Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intraoperative Protocols
2.3. Definition of a Significant Decline in the GFR
2.4. Primary and Secondary Outcomes
2.5. Data Collection and Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Bachir, B.G.; Kassouf, W. Urinary diversions: Advantages and disadvantages of the major types of diversions. Curr. Opin. Support Palliat Care 2013, 7, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.J.; Gilbert, W.B.; Koch, M.O.; Smith, J.A., Jr. Continent urinary reconstruction versus ileal conduit: A contemporary single-institution comparison of perioperative morbidity and mortality. Urology 2000, 55, 852–855. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, R.; Sinha, R.J.; Gupta, D.K. Prospective comparison of quality-of-life outcomes between ileal conduit urinary diversion and orthotopic neobladder reconstruction after radical cystectomy: A statistical model. BJU Int. 2014, 113, 726–732. [Google Scholar] [CrossRef]
- Jin, X.D.; Roethlisberger, S.; Burkhard, F.C.; Birkhaeuser, F.; Thoeny, H.C.; Studer, U.E. Long-term renal function after urinary diversion by ileal conduit or orthotopic ileal bladder substitution. Eur. Urol. 2012, 61, 491–497. [Google Scholar] [CrossRef]
- Ahmed, Y.E.; Hussein, A.A.; May, P.R.; Ahmad, B.; Ali, T.; Durrani, A.; Khan, S.; Kumar, P.; Guru, K.A. Natural History, Predictors and Management of Ureteroenteric Strictures after Robot Assisted Radical Cystectomy. J. Urol. 2017, 198, 567–574. [Google Scholar] [CrossRef]
- Large, M.C.; Cohn, J.A.; Kiriluk, K.J.; Dangle, P.; Richards, K.A.; Smith, N.D.; Steinberg, G.D. The impact of running versus interrupted anastomosis on ureterointestinal stricture rate after radical cystectomy. J. Urol. 2013, 190, 923–927. [Google Scholar] [CrossRef]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 2009, 55, 164–174. