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum. Mol. Genet. 2001, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Mollema, N.; Gaule, M.; Yuan, Y.; Sachs, A.J.; Nystuen, A.M.; Naggert, J.K.; Nishina, P.M. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp. Eye Res. 2009, 89, 365–372. [Google Scholar] [CrossRef]
- Haider, N.B.; Demarco, P.; Nystuen, A.M.; Huang, X.; Smith, R.S.; McCall, M.A.; Naggert, J.K.; Nishina, P.M. The transcription factorNr2e3functions in retinal progenitors to suppress cone cell generation. Vis. Neurosci. 2006, 23, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Montana, C.L.; Kolesnikov, A.V.; Shen, S.Q.; Myers, C.A.; Kefalov, V.; Corbo, J.C. Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mookherjee, S.; Chaitankar, V.; Hiriyanna, S.; Kim, J.-W.; Brooks, M.; Ataeijannati, Y.; Sun, X.; Dong, L.; Li, T.; et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 2017, 8, 14716. [Google Scholar] [CrossRef]
- Zhu, J.; Ming, C.; Fu, X.; Duan, Y.; Hoang, D.A.; Rutgard, J.; Zhang, R.; Wang, W.; Hou, R.; Zhang, D.; et al. Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors. Cell Res. 2017, 27, 830–833. [Google Scholar] [CrossRef]
- Nakamura, P.A.; Tang, S.; Shimchuk, A.A.; Ding, S.; Reh, T.A. Potential of Small Molecule–Mediated Reprogramming of Rod Photoreceptors to Treat Retinitis Pigmentosa. Investig. Opthalmology Vis. Sci. 2016, 57, 6407–6415. [Google Scholar] [CrossRef]
- A Nakamura, P.; A Shimchuk, A.; Tang, S.; Wang, Z.; DeGolier, K.; Ding, S.; Reh, T.A. Small molecule Photoregulin3 prevents retinal degeneration in the RhoP23H mouse model of retinitis pigmentosa. eLife 2017, 6. [Google Scholar] [CrossRef]
- Naessens, S.; Ruysschaert, L.; Lefever, S.; Coppieters, F.; De Baere, E. Antisense Oligonucleotide-Based Downregulation of the G56R Pathogenic Variant Causing NR2E3-Associated Autosomal Dominant Retinitis Pigmentosa. Genes 2019, 10, 363. [Google Scholar] [CrossRef]
- Li, S.; Datta, S.; Brabbit, E.; Love, Z.; Woytowicz, V.; Flattery, K.; Capri, J.; Yao, K.; Wu, S.; Imboden, M.; et al. Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa. Gene Ther. 2020, 1–19. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef] [PubMed]
- E Roger, J.; Ranganath, K.; Zhao, L.; Cojocaru, R.I.; Brooks, M.; Gotoh, N.; Veleri, S.; Hiriyanna, A.; Rachel, R.A.; Campos, M.M.; et al. Preservation of cone photoreceptors after a rapid yet transient degeneration and remodeling in cone-only Nrl-/- mouse retina. J. Neurosci. 2012, 32, 528–541. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Treatment Strategy | Advantages | Disadvantages | Clinical Trial Examples | Noteworthy Outcomes |
|---|---|---|---|---|
| Neuroprotection | -Simplicity of drug delivery/noninvasive -FDA-approved agents | -Unproven in neurodegenerative disease -Importance of timing early in disease process | -CNTF [27,28,29] -NGF [30] -Valproic acid [31] -NAC [32] -RdCVF [33] | -CNTF intraocular implant: inferior to sham control -Topical NGF: vision improved in minority of patients -PO valproic acid: negative results vs. placebo |
| Regenerative medicine/cell transplant | -Theoretical capacity to replace lost cells/tissues -Future ability to reprogram patient-derived stem cells to avoid immune rejection | -May require surgical intervention -Uncertain potential for cellular integration and function -Potential for immune rejection in allografts | -Bone-marrow derived stem cells [36] -Human embryonic stem cell derived retinal/neural progenitor cells | -No long-term benefits reported from BMDSCs -hESC retinal/neural progenitor cell transplants recruiting/underway (NCT02384293, 02464436, 03073733, 02320812) |
| Optogenetics/prostheses | -Ability to increase light sensitivity | -Poor visual resolution -Unproven durability | -Channelrhodopsins [39,40,41] -Photoswitches [40] -Retinal prostheses [42,43] | -Channelrhodopsin trials recruiting/underway -Argus II retinal prosthesis– improved 5yr visual function -Alpha IMS retinal prosthesis recent CE approval in EU |
| Photoreceptor reprogramming | -Conferring resistance to degenerative photoreceptor loss | -Modification of intrinsic visual physiology -Potential changes in visual perception -Requires timely intervention |
| Study | Model(s) | Experimental Manipulation | Timing | Effects on Visual Physiology | Effects on Retinal Degeneration | Effects on Gene/Protein Expression |
|---|---|---|---|---|---|---|
| Mears 2001 PMID 11694879 [20] | wt mice | Germline Nrl deletion | Germline knockout | -Absent ERG scotopic rod response -2-3x increased ERG photopic cone response -6x increased ERG 400nm S-cone response | -ONL photoreceptor nuclei appear cone-like, formation of rosette-like structures | -Absent Nr2e3, Pdeb, Rho, Gnat1 (rod gene) expression -Increased Opn1sw, Gnat2, Car (cone gene) expression |