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Tighiouart, H.; Sang, Y.; Ballew, S.; Mondal, H.; Matsushita, K.; Coresh, J.; Levey, A.S.; Inker, L.A. GFR decline and subsequent risk of established kidney outcomes: A meta-analysis of 37 randomized controlled trials. Am. J. Kidney Dis. 2014, 64, 860–866. [Google Scholar] [CrossRef]
- Matsushita, K.; Chen, J.; Sang, Y.; Ballew, S.H.; Shimazaki, R.; Fukagawa, M.; Imai, E.; Coresh, J.; Hishida, A. Risk of end-stage renal disease in Japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int. 2016, 90, 1109–1114. [Google Scholar] [CrossRef]
- Shimko, M.S.; Tollefson, M.K.; Umbreit, E.C.; Farmer, S.A.; Blute, M.L.; Frank, I. Long-term complications of conduit urinary diversion. J. Urol. 2011, 185, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Rouanne, M.; Perreaud, A.; Letang, N.; Yonneau, L.; Neuzillet, Y.; Herve, J.M.; Botto, H.; Lebret, T. Trends in renal function after radical cystectomy and ileal conduit diversion: New insights regarding estimated glomerular filtration rate variations. Clin. Genitourin Cancer 2015, 13, e139–e144. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.J.; Kim, J.; Kim, H.G.; Koh, G.H.; Hwang, J.H.; Kim, Y.K. Risk factors of postoperative major adverse cardiac events after radical cystectomy: Implication of diastolic dysfunction. Sci. Rep. 2019, 9, 14096. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.G.; Kim, J.Y.; Yu, J.; Lim, J.; Hwang, J.H.; Kim, Y.K. Efficacy and Safety of Stroke Volume Variation-Guided Fluid Therapy for Reducing Blood Loss and Transfusion Requirements During Radical Cystectomy: A Randomized Clinical Trial. Medicine 2016, 95, e3685. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.G.; You, D.; Kim, J.W.; Song, C.; Hong, J.H.; Ahn, H.; Kim, C.S. Outcomes of single lymph node positive urothelial carcinoma after radical cystectomy. J. Urol. 2011, 185, 2085–2090. [Google Scholar] [CrossRef]
- Jeong, I.G.; You, D.; Kim, J.; Kim, S.C.; Hong, J.H.; Ahn, H.; Kim, C.S. Factors associated with non-orthotopic urinary diversion after radical cystectomy. World J. Urol. 2012, 30, 815–820. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Jeong, T.D.; Lee, W.; Yun, Y.M.; Chun, S.; Song, J.; Min, W.K. Development and validation of the Korean version of CKD-EPI equation to estimate glomerular filtration rate. Clin. Biochem. 2016, 49, 713–719. [Google Scholar] [CrossRef]
- Lane, B.R.; Babineau, D.C.; Poggio, E.D.; Weight, C.J.; Larson, B.T.; Gill, I.S.; Novick, A.C. Factors predicting renal functional outcome after partial nephrectomy. J. Urol. 2008, 180, 2363–2368. [Google Scholar] [CrossRef]
- Gilbert, J.; Lovibond, K.; Mooney, A.; Dudley, J. Renal replacement therapy: Summary of NICE guidance. BMJ 2018, 363, k4303. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Comperat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Roupret, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Okusa, M.D.; Davenport, A. Reading between the (guide)lines—The KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int. 2014, 85, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.W.; Choi, S.S.; Kong, Y.G.; Yu, J.; Lim, J.; Hwang, J.H.; Kim, Y.K. Incidence and Risk Factors of Acute Kidney Injury after Radical Cystectomy: Importance of Preoperative Serum Uric Acid Level. Int. J. Med. Sci. 2015, 12, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E.; de Petriconi, R.; Gottfried, H.W.; Kleinschmidt, K.; Mattes, R.; Paiss, T. The ileal neobladder: Complications and functional results in 363 patients after 11 years of followup. J. Urol. 1999, 161, 422–427. [Google Scholar] [CrossRef]