| Montana 2013 PMID 23319618 [75] | -Nrlfl/fl CAG-Cre -Nrlfl/fl CAG-Cre, Rho-/- germline mice | -Tamoxifen injection inducing Cre-recombinase expression | -KO P42, analysis P63 (Nrlfl/fl CAG-Cre) -KO P25-P28, analysis P90 (Nrlfl/fl CAG-Cre Rho-/-) | -Nrl KO: significantly decreased scotopic (rod) and increased photopic (cone) function in vivo; 35x desensitization and rapid inactivation of photoresponse -Rho-/- Nrl KO: increased photopic ERG cone function | -Nrl KO: variable ONL waviness, no rosettes -Rho-/- Nrl KO: increased ONL cell density, cone opsin expression | -Nrl KO: absent Nr2e3, Rho, Gnat1, Gnb1 (rod gene) expression; increased Gnat2, Gnb3 (cone gene) expression |
| Yu 2017 PMID 28291770 [76] | -wt mice -Rho-/- -Pde6β-/- -RhoP347S | -Subretinal injection of AAV8-CRISPR/Cas9 Nrl sgRNA construct | -Injection P14, analysis P90-P105 | -wt Nrl KO: decreased scotopic ERG rod function, stable ERG cone function -Rho-/- Nrl KO: slowed decline in photopic ERG function -Pde6β--/- Nrl KO: preserved photopic ERG function -RhoP347S Nrl KO: slower decline of photopic ERG function, improved optomotor response | -wt Nrl KO: no significant retinal structure changes -Rho-/- Nrl KO, Pde6β--/- Nrl KO, RhoP347S Nrl KO: preserved ONL cell density | -wt Nrl KO: mild decreased rod gene expression, increased Gnb3, Arr3 (cone) gene expression -Rho-/- Nrl KO: increased S-opsin staining -Pde6β--/- Nrl KO: increased cone arrestin, S-opsin staining - RhoP347S Nrl KO: increased S-opsin staining |
| Zhu 2017 PMID 28429769 [77] | -wt mice- Pde6β-/- | -Subretinal injection of AAV-CRISPR/Cas9 Nrl or Nr2e3 double sgRNA construct | -Injection P7, immunohistochemistry P30 or P50, ERG P50 or P60 | -Pde6β-/- Nrl or Nr2e3 KO: increased photopic ERG function, no effect on scotopic ERG a-wave, small increase in scotopic ERG b-wave | -Pde6β-/- Nrl or Nr2e3 KO: increased ONL thickness | -wt and Pde6β-/- Nrl or Nr2e3 KO: increased cone arrestin+ cells in ONL |
| Haider 2000 PMID 10655056 [68] | Human ESCS patients with NR2E3 mutations | none | Germline | -12 degree visual field testing: decreased sensitivity to rod and L/M cone stimuli, 30x increased sensitivity to S-cone stimuli -increased ERG response to 450nm stimulus | -variable OCT abnormalities including foveal cysts | |
| Haider 2001 PMID 11487564 [14] | wt mice | Nr2e3 knockout | Germline | -Disrupted ONL, whorl formation -Increased cone cells, including 203x increased blue-opsin+ cones | See Corbo and Cepko 2005 PMID 16110338 – hybrid rod-cone gene expression in Nr2e3-/- mouse retina | |
| Milam 2002 PMID 11773633 [44] | Human ESCS patients with NR2E3 mutations | none | Germline | -Visual field testing: supranormal S cone function and S:L/M cone function | -Fewer layers of ONL photoreceptor nuclei -No rods detected -Increased S-opsin staining | |
| Nakamura 2017 PMID 29148976 [79] | -wt mice -RhoP23H | Intraperitoneal injection of Nr2e3 inhibitor PR3 | Injection P12-P14 or P21, analysis P14 or P21 | -RhoP23H: significantly increased scotopic and photopic ERG function | -wt: increased S-opsin+ cells, truncated photoreceptor outer segments -RhoP23H: protection against ONL photoreceptor loss/increased ONL thickness | -wt: reduction of rod gene expression, unchanged cone gene expression -RhoP23H: increased Rcvrn and Rho expression |
| Li 2020 PMID 32123325 [81] | -wt mice -Pde6β--/- -Rho-/- -RhoP23H -Cep290-/- -Nr2e3-/- | Subretinal injection of AAV8-Nr2e3 overexpression vector | -Injection P0, analysis P30 or P90-P120 -Injection P21, analysis P80-P110 | -wt mice: no ERG changes -Pde6β-/-, Rho-/-, RhoP23H, Cep290-/-: partial rescue of scotopic ERG function | -wt mice: no retinal changes -RhoP23H, Cep290-/-, Nr2e3-/-: improved fundus exam findings -Rho-/-, RhoP23H, Cep290-/-, Nr2e3-/-: preserved retinal integrity | -Rho-/-, RhoP23H, Cep290-/-, Nr2e3-/-: increased rhodopsin and blue/green opsin expression; extensive gene expression changes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, S.M.; Skowronska-Krawczyk, D.; Chao, D.L. Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa. J. Clin. Med. 2020, 9, 2224. https://doi.org/10.3390/jcm9072224
Moore SM, Skowronska-Krawczyk D, Chao DL. Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa. Journal of Clinical Medicine. 2020; 9(7):2224. https://doi.org/10.3390/jcm9072224
Chicago/Turabian StyleMoore, Spencer M., Dorota Skowronska-Krawczyk, and Daniel L. Chao. 2020. "Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa" Journal of Clinical Medicine 9, no. 7: 2224. https://doi.org/10.3390/jcm9072224
APA StyleMoore, S. M., Skowronska-Krawczyk, D., & Chao, D. L. (2020). Targeting of the NRL Pathway as a Therapeutic Strategy to Treat Retinitis Pigmentosa. Journal of Clinical Medicine, 9(7), 2224. https://doi.org/10.3390/jcm9072224