- Bricker, E.M. Bladder substitution after pelvic evisceration. Surg. Clin. North. Am. 1950, 30, 1511–1521. [Google Scholar] [CrossRef]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical Risk Factors, Morbidity, and Mortality in Elderly Patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- Law, S.; Wong, K.-H.; Kwok, K.-F.; Chu, K.-M.; Wong, J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann. Surg. 2004, 240, 791–800. [Google Scholar] [CrossRef]
- Studer, U.E.; Zingg, E.J. Ileal orthotopic bladder substitutes. What we have learned from 12 years’ experience with 200 patients. Urol. Clin. North. Am. 1997, 24, 781–793. [Google Scholar] [CrossRef]
- Hautmann, R.E.; de Petriconi, R.C.; Volkmer, B.G. 25 years of experience with 1,000 neobladders: Long-term complications. J. Urol. 2011, 185, 2207–2212. [Google Scholar] [CrossRef]
- Lantz, A.G.; Saltel, M.E.; Cagiannos, I. Renal and functional outcomes following cystectomy and neobladder reconstruction. Can. Urol. Assoc. J. 2010, 4, 328–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joung, K.W.; Kong, Y.G.; Yoon, S.H.; Kim, Y.J.; Hwang, J.H.; Hong, B.; Kim, Y.K. Comparison of postoperative acute kidney injury between ileal conduit and neobladder urinary diversions after radical cystectomy: A propensity score matching analysis. Medicine 2016, 95, e4838. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, S.; Noto, L.; Schiavon, R.; Bonciarelli, M.; Petrosino, L.; Cavallini, L.; Zambello, A.; Olivo, G. Metabolic aspects of intestinal urinary diversion. Comparison with ileo-cecal bladder substitution and ileal conduct. Clin. Ter. 1994, 144, 223–229. [Google Scholar] [PubMed]
- Ku, J.H.; Lerner, S.P. Variables Affecting Long-term Maintenance of Renal Function Following Ileal Based Urinary Diversion. Eur. Urol. 2012, 61, 498–500. [Google Scholar] [CrossRef]
- Hautmann, R.E.; Abol-Enein, H.; Davidsson, T.; Gudjonsson, S.; Hautmann, S.H.; Holm, H.V.; Lee, C.T.; Liedberg, F.; Madersbacher, S.; Manoharan, M.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Urinary diversion. Eur. Urol. 2013, 63, 67–80. [Google Scholar] [CrossRef]
- Eisenberg, M.S.; Thompson, R.H.; Frank, I.; Kim, S.P.; Cotter, K.J.; Tollefson, M.K.; Kaushik, D.; Thapa, P.; Tarrell, R.; Boorjian, S.A. Long-term renal function outcomes after radical cystectomy. J. Urol. 2014, 191, 619–625. [Google Scholar] [CrossRef]
- Zabell, J.R.; Adejoro, O.; Konety, B.R.; Weight, C.J. Risk of End Stage Kidney Disease after Radical Cystectomy According to Urinary Diversion Type. J. Urol. 2015, 193, 1283–1287. [Google Scholar] [CrossRef]
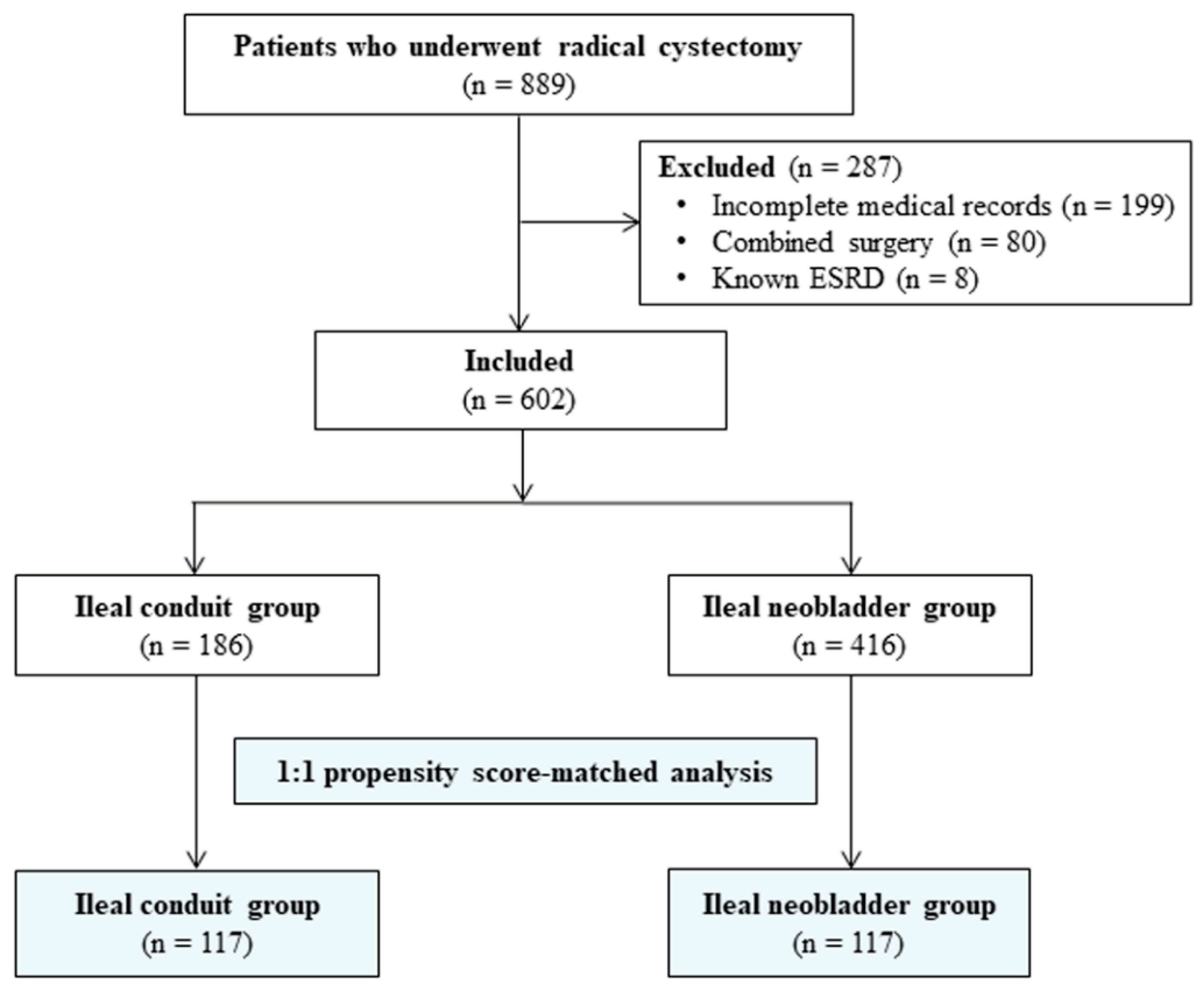

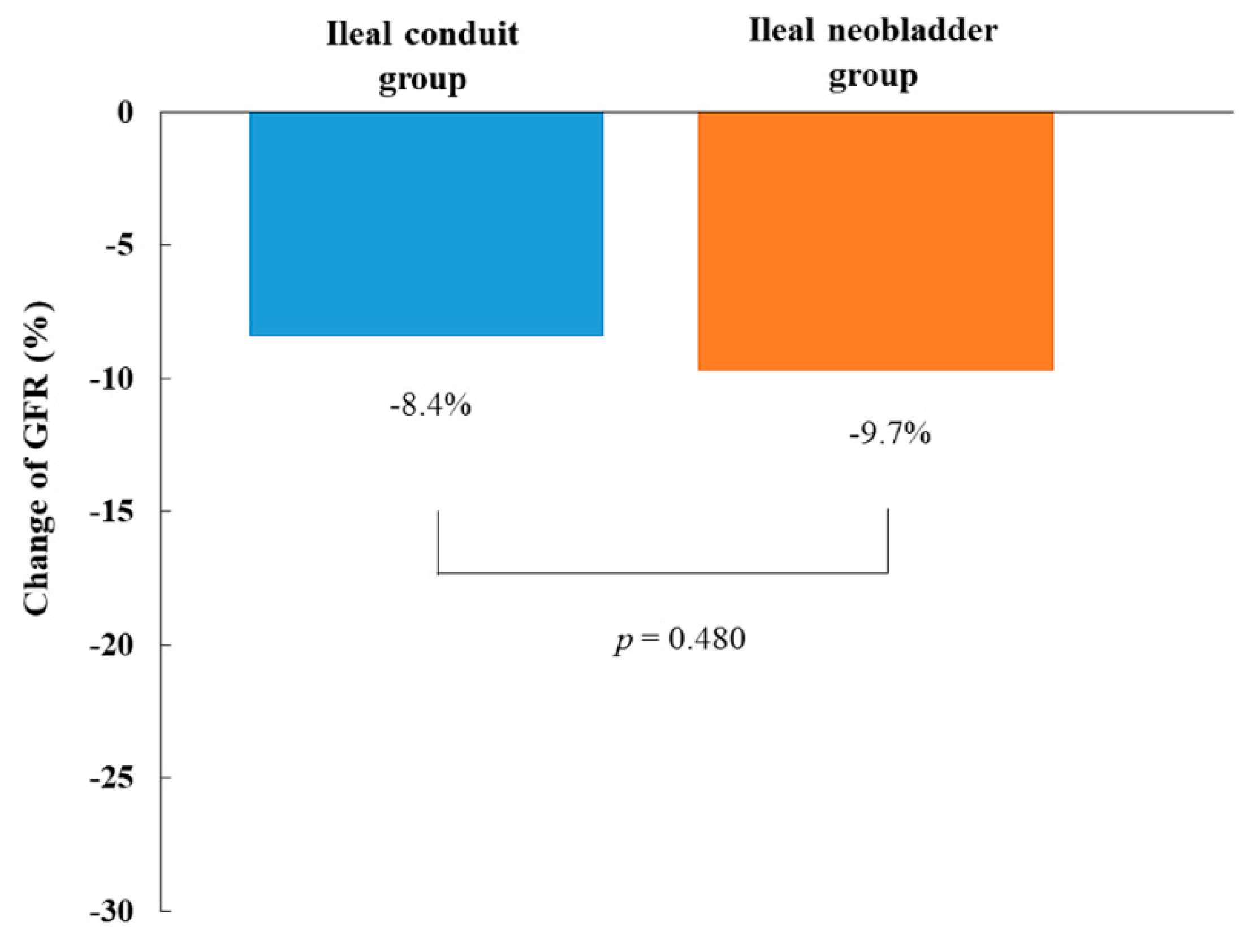
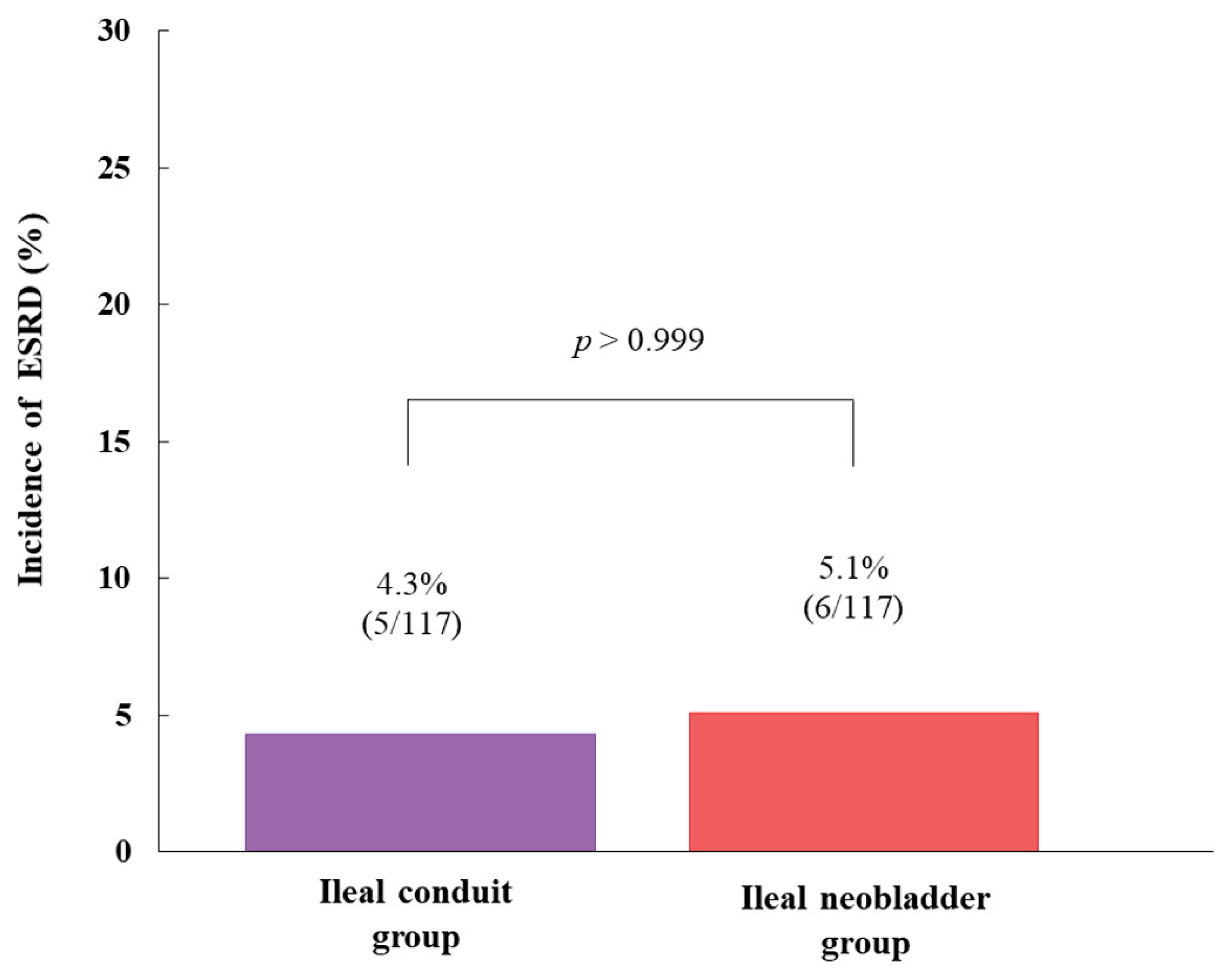
| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Ileal Conduit Group (n = 186) | Ileal Neobladder Group (n = 416) | SMD | p-Value | Ileal Conduit Group (n = 117) | Ileal Neobladder Group (n = 117) | SMD | p-Value |
| Sex (male) | 125 (67.2) | 387 (93.0) | 1.013 | <0.001 | 21 (17.9) | 22 (18.8) | 0.034 | >0.999 |
| Age (years) | 69 ± 10 | 61 ± 9 | −0.812 | <0.001 | 67 ± 10 | 66 ± 9 | −0.138 | 0.212 |
| Body mass index (kg/m2) | 23.7 ± 3.1 | 24.6 ± 3.1 | 0.277 | 0.002 | 23.8 ± 3.1 | 23.8 ± 3.3 | −0.011 | 0.931 |
| ASA Physical Status | −0.335 | 0.002 | <0.001 | >0.999 | ||||
| ≤2 | 160 (86.0) | 391 (94.0) | 105 (89.7) | 105 (89.7) | ||||
| 3 | 26 (14.0) | 25 (6.0) | 12 (10.3) | 12 (10.3) | ||||
| Diabetes mellitus | 37 (19.9) | 70 (16.8) | −0.082 | 0.419 | 20 (17.1) | 20 (17.1) | <0.001 | >0.999 |
| Hypertension | 96 (51.6) | 153 (36.5) | −0.307 | <0.001 | 52 (44.4) | 45 (38.5) | −0.124 | 0.401 |
| Atrial fibrillation | 8 (4.3) | 5 (1.2) | −0.284 | 0.028 | 2 (1.7) | 1 (0.9) | −0.078 | >0.999 |
| Coronary artery disease | 12 (6.5) | 14 (3.4) | −0.171 | 0.126 | 6 (5.1) | 6 (5.1) | <0.001 | >0.999 |
| Cerebrovascular disease | 9 (4.8) | 7 (1.7) | −0.245 | 0.032 | 4 (3.4) | 5 (4.3) | 0.066 | >0.999 |
| COPD | 5 (2.7) | 15 (3.6) | 0.049 | 0.633 | 5 (4.3) | 2 (1.7) | −0.137 | 0.453 |
| Chronic kidney disease | 17 (9.1) | 7 (1.7) | −0.499 | <0.001 | 3 (2.6) | 6 (5.1) | 0.139 | 0.508 |
| Medications | ||||||||
| ACEi or ARB | 33 (17.7) | 51 (12.3) | −0.167 | 0.076 | 17 (14.5) | 13 (11.1) | −0.104 | 0.503 |
| Diuretic | 14 (7.5) | 11 (2.6) | −0.304 | 0.008 | 5 (4.3) | 5 (4.3) | <0.001 | >0.999 |
| Calcium channel blocker | 62 (33.3) | 98 (23.6) | −0.230 | 0.013 | 32 (27.4) | 30 (25.6) | −0.040 | 0.878 |
| Beta blocker | 14 (7.5) | 20 (4.8) | −0.127 | 0.251 | 8 (6.8) | 7 (6.0) | −0.040 | >0.999 |
| Plavix | 8 (4.3) | 3 (0.7) | −0.423 | 0.005 | 2 (1.7) | 2 (1.7) | <0.001 | >0.999 |
| Aspirin | 22 (11.8) | 18 (4.3) | −0.368 | 0.001 | 9 (7.7) | 7 (6.0) | −0.084 | 0.774 |
| Tumor stage | −0.118 | 0.198 | <0.001 | >0.999 | ||||
| ≤2 | 129 (69.4) | 310 (74.5) | 85 (72.6) | 85 (72.6) | ||||
| ≥3 | 57 (30.6) | 106 (25.5) | 32 (27.4) | 32 (27.4) | ||||
| Tumor grade | −0.013 | >0.999 | <0.001 | >0.999 | ||||
| 2 | 8 (4.3) | 19 (4.6) | 5 (4.3) | 5 (4.3) | ||||
| 3 | 178 (95.7) | 397 (95.4) | 112 (95.7) | 112 (95.7) | ||||
| Neo-adjuvant chemotherapy | 39 (21.0) | 79 (19.0) | −0.050 | 0.580 | 24 (20.5) | 28 (23.9) | 0.087 | 0.618 |
| Hydronephrosis | 36 (19.4) | 28 (6.7) | −0.503 | <0.001 | 17 (14.5) | 13 (11.1) | −0.136 | 0.572 |
| Preoperative laboratory data | ||||||||
| White blood cell (103/µL) | 6.5 ± 2.2 | 6.7 ± 2.5 | 0.046 | 0.589 | 6.6 ± 2.3 | 6.6 ± 3.0 | 0.026 | 0.846 |
| Neutrophil (%) | 57.9 ± 11.8 | 56.5 ± 11.5 | −0.124 | 0.166 | 57.1 ± 11.2 | 56.9 ± 11.2 | −0.019 | 0.887 |
| Lymphocyte (%) | 29.9 ± 10.3 | 31.3 ± 9.9 | 0.139 | 0.119 | 30.4 ± 9.5 | 30.4 ± 10.3 | 0.003 | 0.980 |
| Hemoglobin (g/dL) | 11.7 ± 1.8 | 12.8 ± 1.9 | 0.608 | <0.001 | 12.0 ± 1.8 | 12.1 ± 1.8 | 0.050 | 0.663 |
| Platelet (103/µL) | 237.0 ± 83.9 | 245.4 ± 80.7 | 0.104 | 0.242 | 236.1 ± 84.9 | 248.6 ± 90.9 | 0.155 | 0.284 |
| Creatinine (mg/dL) | 1.1 ± 0.4 | 1.0 ± 0.2 | −0.503 | <0.001 | 1.0 ± 0.3 | 1.0 ± 0.3 | −0.018 | 0.921 |
| GFR (mL/min/1.73 m2) | 86.8 ± 17.7 | 97.4 ± 17.1 | 0.619 | <0.001 | 91.0 ± 18.3 | 91.5 ± 19.0 | 0.032 | 0.820 |
| Albumin (g/dL) | 3.6 ± 0.4 | 3.9 ± 0.4 | 0.721 | <0.001 | 3.7 ± 0.4 | 3.6 ± 0.4 | −0.066 | 0.612 |
| AST (IU/L) | 21.3 ± 7.7 | 22.9 ± 8.7 | 0.178 | 0.038 | 21.6 ± 8.0 | 20.9 ± 6.5 | −0.082 | 0.431 |
| ALT (IU/L) | 17.2 ± 9.8 | 22.5 ± 15.7 | 0.337 | <0.001 | 18.3 ± 9.9 | 17.6 ± 11.8 | −0.042 | 0.636 |
| Sodium (mmol/L) | 140 ± 3 | 140 ± 3 | 0.016 | 0.859 | 140 ± 3 | 140 ± 2 | −0.038 | 0.762 |
| Potassium (mmol/L) | 4.3 ± 0.4 | 4.4 ± 0.4 | 0.043 | 0.638 | 4.3 ± 0.3 | 4.3 ± 0.4 | −0.029 | 0.823 |
| Chloride (mmol/L) | 105 ± 4 | 104 ± 3 | −0.187 | 0.042 | 105 ± 3 | 105 ± 3 | −0.081 | 0.513 |
| Uric acid (mg/dL) | 5.1 ± 1.6 | 5.4 ± 1.5 | 0.194 | 0.032 | 5.2 ± 1.4 | 5.2 ± 1.6 | 0.060 | 0.639 |
| Variables | All Patients (n = 234) | Ileal Conduit Group (n = 117) | Ileal Neobladder Group (n = 117) | p-Value |
|---|---|---|---|---|
| Operation time (minute) | 435 ± 111 | 434 ± 118 | 436 ± 104 | 0.929 |
| Crystalloid amount (mL) | 3542 ± 1396 | 3637 ± 1629 | 3446 ± 1114 | 0.314 |
| Colloid amount (mL) | 547 ± 432 | 579 ± 513 | 515 ± 403 | 0.285 |
| Red blood cell transfusion rate | 128 (54.7) | 65 (55.6) | 63 (53.8) | 0.896 |
| Hospital stay (days) | 26 ± 28 | 25 ± 33 | 27 ± 21 | 0.628 |
| Intensive care unit admission rate | 54 (23.1) | 30 (25.6) | 24 (20.5) | 0.392 |
| Intensive care unit stay (days) | 0.2 ± 0.5 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.240 |
| Acute kidney injury | 64 (27.4) | 35 (29.9) | 29 (24.8) | 0.451 |
| Ureterointestinal stricture | 20 (8.5) | 5 (4.3) | 15 (12.8) | 0.031 |
| Adjuvant chemotherapy | 99 (42.3) | 52 (44.4) | 47 (40.2) | 0.615 |
| Variables | All Patients (n = 234) | Ileal Conduit Group (n = 117) | Ileal Neobladder Group (n = 117) | p-Value |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 12.5 ± 2.0 | 12.6 ± 2.0 | 12.3 ± 2.0 | 0.294 |
| Sodium (mmol/L) | 138 ± 3 | 138 ± 3 | 138 ± 4 | 0.866 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.5 | 0.880 |
| Chloride (mmol/L) | 105 ± 4 | 104 ± 3 | 105 ± 4 | 0.475 |
| Uric acid (mg/dL) | 5.5 ± 1.4 | 5.6 ± 1.4 | 5.5 ± 1.5 | 0.701 |
| Creatinine (mg/dL) | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.4 | 0.289 |
| GFR (mL/min/1.73 m2) | 82.0 ± 17.1 | 82.6 ± 19.8 | 81.4 ± 13.8 | 0.586 |
| Mortality | 5 (2.1) | 2 (1.7) | 3 (2.6) | >0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Hong, B.; Park, J.-Y.; Lee, Y.; Hwang, J.-H.; Kong, Y.-G.; Kim, Y.-K. Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis. J. Clin. Med. 2020, 9, 2236. https://doi.org/10.3390/jcm9072236
Yu J, Hong B, Park J-Y, Lee Y, Hwang J-H, Kong Y-G, Kim Y-K. Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2020; 9(7):2236. https://doi.org/10.3390/jcm9072236
Chicago/Turabian StyleYu, Jihion, Bumsik Hong, Jun-Young Park, Yongsoo Lee, Jai-Hyun Hwang, Yu-Gyeong Kong, and Young-Kug Kim. 2020. "Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 9, no. 7: 2236. https://doi.org/10.3390/jcm9072236
APA StyleYu, J., Hong, B., Park, J.-Y., Lee, Y., Hwang, J.-H., Kong, Y.-G., & Kim, Y.-K. (2020). Comparison of a Significant Decline in the Glomerular Filtration Rate between Ileal Conduit and Ileal Neobladder Urinary Diversions after Radical Cystectomy: A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 9(7), 2236. https://doi.org/10.3390/jcm9072236






